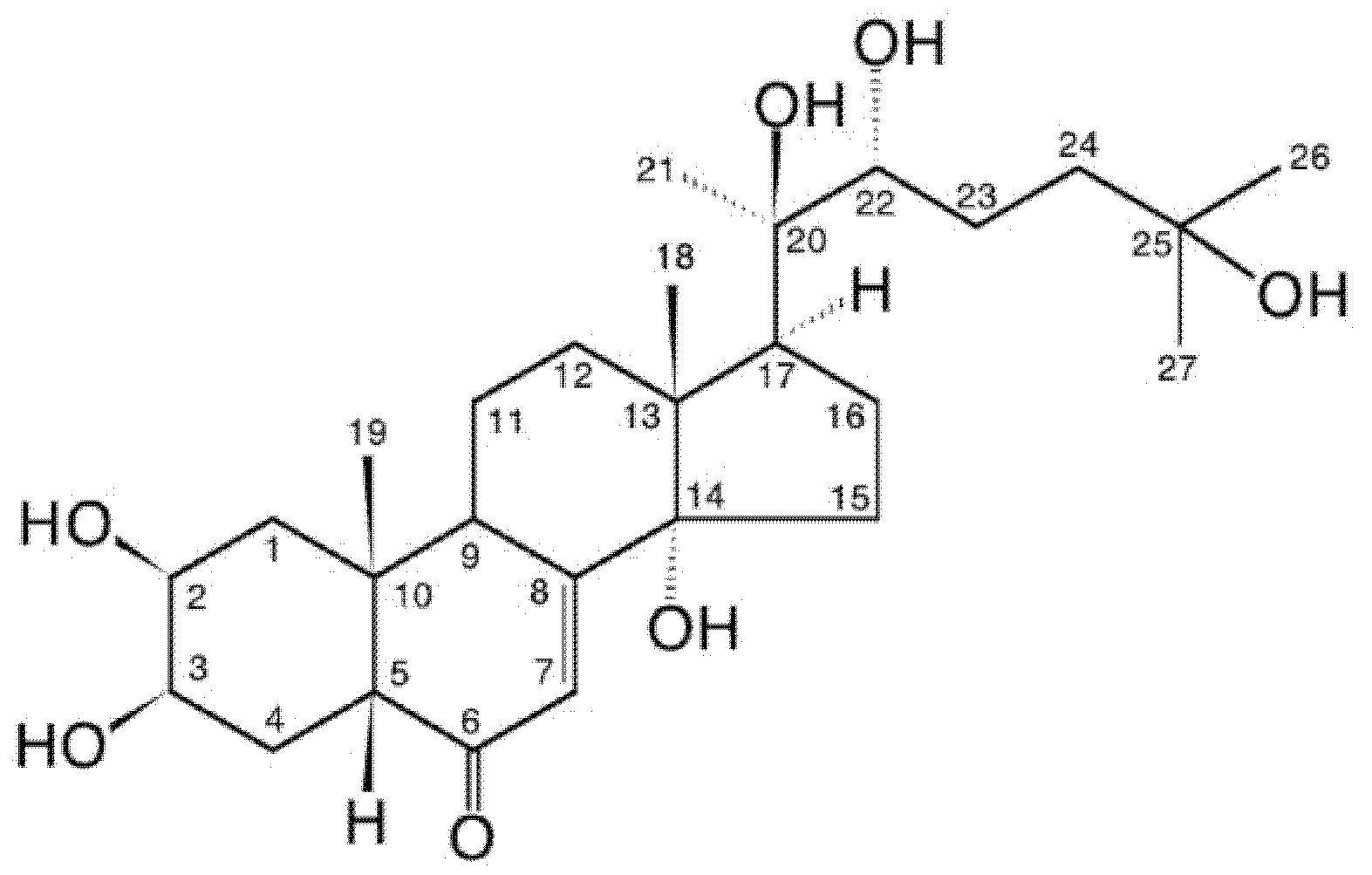
Ecdysteroids
Definition
1. Introduction
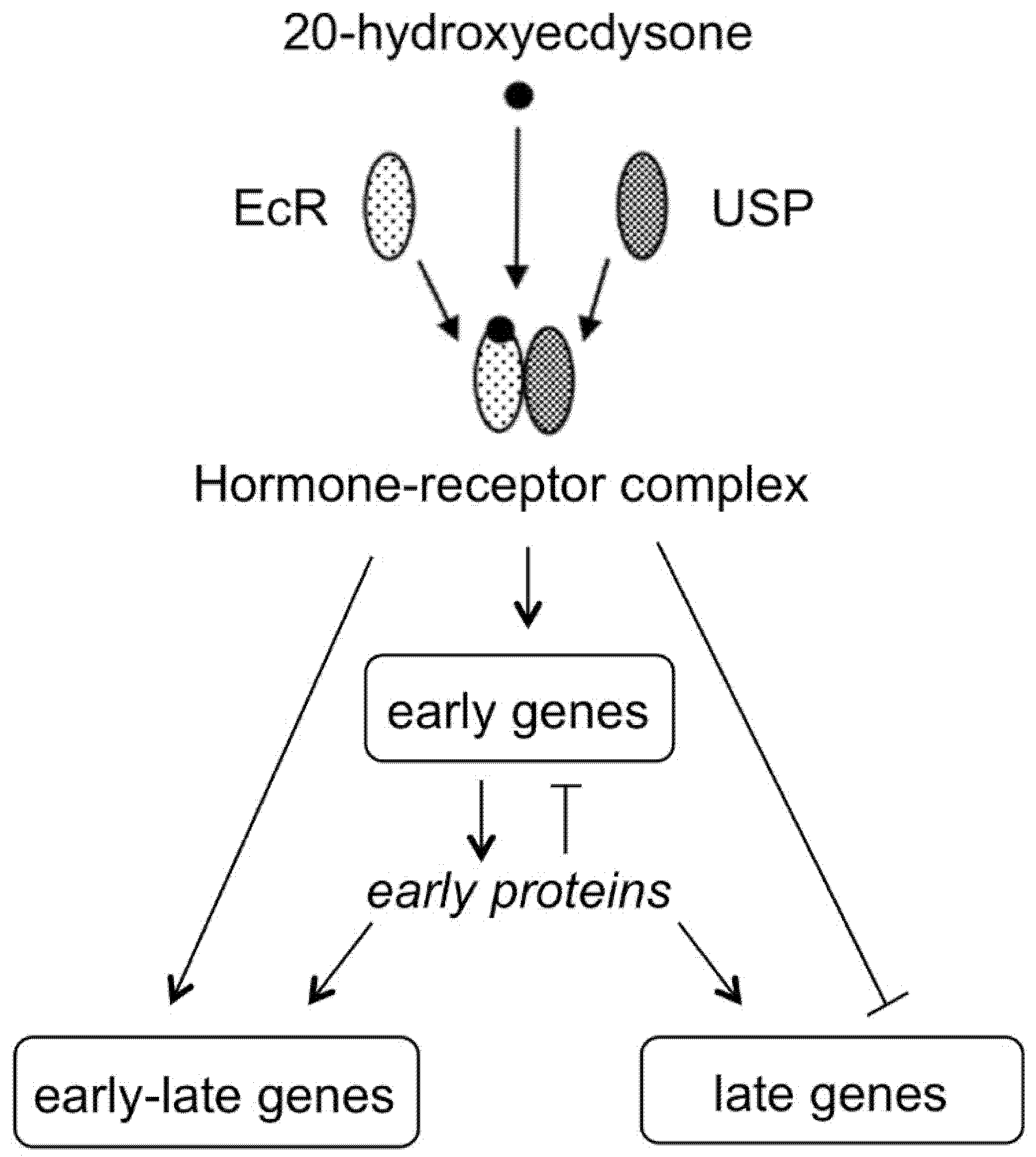
2. Zooecdysteroids Are Arthropod Hormones
3. Phytoecdysteroids Are Plant Secondary Metabolites
3.1. Discovery
3.2. Distribution in the Plant World
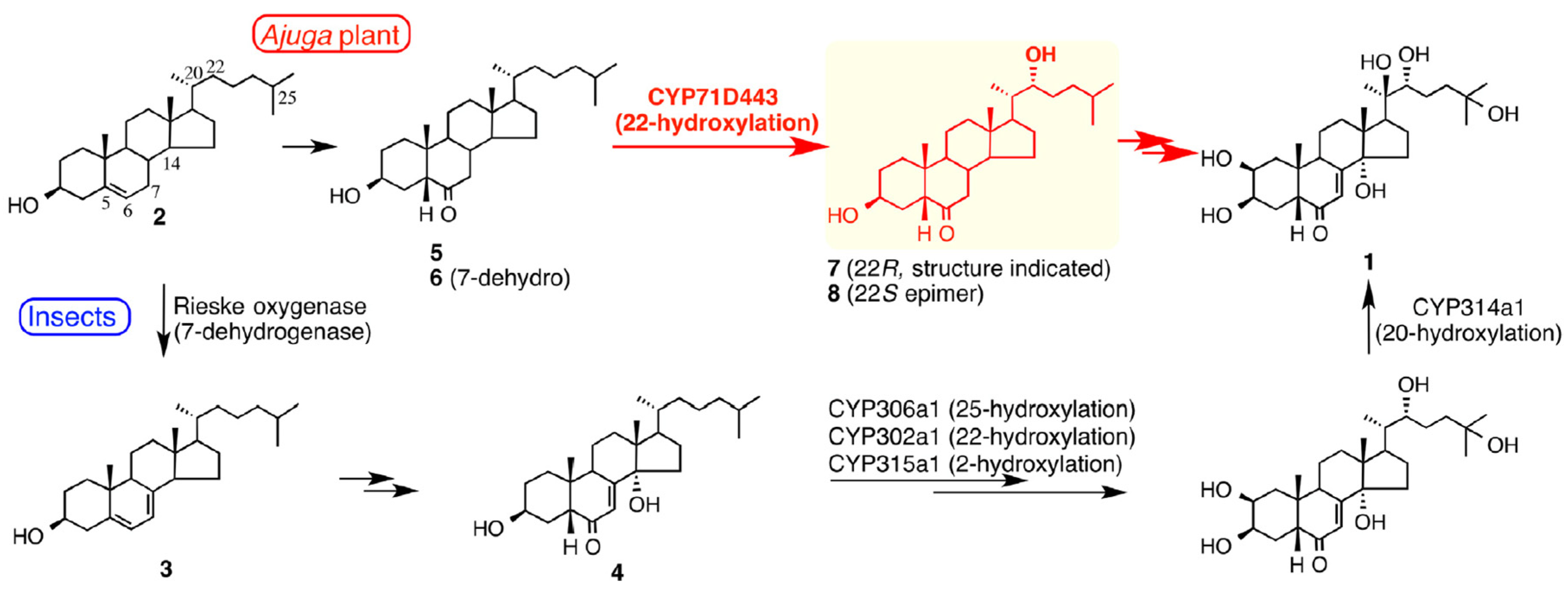
3.3. Range of Analogues and Biosynthesis
3.4. High-Accumulating Species
3.5. Distribution within Ecdysteroid-Containing Plants
3.6. Physiological Roles in Plants?
3.7. Allelochemical Role
3.8. Ecdysteroids for the Protection of Crop Species
3.9. Practical Uses of Phytoecdysteroids
3.9.1. In Sericulture
3.9.2. In Apiculture
3.9.3. In Aquaculture
3.10. Similarity to Brassinosteroids
4. Methods of Purification, Analysis and Quantification of Ecdysteroids
