On-Chip Liquid Chromatography
Definition
1. Introduction
2. Approaches
2.1. Open-Tubular Chromatography
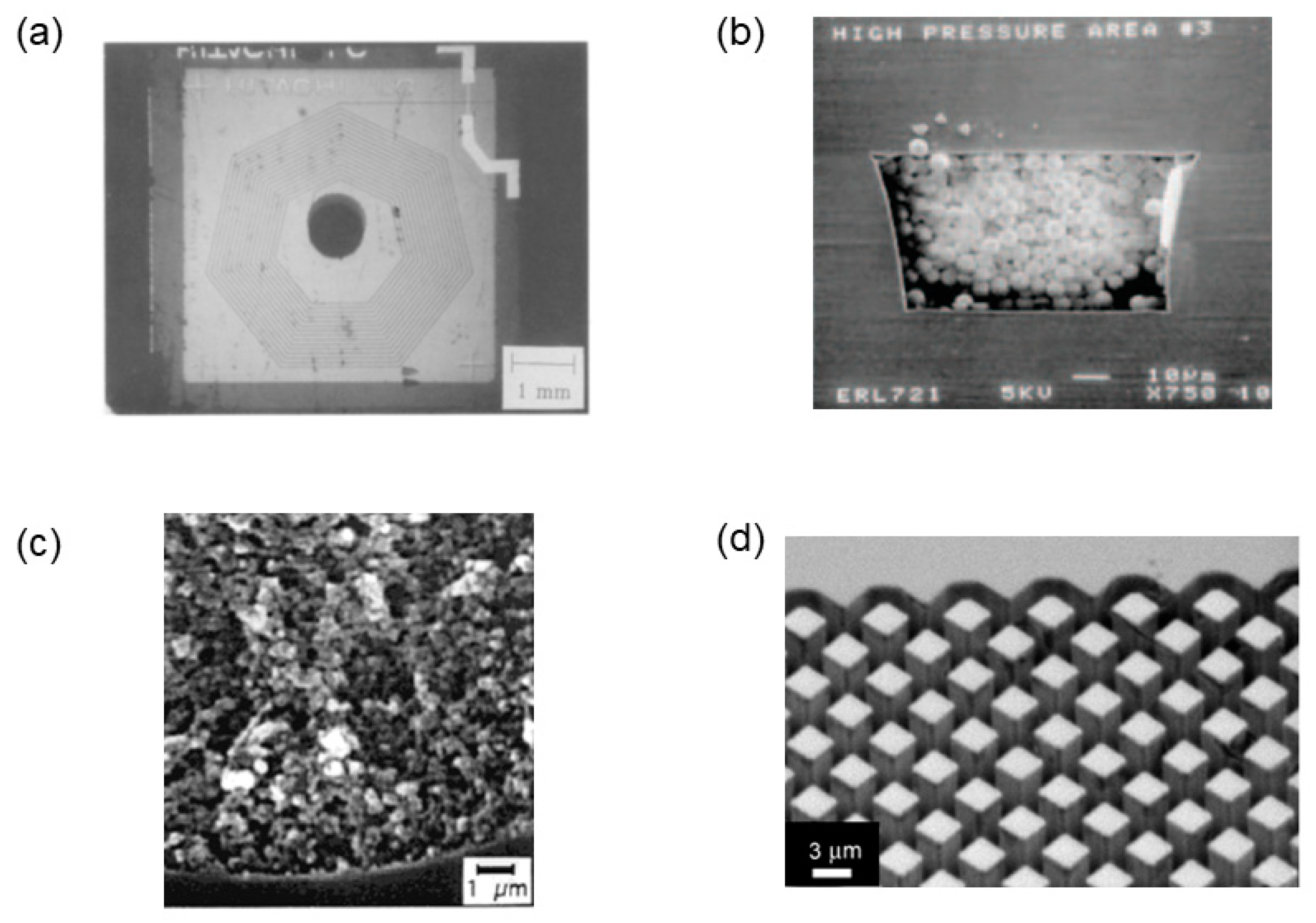
2.2. Packed-Particle Columns
2.3. Monoliths
2.4. Pillar Array
3. Conclusions
Funding
Conflicts of Interest
Entry Link on the Encyclopedia Platform
References
- Manz, A.; Miyahara, Y.; Miura, J.; Watanabe, Y.; Miyagi, H.; Sato, K. Design of an open-tubular column liquid chromatograph using silicon chip technology. Sens. Actuators B Chem. 1990, 1, 249–255. [Google Scholar] [CrossRef]
- Yin, H.F.; Killeen, K.; Brennen, R.; Sobek, D.; Werlich, M.; van de Goor, T. Microfluidic chip for peptide analysis with an integrated HPLC column, sample enrichment column, and nanoelectrospray tip. Anal. Chem. 2005, 77, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Ericson, C.; Holm, J.; Ericson, T.; Hjertén, S. Electroosmosis- and pressure-driven chromatography in chips using continuous beds. Anal. Chem. 2000, 72, 81–87. [Google Scholar] [CrossRef]
- Aoyama, C.; Saeki, A.; Noguchi, M.; Shirasaki, Y.; Shoji, S.; Funatsu, T.; Mizuno, J.; Tsunoda, M. Use of folded micromachined pillar array column with low-dispersion turns for pressure-driven liquid chromatography. Anal. Chem. 2010, 82, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Toh, G.M.; Corcoran, R.C.; Dutta, D. Sodium silicate based sol–gel structures for generating pressure-driven flow in microfluidic channels. J. Chromatogr. A 2010, 1217, 5004–5011. [Google Scholar] [CrossRef] [PubMed]
- Schlund, M.; Gilbert, S.E.; Schnydrig, S.; Renaud, P. Continuous sampling and analysis by on-chip liquid/solid chromatography. Sens. Actuators B 2007, 123, 1133–1141. [Google Scholar] [CrossRef][Green Version]
- Collins, D.A.; Nesterenko, E.P.; Brabazon, D.; Paull, B. Controlled ultraviolet (UV) photoinitiated fabrication of monolithic porous layer open tubular (monoPLOT) capillary columns for chromatographic applications. Anal. Chem. 2012, 84, 3465–3472. [Google Scholar] [CrossRef]
- Tanaka, T.; Izawa, K.; Okochi, M.; Lim, T.-K.; Watanabe, S.; Harada, M.; Matsunaga, T. On-chip type cation-exchange chromatography with ferrocene-labeled anti-hemoglobin antibody and electrochemical detector for determination of hemoglobin A1c level. Anal. Chim. Acta 2009, 638, 186–190. [Google Scholar] [CrossRef]