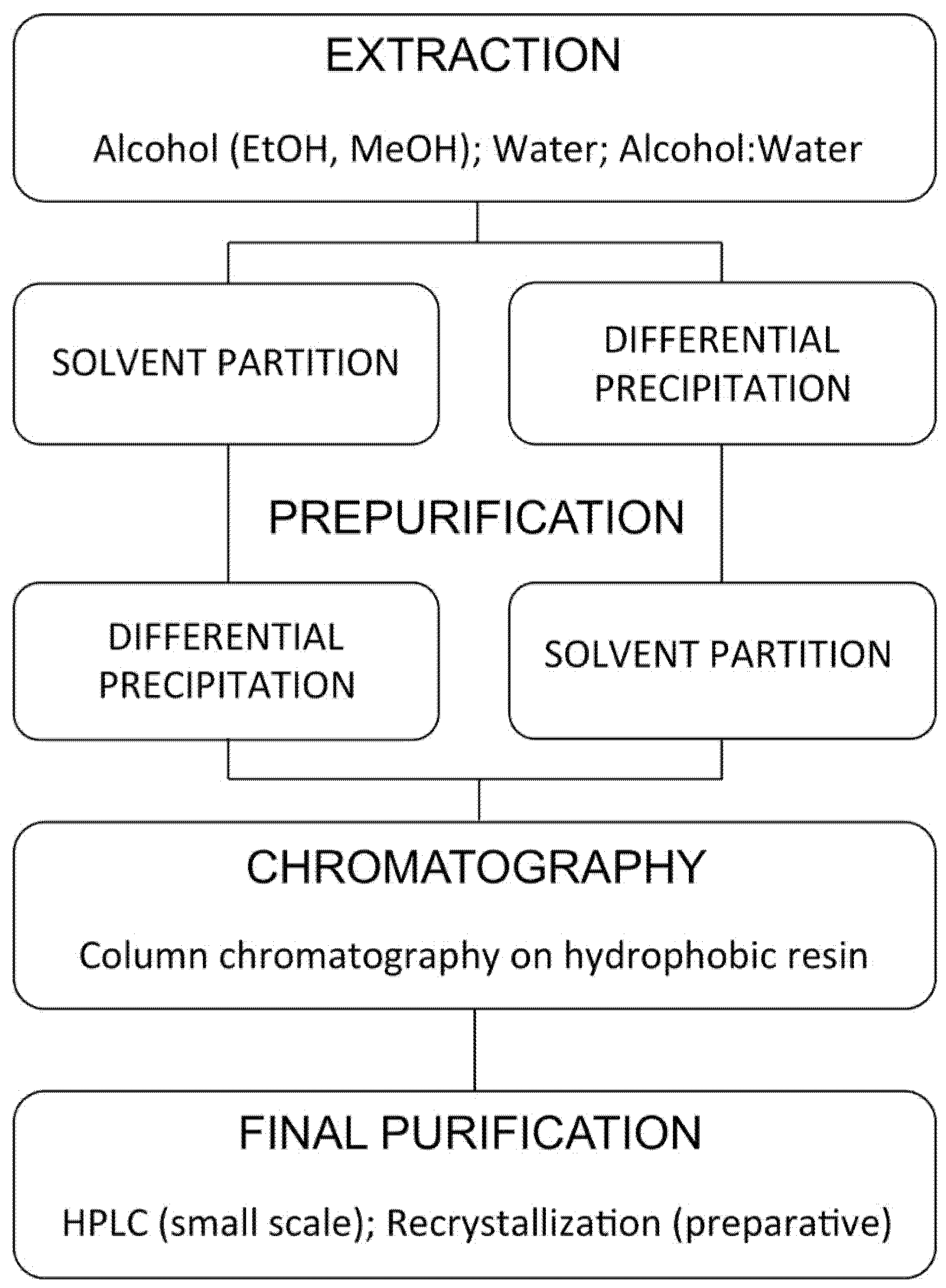
4.1. Extraction
4.2. Partial Purification
Solvent Partitioning
4.3. Solid-Phase Extraction
4.4. Chromatography
Thin-Layer Chromatography (TLC)
4.5. Open-Column/Flash Chromatography
4.6. Droplet Counter-Current Chromatography (DCCC)
4.7. Centrifugal Partition Chromatography (CPC)
4.8. High-Performance Liquid Chromatography (HPLC)
4.9. Supercritical-Fluid Chromatography (SFC)
4.10. Spectral Identification
4.10.1. UV Spectra
4.10.2. Mass Spectroscopy (MS)
4.10.3. Nuclear Magnetic Resonance Spectroscopy (NMR)
4.11. Hyphenated Techniques
4.12. Immunoassays
4.13. Bioassays
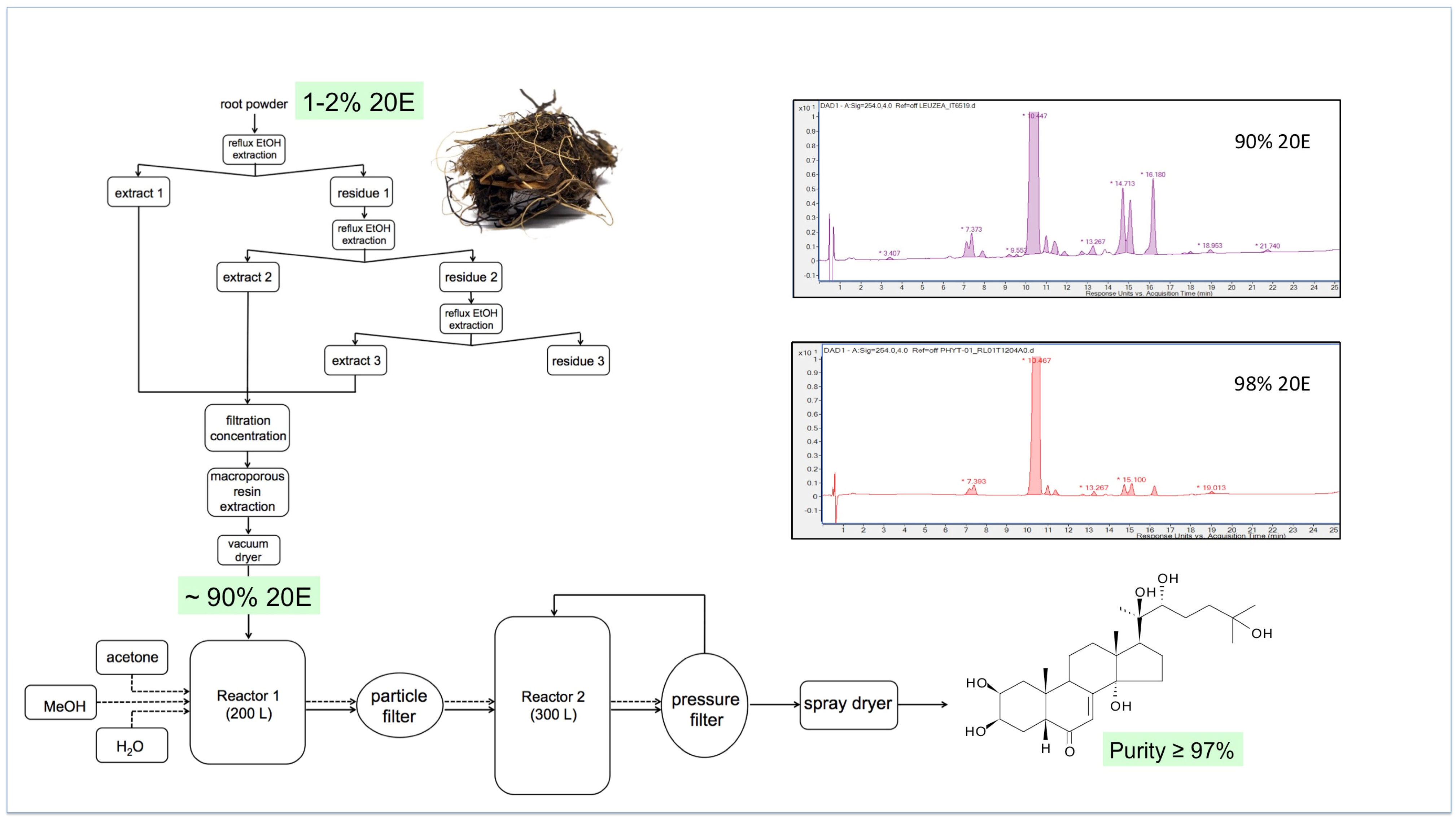
5. Sourcing of Large Amounts of Ecdysteroids for Pharmaceutical and Clinical Purposes
Sourcing from Plants
- It is perhaps worth reminding ourselves first of the criteria that an ideal plant source should fulfil:
- The plant should accumulate a high amount of 20E;
- The plant should have a simple ecdysteroid profile (ideally just 20E);
- The plant should be easy and rapid to grow in accessible areas of the world;
- The ecdysteroids should be present in the aerial portions (allowing the roots to regenerate the plant);
- The plant matrix should be amenable to the ready purification of ecdysteroids;
- Purification and isolation of 20E should not involve expensive chromatographic methods;
- The plant should not be susceptible to pests and diseases;
- The species should not be rare or protected;
- Culture, harvesting and processing costs should be low; initial processing should take place at the culture site.
6. Alternatives for Large-Scale Production of Ecdysteroids and Their Current Viability
6.1. Chemical Synthesis
6.2. Cell/Callus Cultures
6.3. Hairy-Root Cultures
6.4. Plant “Milking”
6.5. Biofermentation
7. Ecdysteroids: Presence in Human Food and Traditional Medicinal Plants
8. Ecdysteroids: A Multifaceted Human Medicine?
9. Conclusions
- Ecdysteroids are widespread in nature. They are the steroid hormones of arthropods and other invertebrates and are, in view of the predominance of these animals in the world, the most frequently occurring steroid hormones, even if vertebrate steroid hormones receive more scientific attention. They also occur in plants, where their occurrence is not universal (although the genetic capacity to produce them may be), but when they do occur, they can accumulate to high concentrations.
- The analysis, identification and quantification of ecdysteroids are highly advanced, and new analogues (particularly in plants) are continuing to be identified. Perhaps uniquely, the data and literature concerning ecdysteroids have been compiled, are readily available online and are continuously updated (www.ecdybase.com).
- The mode of action of ecdysteroids in some model arthropod species is now fairly well understood, but, in view of the enormous diversity of arthropods, much certainly remains to be discovered. The control of development by ecdysteroids in other invertebrates is still poorly understood.
- Phytoecdysteroids appear to possess allelochemical functions in the plants where they occur by acting as either antifeedants or endocrine disruptors to invertebrate predators. The wide diversity of phytoecdysteroid analogues would appear to be a consequence of this competition for survival between ecdysteroid-containing plants and the behavioural and biochemical strategies of invertebrate predator species to cope with this class of molecules.
- Ecdysteroids have many positive pharmacological effects on mammals. The modes of action by which these effects occur are now starting to be understood. This aspect of ecdysteroid research is currently the one with the greatest potential, as indicated by the steady rise in the number of clinical trials being performed to assess the medical uses of ecdysteroids.
10. Prospects and Applications
- The biosynthesis of ecdysteroids in arthropods and plants remains to be fully elucidated. Final clarification of the biochemical pathways, enzymology and regulation remains a major challenge, which would considerably facilitate many strategies for the agricultural and medical applications of ecdysteroids.
- Although high concentrations of exogenous ecdysteroids are generally deleterious to invertebrate development, it is clear that low doses can have beneficial developmental and commercial consequences for the rearing of silkworms, bees, prawns and crabs. This area can be expected to develop considerably into established cottage industries as our knowledge of local ecdysteroid-containing plants grows, fueled by the general commercial availability of purified 20E at a reasonable cost.
- Certain plant species are high accumulators of ecdysteroids (mainly 20E) and are thus very good commercial sources of these compounds, especially in the current absence of other viable strategies to obtain ecdysteroids. The availability of reliable, pure preparations of ecdysteroids is essential for continuing clinical trials and for the ultimate commercialisation of medical drugs with ecdysteroids as their API.
- The current emphasis is on medical applications that concern the potential anabolic effects of ecdysteroids, especially those concerning muscle wasting (e.g., sarcopenia, Duchenne muscular dystrophy). Such research will continue apace, but it is to be expected that other medical conditions could benefit from other beneficial pharmacological effects of ecdysteroids (see Table 3).
- Many nutritional supplements that claim that they contain specified analogues at specified levels are commercially available. However, quantitative and qualitative analyses demonstrate that very few of these contain the stated analogue (20E or turkesterone) at the required level; they may contain other unstated analogues. Further, ecdysteroid contents and profiles can vary from batch to batch. This area needs regulation as purchasers are not receiving what they thought; there is no information on the identity or safety of other plant components present and these poor and variable preparations could result in ecdysteroids unfairly receiving a bad reputation.
- Evidence is beginning to accumulate that the pharmacological effects are not specific to ecdysteroids but may be a more general property of polyhydroxylated steroids (as proposed by Karel Sláma in 1993 [212]) since anabolic, hypoglycaemic and wound-healing effects are also associated with brassinosteroids in rodents [213,214,215] and withaferin A (withanolide A; Figure 15) protects against high-fat-induced obesity in mice [216]. This lack of absolute specificity may explain why relatively high concentrations of polyhydroxylated steroids are required for activity. If this is substantiated, it is probable that 20E would be the polyhydroxylated steroid of choice because of its commercial availability in large amounts and its demonstrated lack of toxicity in mammals.
Author Contributions
Funding
Conflicts of Interest
Entry Link on the Encyclopedia Platform
References
- Koolman, J. (Ed.) Ecdysone: From Chemistry of Mode of Action; Thieme Verlag: Stuttgart, Germany, 1989; 482p. [Google Scholar]
- Dinan, L.; Savchenko, T.; Whiting, P. On the distribution of phytoecdysteroids in plants. Cell Mol. Life Sci. 2001, 58, 1121–1132. [Google Scholar] [CrossRef]
- Dinan, L.; Harmatha, J.; Volodin, V.; Lafont, R. Phytoecdysteroids: Diversity, biosynthesis and distribution. In Ecdysone: Structures and Functions; Smagghe, G., Ed.; Springer Science & Business Media B.V.: Berlin, Germany, 2009; pp. 3–45. [Google Scholar]
- Lafont, R.; Harmatha, J.; Marion-Poll, F.; Dinan, L.; Wilson, I.D. The Ecdysone Handbook, 3rd ed.; Cybersales: Prague, Czech Republic, 2002; Available online: http://ecdybase.org/ (accessed on 1 April 2021).