- Kleinnijenhuis, A.J.; Ingola, M.; Toersche, J.H.; van Holthoon, F.L.; van Dongen, W.D. Quantitative bottom up analysis of infliximab in serum using protein A purification and integrated μLC-electrospray chip IonKey MS/MS technology. Bioanalysis 2016, 8, 891–904. [Google Scholar] [CrossRef]
- Santbergen, M.J.C.; van der Zande, M.; Gerssen, A.; Bouwmeester, H.; Nielen, M.W.F. Dynamic in vitro intestinal barrier model coupled to chip-based liquid chromatography mass spectrometry for oral bioavailability studies. Anal. Bioanal. Chem. 2020, 412, 1111–1122. [Google Scholar] [CrossRef]
- Costa, J.; Pronto-Laborinho, A.; Pinto, S.; Gromicho, M.; Bonucci, S.; Tranfield, E.; Correia, C.; Alexandre, B.M.; de Carvalho, M. Investigating LGALS3BP/90 K glycoprotein in the cerebrospinal fluid of patients with neurological diseases. Sci. Rep. 2020, 10, 5649. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, S.; Kraiczek, K.; Mora, J.A.; Dittmann, M.; Rozing, G.P.; Tallarek, U. Separation efficiency of particle-packed HPLC microchips. Anal. Chem. 2008, 80, 5945–5950. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Höltzel, A.; Ehlert, S.; Mora, J.A.; Kraiczek, K.; Dittmann, M.; Rozing, G.P.; Tallarek, U. Impact of conduit geometry on the performance of typical particulate microchip packings. Anal. Chem. 2009, 81, 10193–10200. [Google Scholar] [CrossRef]
- Thurmann, S.; Mauritz, L.; Heck, C.; Belder, D. High-performance liquid chromatography on glass chips using precisely defined porous polymer monoliths as particle retaining elements. J. Chromatogr. A 2014, 1370, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kecskemeti, A.; Gasper, A. Particle-based liquid chromatographic separations in microfluidic devices—A review. Anal. Chim. Acta 2018, 1021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; O’Hare, D. Monolithic nano-porous polymer in microfluidic channels for lab-chip liquid chromatography. Nano Converg. 2018, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Lönnberg, M.; Carlsson, J. Lab-on-a-chip technology for determination of protein isoform profiles. J. Chromatogr. A. 2006, 1127, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, C.-F.; Tsao, C.-W.; Chang, C.-C.; Chu, C.-C.; DeVoe, D.L. Polymer microchips integrating solid-phase extraction and high-performance liquid chromatography using reversed-phase polymethacrylate monoliths. Anal. Chem. 2009, 81, 2545–2554. [Google Scholar] [CrossRef]