- Butenandt, A.; Karlson, P. Über die Isolierung eines Metamorphose-Hormons der Insekten in kristallisierter Form. Z. Naturforsch. 1954, 9b, 389–391. [Google Scholar] [CrossRef]
- Huber, R.; Hoppe, W. Zur Chemie des Ecdysons, VII: Die Kristall- und Molekülstrukturanalyse des Insektenverpuppungshormons ecdyson mit der automatisierten Faltmolekülmethode. Chem. Ber. 1965, 98, 2403–2424. [Google Scholar] [CrossRef] [PubMed]
- Kannangara, J.R.; Mirth, C.K.; Warr, C.G. Regulation of ecdysone production in Drosophila by neuropeptides and peptide hormones. Open Biol. 2021, 11, 200373. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.I. Insect Endocrinology; Academic Press: London, UK, 2012; 577p. [Google Scholar]
- Belles, X. Insect Metamorphosis. From Natural History to Regulation of Developement and Evolution; Academic Press: London, UK, 2020; 289p. [Google Scholar]
- Denlinger, D.L.; Yocum, G.D.; Rinehart, J.P. Hormonal control of diapause. In Insect Endocrinology; Gilbert, L.I., Ed.; Academic Press: London, UK, 2012; pp. 430–463. [Google Scholar]
- Lafont, R.; Dauphin-Villemant, C.; Warren, J.T.; Rees, H. Ecdysteroid chemistry and biochemistry. In Insect Endocrinology; Gilbert, L.I., Ed.; Academic Press: London, UK, 2012; pp. 106–176. [Google Scholar]
- Yamanaka, N.; Okamoto, N. Molecular functions of ecdysteroids in insects. In Advances in Invertebrate (Neuro)endocrinology; Saleuddin, S., Lange, A.B., Orchard, I., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2020; Volume 2, pp. 77–127. [Google Scholar]
- Pan, X.; Connacher, R.P.; O’Connor, M.B. Control of the insect metamorphic transition by ecdysteroid production and secretion. Curr. Opin. Insect Sci. 2021, 43, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Clever, U.; Karlson, P. Induktion von Puff-Verändungen in den Speichedrüsenchromosomen von Chironomus tentans durch Ecdyson. Exp. Cell. Res. 1960, 20, 623–626. [Google Scholar] [CrossRef]
- Karlson, P. Chemistry and biochemistry of insect hormones. Angew. Chem. Internat. Edit. 1963, 2, 175–182. [Google Scholar] [CrossRef]
- Ashburner, M.; Chihara, C.; Meltzer, P.; Richards, G. Temporal control of puffing activity in polytene chromosomes. Cold Spring Harb. Symp. Quant. Biol. 1974, 38, 662–665. [Google Scholar] [CrossRef]
- Thummel, C.S. Ecdysone-regulated puff genes 2000. Insect Biochem. Molec. Biol. 2002, 32, 113–120. [Google Scholar] [CrossRef]
- Nakanishi, K.; Koreeda, M.; Sasaki, S.; Chang, M.L.; Hsu, H.Y. Insect hormones. The structure of ponasterone A, an insect moulting hormone from the leaves of Podocarpus nakaii Hay. J. Chem. Soc. Chem. Commun. 1966, 915–917. [Google Scholar] [CrossRef]
- Takemoto, T.; Ogawa, S.; Nishimoto, N. Isolation of the moulting hormones of insects from Achyranthes radix. Yakugaku Zasshi 1967, 87, 325–327. [Google Scholar] [CrossRef][Green Version]
- Galbraith, M.N.; Horn, D.H.S. An insect-moulting hormone from a plant. J. Chem. Soc. Chem. Commun. 1966, 905–906. [Google Scholar] [CrossRef]
- Jizba, J.; Herout, V.; Sorm, F. Isolation of ecdysterone (crustecdysone) from Polypodium vulgare L. rhizomes. Tetrahedron Lett. 1967, 8, 1689–1691. [Google Scholar] [CrossRef]
- Heinrich, G.; Hoffmeister, H. Ecdyson als Begleitsubstanz des Ecdysterons in Polypodium vulgare L. Experientia 1967, 23, 995. [Google Scholar] [CrossRef] [PubMed]
- Kaplanis, J.N.; Thompson, M.J.; Robbins, W.E.; Bryce, M. Insect hormones: Alpha ecdysone and 20-hydroxyecdysone in bracken fern. Science 1967, 157, 1436–1438. [Google Scholar] [CrossRef]
- Hocks, P.; Wiechert, R. 20-Hydroxyecdyson, isoliert aus Insekten. Tetrahedron Lett. 1966, 7, 2989–2993. [Google Scholar] [CrossRef]
- Koreeda, M.; Nakanishi, K. 5β-Hydroxy-ecdysones and a revision of the structure of ponasterone C. Chem. Commun. 1970, 351–352. [Google Scholar] [CrossRef]
- Blunt, J.W.; Lane, G.A.; Munro, M.H.G.; Russell, G.B. The absolute configuration at C-24 of the ecdysteroids dacrysterone, pterosterone and ponasterone C. Aust. J. Chem. 1979, 32, 779–782. [Google Scholar] [CrossRef]
- Matsuoka, T.; Imai, S.; Sakai, M.; Kamada, M. Studies on phytoecdysones—A review of our works. Ann. Rep. Takeda Res. Lab 1969, 28, 221–271. [Google Scholar]
- Yen, K.-Y.; Yang, L.-L.; Okuyami, T.; Hikino, H.; Takemoto, T. Screening of Formosan ferns for phytoecdysones. I. Chem. Pharm. Bull. 1974, 22, 805–808. [Google Scholar] [CrossRef]
- Volodin, V.; Chadin, I.; Whiting, P.; Dinan, L. Screening plants of European North-East Russia for ecdysteroids. Biochem. Syst. Ecol. 2002, 30, 525–578. [Google Scholar] [CrossRef]
- Lafont, R.; Ho, R.; Raharivelomanana, P.; Dinan, L. Ecdysteroids in Ferns: Distribution, Diversity, Biosynthesis, and Functions. In Working with Ferns: Issues and Applications; Fernández, H., Kumar, A., Revilla, M.A., Eds.; Springer Science+Business Media: New York, NY, USA, 2011; pp. 305–319. [Google Scholar]
- Kovganko, N.V. Ecdysteroids and related compounds in fungi. Chem. Nat. Comp. 1999, 35, 597–611. [Google Scholar] [CrossRef]
- Dinan, L.; Mamadalieva, N.; Lafont, R. The occurrence of ecdysteroids and ecdysteroid-related compounds in edible mushroom species. In Ecdybase.org; Cybersales: Prague, Czech Republic, 2019. [Google Scholar]
- Dinan, L. Distribution and levels of phytoecdysteroids within individual plants of species of Chenopodiaceae. Eur. J. Entomol. 1995, 92, 295–300. [Google Scholar]
- Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; Stevens, P.F.; The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- Dinan, L. Phytoecdysteroids: Biological aspects. Phytochemistry 2001, 57, 325–339. [Google Scholar] [CrossRef]
- Tsukagoshi, Y.; Ohyama, K.; Seki, H.; Akashi, T.; Muranaka, T.; Suzuki, H.; Fujimoto, Y. Functional characterization of CYP71D443, a cytochrome P450 catalyzing C-22 hydroxylation in the 20-hydroxyecdysone biosynthesis of Ajuga hairy roots. Phytochemistry 2016, 127, 23–28. [Google Scholar] [CrossRef]
- Dinan, L. Ecdysteroid structure-activity relationships. In Studies in Natural Product Chemistry 29; Atta-ur-Rahman, Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2003; pp. 3–71. [Google Scholar]
- Báthori, M.; Tóth, N.; Hunyadi, A.; Márki, A.; Zador, E. Phytoecdysteroids and anabolic-androgenic steroids—Structure and effects on Humans. Curr. Med. Chem. 2008, 15, 75–91. [Google Scholar] [CrossRef]
- Harmatha, J.; Budesinsky, M.; Vokac, K. Photochemical transformation of 20-hydroxyecdysone: Production of monomeric and dimeric ecdysteroid analogues. Steroids 2002, 67, 127–135. [Google Scholar] [CrossRef]
- Hunyadi, A.; Csábi, J.; Martins, A.; Molnár, J.; Balázs, A.; Tóth, G. Backstabbing P-gp: Side-chain cleaved ecdysteroid 2,3-dioxolanes hyper-sensitize MDR cancer cells to doxorubicin without efflux inhibition. Molecules 2017, 22, 199. [Google Scholar] [CrossRef]
- Issaadi, H.M.; Béni, Z.; Tóth, T.; Dékány, M.; Hsieh, T.-J.; Balogh, G.T.; Hunyadi, A. Diversity-oriented synthesis through gamma radiolysis: Preparation of unusual ecdysteroid derivatives activating Akt and AMPK in skeletal muscle cells. Bioorg. Chem. 2021, 112, 104951. [Google Scholar] [CrossRef]
- Dinan, L.; Balducci, C.; Guibout, L.; Lafont, R. Database of Human food plants and whether they have been assessed for the presence or absence of ecdysteroids. In Ecdybase.org; Cybersales: Prague, Czech Republic, 2019. [Google Scholar]
- Dinan, L.; Mamadalieva, N.; Lafont, R. Dietary phytoecdysteroids. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 1–54. [Google Scholar] [CrossRef]
- Dinan, L.; Balducci, C.; Guibout, L.; Lafont, R. Small-scale analysis of phytoecdysteroids in seeds by HPLC-DAD-MS for the identification and quantification of specific analogues, dereplication and chemotaxonomy. Phytochem. Anal. 2020, 31, 643–661. [Google Scholar] [CrossRef]
- Dinan, L.; Lafont, R. Compilation of the literature reports for the screening of vascular plants, algae, fungi and non-arthropod invertebrates for the presence of ecdysteroids. In Ecdybase.org; Cybersales: Prague, Czech Republic, 2019. [Google Scholar]
- Hendrix, S.D.; Jones, R.L. The activity of β-ecdysone in four gibberellin bioassays. Plant Physiol. 1972, 50, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Dreier, S.I.; Towers, G.H.N. Activity of ecdysterone in selected plant growth bioassays. J. Plant Physiol. 1988, 132, 509–512. [Google Scholar] [CrossRef]
- Machackova, I.; Vagner, M.; Sláma, K. Comparison between the effects of 20-hydroxyecdysone and phytohormones on growth and development in plants. Eur. J. Entomol. 1995, 92, 309–316. [Google Scholar]
- Tarkowská, D.; Strnad, M. Plant ecdysteroids: Plant sterols with intriguing distributions, biological effects and relations to plant hormones. Planta 2016, 244, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, A.; Lamhamdi, M.; Sayah, F.; Chibi, F. Effect of plant hormones and 20-hydroxyecdysone on tomato (Lycopersicum esculentum) seed germination and seedlings growth. Afr. J. Biotechnol. 2007, 6, 2792–2802. [Google Scholar]
- Turk, H. Progesterone promotes mitochondrial respiration at the biochemical and molecular level in germinating maize seed. Plants 2021, 10, 1326. [Google Scholar] [CrossRef]
- Bajguz, A.; Koronka, A. Effect of ecdysone application on the growth and biochemical changes in Chlorella vulgaris cells. Plant Physiol. Biochem. 2001, 39, 707–715. [Google Scholar] [CrossRef]
- Bajguz, A.; Dinan, L. Effects of ecdysteroids on Chlorella vulgaris. Physiol. Plant. 2004, 121, 349–357. [Google Scholar] [CrossRef]
- Maršálek, B.; Šimek, M.; Smith, R.J. The effect of ecdysone on the cyanobacterium Nostoc 6720. Z. Naturforsch. 1992, 47c, 726–730. [Google Scholar] [CrossRef]
- Uhlik, O.; Kamlar, M.; Kohout, L.; Jezek, R.; Harmatha, J.; Macek, T. Affinity chromatography reveals RuBisCO as an ecdysteroid-binding protein. Steroids 2008, 73, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Marion-Poll, F.; Dinan, L.; Lafont, R. The role of phytoecdysteroids in the battle against phytophagous insects. In Biopesticides of Plant Origin; Regnault-Roger, C., Philogène, B.J.R., Vincent, C., Eds.; Lavoisier: Paris, France, 2008; pp. 301–319. [Google Scholar]
- Marion-Poll, F. Chapter 4: Tasting toxicants as bitter: Phytoecdysteroids. In SEB Experimental Biological Series; Taste, I., Newland, P., Cobb, M., Marion-Poll, F., Eds.; Taylor and Francis: London, UK, 2009; Volume 63, pp. 127–138. [Google Scholar]
- Bergamasco, R.; Horn, D.H.S. Distribution and role of insect hormones in plants. In Endocrinology of Insects; A. R. Liss Inc.: New York, NY, USA, 1983; pp. 627–654. [Google Scholar]
- Kubo, I.; Hanke, F.J. Chemical methods for isolating and identifying phytochemicals biologically active in insects. In Insect Plant Interactions; Miller, J.R., Miller, T.A., Eds.; Springer: New York, NY, USA, 1986; pp. 225–249. [Google Scholar]
- Blackford, M.; Clarke, B.; Dinan, L. Tolerance of Spodoptera littoralis (Lepidoptera: Noctuidae) to ingested phytoecdysteroids. J. Insect Physiol. 1996, 42, 931–936. [Google Scholar] [CrossRef]