- Wang, X.-L.; Zhu, Y.; Fang, Q. Valveless gated injection for microfluidic chip-based liquid chromatography system with polymer monolithic column. J. Chromatogr. A 2012, 1246, 123–128. [Google Scholar] [CrossRef]
- Wouters, B.; De Vos, J.; Desmet, G.; Terryn, H.; Schoenmakers, P.J.; Eeltink, S. Design of a microfluidic device for comprehensive spatial two-dimensional liquid chromatography. J. Sep. Sci. 2015, 38, 1123–1129. [Google Scholar] [CrossRef]
- Gupta, V.; Talebi, M.; Deverell, J.; Sandron, S.; Nesterenko, P.N.; Heery, B.; Thompson, F.; Beirne, S.; Wallace, G.G.; Paull, B. 3D printed titanium micro-bore columns containing polymer monoliths for reversed-phase liquid chromatography. Anal. Chim. Acta 2016, 910, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Beirne, S.; Nesterenko, P.N.; Paull, B. Investigating the effect of column geometry on separation efficiency using 3D printed liquid chromatographic columns containing polymer monolithic phases. Anal. Chem. 2018, 90, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.H. Band dispersion in chromatography – a new view of A-term dispersion. J. Chromatogr. A. 1999, 831, 3–15. [Google Scholar] [CrossRef]
- He, B.; Tait, N.; Regnier, F. Fabrication of nanocolumns for liquid chromatography. Anal. Chem. 1998, 70, 3790–3797. [Google Scholar] [CrossRef]
- De Malsche, W.; Eghbali, H.; Clicq, D.; Vangelooven, J.; Gardeniers, H.; Desmet, G. Pressure-driven reverse-phase liquid chromatography separations in ordered nonporous pillar array columns. Anal. Chem. 2007, 79, 5915–5926. [Google Scholar] [CrossRef]
- Song, Y.; Takatsuki, K.; Isokawa, M.; Sekiguchi, T.; Mizuno, J.; Funatsu, T.; Shoji, S.; Tsunoda, M. Fast and quantitative analysis of branched-chain amino acids in biological samples using a pillar array column. Anal. Bioanal. Chem. 2013, 405, 7993–7999. [Google Scholar] [CrossRef]
- Song, Y.; Takatsuki, K.; Sekiguchi, T.; Funatsu, T.; Shoji, S.; Tsunoda, M. Rapid quantitative method for the detection of phenylalanine and tyrosine in human plasma using pillar array columns and gradient elution. Amino Acids 2016, 48, 1731–1735. [Google Scholar] [CrossRef]
- Fujiwara, T.; Funatsu, T.; Tsunoda, M. Fast analysis using pillar array columns: Quantification of branched-chain α-keto acids in human plasma samples. J. Pharm. Biomed. Anal. 2021, 198, 114019. [Google Scholar] [CrossRef]
- Kuroki, H.; Koyama, H.; Nakatani, Y.; Funatsu, T.; Horiike, S.; Tsunoda, M. Development of an automated sample injection system for pillar array columns. Chromatography 2021, 41, 59–62. [Google Scholar] [CrossRef]
- Isokawa, M.; Takatsuki, K.; Song, Y.; Shih, K.; Nakanishi, K.; Xie, Z.; Yoon, D.H.; Sekiguchi, T.; Funatsu, T.; Shoji, S.; et al. Liquid chromatography chip with low-dispersion and low-pressure-drop turn structure utilizing a distribution-controlled pillar array. Anal. Chem. 2016, 88, 6485–6491. [Google Scholar] [CrossRef]
- Song, Y.; Noguchi, M.; Takatsuki, K.; Sekiguchi, T.; Mizuno, J.; Funatsu, T.; Shoji, S.; Tsunoda, M. Integration of pillar array columns into a gradient elution system for pressure-driven liquid chromatography. Anal. Chem. 2012, 84, 4739–4745. [Google Scholar] [CrossRef] [PubMed]
- De Malsche, W.; Clicq, D.; Verdoold, V.; Gzil, P.; Desmet, G. Integration of porous layers in ordered pillar arrays for liquid chromatography. Lab Chip 2007, 7, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Detobel, F.; De Bruyne, S.; Vangelooven, J.; De Malsche, W.; Aerts, T.; Terryn, H.; Gardeniers, H.; Eeltink, S.; Desmet, G. Fabrication and chromatographic performance of porous-shell pillar-array columns. Anal. Chem. 2010, 82, 7208–7217. [Google Scholar] [CrossRef]
- Fonverne, A.; Ricoul, F.; Demesmay, C.; Delattre, C.; Fournier, A.; Dijon, J.; Vinet, F. In Situ synthesized carbon nanotubes as a new nanostructured stationary phase for microfabricated liquid chromatographic column. Sens. Actuators B 2008, 129, 510–517. [Google Scholar] [CrossRef]
- Müller, J.B.; Geyer, P.E.; Colaço, A.R.; Treit, P.V.; Strauss, M.T.; Oroshi, M.; Doll, S.; Winter, S.V.; Bader, J.M.; Köhler, N.; et al. The proteome landscape of the kingdoms of life. Nature 2020, 582, 592–596. [Google Scholar] [CrossRef]
- Cho, B.G.; Jiang, P.; Goli, M.; Gautam, S.; Mechref, Y. Using micro pillar array columns (μPAC) for the analysis of permethylated glycans. Analyst 2021, 146, 4374–4383. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsunoda, M. On-Chip Liquid Chromatography. Encyclopedia 2022, 2, 617-624. https://doi.org/10.3390/encyclopedia2010041
Tsunoda M. On-Chip Liquid Chromatography. Encyclopedia. 2022; 2(1):617-624. https://doi.org/10.3390/encyclopedia2010041
Chicago/Turabian StyleTsunoda, Makoto. 2022. "On-Chip Liquid Chromatography" Encyclopedia 2, no. 1: 617-624. https://doi.org/10.3390/encyclopedia2010041
APA StyleTsunoda, M. (2022). On-Chip Liquid Chromatography. Encyclopedia, 2(1), 617-624. https://doi.org/10.3390/encyclopedia2010041