- Blackford, M.J.P.; Clarke, B.S.; Dinan, L. Distribution and metabolism ofexogenous ecdysteroids in the Egyptian Cotton Leafworm Spodoptera littoralis (Lepidoptera: Noctuidae). Arch. Insect Biochem. Physiol. 1997, 34, 329–346. [Google Scholar] [CrossRef]
- Blackford, M.; Dinan, L. The tomato moth Lacanobia oleracea (Lepidoptera: Noctuidae) detoxifies ingested 20-hydroxyecdysone, but is susceptible to the ecdysteroid agonists RH-5849 and RH-5992. Insect Biochem. Molec. Biol. 1997, 27, 167–177. [Google Scholar] [CrossRef]
- Blackford, M.; Dinan, L. The effects of ingested ecdysteroid agonists (20-hydroxyecdysone, RH5849 and RH5992) and an ecdysteroid antagonist (cucurbitacin B) on larval development of two polyphagous lepidopterans (Acherontia atropos and Lacanobia oleracea). Entomol. Exp. Appl. 1997, 83, 263–276. [Google Scholar] [CrossRef]
- Blackford, M.; Dinan, L. The effects of ingested 20-hydroxyecdysone on the larvae of Aglais urticae, Inachis io, Cynthia cardui (Lepidoptera: Nymphalidae) and Tyria jacobaeae (Lepidoptera: Actiidae). J. Insect Physiol. 1997, 43, 315–327. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Grebenok, R.J.; Galbraith, D.W.; Bowers, W.S. Damage-induced accumulation of phytoecdysteroids in spinach: A rapid root response involving the octadecanoic acid pathway. J. Chem. Ecol. 1998, 24, 339–360. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Grebenok, R.J.; Galbraith, D.W.; Bowers, W.S. Insect-induced synthesis of phytoecdysteroids in spinach, Spinacia oleracea. J. Chem. Ecol. 1999, 25, 1739–1757. [Google Scholar] [CrossRef]
- Soriano, I.R.; Riley, I.T.; Potter, M.J.; Bowers, W.S. Phytoecdysteroids: A novel defense against plant-parasitic nematodes. J. Chem. Ecol. 2004, 30, 1885–1899. [Google Scholar] [CrossRef]
- Prasuna, A.L.; Osmani, Z. Inhibitory effects of ecdysterone on the adult emergence of Achaea janata L. Sci. Cult. 1983, 49, 112–114. [Google Scholar]
- Arnault, C.; Sláma, K. Dietary effects of phytoecdysones in the leek-moth, Acrolepiosis assesctella Zell. (Lepidoptera: Acrolepiidae). J. Chem. Ecol. 1986, 12, 1979–1985. [Google Scholar] [CrossRef]
- Tanaka, Y.; Naya, S.-I. Dietary effect of ecdysone and 20-hydroxyecdysone on larval development of two lepidopteran species. Appl. Entomol. Zool. 1995, 30, 285–294. [Google Scholar] [CrossRef]
- Kubo, I.; Klocke, J.A.; Asano, S. Insect ecdysis inhibitors from the East African medicinal plant Ajuga remota (Labiatae). Agric. Biol. Chem. 1981, 45, 1925–1927. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takeda, S. Ecdysone and 20-hydroxyecdysone supplements to the diet affect larval development in the silkworm, Bombyx mori, differently. J. Insect Physiol. 1993, 39, 805–809. [Google Scholar] [CrossRef]
- Zhang, M.; Kubo, I. Metabolic fate of ecdysteroids in larval Bombyx mori and Heliothis virescens. Insect Biochem. Mol. Biol. 1993, 7, 831–843. [Google Scholar] [CrossRef]
- Tanaka, Y.; Asaoka, K.; Takeda, S. Different feeding and gustatory responses to ecdysone and 20-hydroxyecdysone by larvae of the silkworm, Bombyx mori. J. Chem. Ecol. 1994, 20, 125–133. [Google Scholar] [CrossRef]
- Marion-Poll, F.; Descoins, C. Taste detection of phytoecdysteroids in larvae of Bombyx mori, Spodoptera littoralis and Ostrinia nubilalis. J. Insect Physiol. 2002, 48, 467–476. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Grebenok, R.J.; Ohnmeiss, T.E.; Bowers, W.S. Interactions between Spinacia oleracea and Bradysia impatiens: A role for phytoecdysteroids. Arch. Insect Biochem. Physiol. 2002, 51, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Komatsu, S.; Asaka, Y.; de Boer, G. Isolation and identification of apolar metabolites of ingested 20-hydroxyecdysone in frass of Heliothis virescens larvae. J. Chem. Ecol. 1987, 13, 785–794. [Google Scholar] [CrossRef]
- Hoffmann, K.H.; Thiry, E.; Lafont, R. 14-Deoxyecdysteroids in an insect (Gryllus bimaculatus). Z. Naturforsch. 1990, 45c, 703–708. [Google Scholar] [CrossRef]
- Thiry, E.; Hoffmann, K.H. Dynamics of ecdysone and 20-hydroxyecdysone metabolism after injection and ingestion in Gryllus bimaculatus. Zool. Jb. Physiol. 1992, 96, 17–38. [Google Scholar]
- Robinson, P.D.; Morgan, E.D.; Wilson, I.D.; Lafont, R. The metabolism of ingested and injected [3H]ecdysone by the final instar larvae of Heliothis armigera. Physiol. Entomol. 1987, 12, 321–330. [Google Scholar] [CrossRef]
- Mondy, N.; Caissa, C.; Pitoizet, N.; Delbecque, J.-P.; Corio-Costet, M.-F. Effects of the ingestion of Serratula tinctoria extracts, a plant containing phytoecdysteroids, on the development of the vineyard pest Lobesia botrana (Lepidoptera: Tortricidae). Arch. Insect Biochem. Physiol. 1997, 35, 227–235. [Google Scholar] [CrossRef]
- Feyereisen, R.; Lagueux, M.; Hoffmann, J.A. Dynamics of ecdysone metabolism after ingestion and injection in Locusta migratoria. Gen. Comp. Endocrinol. 1976, 29, 319–327. [Google Scholar] [CrossRef]
- Modde, J.-F.; Lafont, R.; Hoffmann, J.A. Ecdysone metabolism in Locusta migratoria larvae and adults. Int. J. Invert. Reprod. Devel. 1984, 7, 161–183. [Google Scholar] [CrossRef]
- Lafont, R. Phytoecdysteroids and moulting control. In New Strategies for Locust Control; Rembold, H., Ed.; ATSAF: Bonn, Germany, 1994; pp. 39–44. [Google Scholar]
- Weirich, G.F.; Thompson, M.J.; Svoboda, J.A. In vitro ecdysteroid conjugation by enzymes of Manduca sexta midgut cytosol. Arch. Insect Biochem. Physiol. 1986, 3, 109–126. [Google Scholar] [CrossRef]
- Weirich, G.E.; Feldlaufer, M.F.; Svoboda, J.A. Ecdysone oxidase and 3-oxoecdysteroid reductases in Manduca sexta: Developmental changes and tissue distribution. Arch. Insect Biochem. Physiol. 1993, 23, 199–211. [Google Scholar] [CrossRef]
- Rharrabe, K.; Alla, S.; Maria, A.; Sayah, F.; Lafont, R. Diversity of detoxification pathways of ingested ecdysteroids among phytophagous insects. Arch. Insect Biochem. Physiol. 2007, 65, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Rharrabe, K.; Maria, A.; Sayah, F.; Lafont, R. Metabolism of ingested 20-hydroxyecdysone in Plodia interpunctella larvae (Lepidoptera, Pyralidae). Moroccan J. Biol. 2008, 4/5, 55–62. [Google Scholar]
- Rharrabe, K.; Sayah, F.; Lafont, R. Dietary effects of four phytoecdysteroids on growth and development of the Indian meal moth, Plodia interpunctella. J. Insect Sci. 2010, 10, 13. [Google Scholar] [CrossRef]
- Dhadialla, T.S.; Carlson, G.R.; Le, D.P. New insecticides with ecdysteroidal and juvenile hormone activity. Ann. Rev. Entomol. 1998, 43, 545–569. [Google Scholar] [CrossRef]
- Chandrakala, M.; Maribashetty, V.; Jyoth, I.H. Application of phytoecdysteroids in sericulture. Curr. Sci. 1998, 74, 341–346. [Google Scholar]
- Trivedy, K.; Nirmal Kumar, S.; Dandin, S.B. Phytoecdysteroid and its use in sericulture. Sericologia 2006, 46, 57–78. [Google Scholar]
- Lafont, R.; Dinan, L. Innovative and future applications for ecdysteroids. In Ecdysone, Structures and Functions; Smagghe, G., Ed.; Georg Thieme-Verlag: Stuttgart, Germany, 2009; pp. 551–578. [Google Scholar]
- Sreejit, C.M.; Sasidharan, K.; Bose, C.; Thomas-Matthew, P.; Banerji, A. Effect of phytoecdysteroids isolated from Diploclisia glaucescens (BLUME) Diels and Coscinium fenestratum (Gaertn.) Colebr. and juvenile hormone analogue isolated from Cullen corylifolium (L.) Medik. on economic parameters of Bombyx mori L. under field condition. Int. J. Curr. Adv. Res. 2018, 7, 10746–10752. [Google Scholar] [CrossRef]
- Kumar, D.; Maheshwari, G.; Pandey, J.P.; Kishore, R.; Rai, S.; Sahay, A. Phyto-ecdysteroids modulated synchronisation of cocoon-spinning in tasar silkworm, Antheraea mylitta D. Singapore J. Sci. Res. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Kholodova, Y.D. Phytoecdysteroids: Biological effects, application in agriculture and complementary medicine. Ukr. Biokhim. Zh. 2001, 73, 21–29. [Google Scholar]
- Kanazawa, A.; Tanaka, N.; Kashiwada, K. Nutritional requirement of prawns-IV. The dietary effect of ecdysones. Bull. Jpn. Soc. Sci. Fish. 1972, 38, 1067–1071. [Google Scholar] [CrossRef][Green Version]
- Cho, G.; Itami, T. Plant extract as cholesterol substitute in shrimp. Aqua Feed. Formul. Beyond 2004, 1, 16–17. Available online: http://www.feedware.com/aqua/magazine/v1i2/additivesV1I22004.pdf (accessed on 1 April 2021).
- Waiho, K.; Ikhwanuddin, M.; Baylon, J.C.; Jalilah, M.; Rukminasari, N.; Fujaya, Y.; Fazhan, H. Moult induction methods in soft-shell crab production. Aquac. Res. 2021, 52, 4026–4042. [Google Scholar] [CrossRef]
- Lehmann, M.; Vorbrodt, H.-M.; Adam, G.; Koolman, J. Antiecdysteroid activity of brassinosteroids. Experientia 1988, 44, 355–356. [Google Scholar] [CrossRef]
- Voigt, B.; Whiting, P.; Dinan, D. The ecdysteroid agonist/antagonist and brassinosteroid-like activities of synthetic brassinosteroid/ecdysteroid hybrid molecules. Cell. Mol. Life Sci. 2001, 58, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Tarkowská, D.; Krampolová, E.; Strnad, M. Plant triterpenoid crosstalk: The interaction of brassinosteroids and phytoecdysteroids in Lepidium sativum. Plants 2020, 9, 1325. [Google Scholar] [CrossRef] [PubMed]
- Thussagunpanit, J.; Jutamanee, K.; Homvisasevongsa, S.; Suksamrarn, A.; Yamagami, A.; Nakano, T.; Asami, T. Characterization of synthetic ecdysteroid analogues as functional mimics of brassinosteroids in plant growth. J. Steroid Biochem. Mol. Biol. 2017, 172, 1–8. [Google Scholar] [CrossRef]
- Kovganko, N.V.; Ananich, S.K. Progress in the chemical synthesis of brassinosteroids. Chem. Nat. Compd. 2002, 38, 122–141. [Google Scholar] [CrossRef]
- Kovganko, N.V.; Kashkan, Z.N.; Chernov, Y.G.; Ananich, S.K.; Sokolov, S.N.; Survillo, V.I. Synthesis of ecdysteroids and related compounds. Chem. Nat. Compd. 2003, 39, 411–437. [Google Scholar] [CrossRef]
- Horn, D.H.S. The Ecdysones. In Naturally Occurring Insecticides; Jacobson, M., Crosby, D.G., Eds.; Marcell Dekker, Inc.: New York, NY, USA, 1971; pp. 333–459. [Google Scholar]
- Lafont, R.; Morgan, E.D.; Wilson, I.D. Chromatographic procedures for ecdysteroids. J. Chromatogr. A 1994, 658, 31–53. [Google Scholar] [CrossRef]
- Báthori, M. Phytoecdysteroids effects on mammalians, isolation and analysis. Mini Rev. Med. Chem. 2002, 2, 285–293. [Google Scholar] [CrossRef]
- Ho, R.; Girault, J.-P.; Cousteau, P.-Y.; Bianchini, J.-P.; Raharivelomana, P.; Lafon, T.R. Isolation of a new class of ecdysteroid conjugates (glucosyl-ferulates) using a combination of liquid chromatographic methods. J. Chromatogr. Sci. 2008, 46, 102–110. [Google Scholar] [CrossRef]
- Sovová, H.; Opletal, L.; Sajfrtová, M.; Bártlová, M. Supercritical fluid extraction of cynaropicrin and 20-hydroxyecdysone from Leuzea carthamoides DC. J. Sep. Sci. 2008, 31, 1387–1392. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, B.; Chen, M.; Chen, T. Supercritical fluid extraction of ecdysterone from the roots of Achyranthes bidentata BL. J. Sep. Sci. 2008, 31, 1393–1398. [Google Scholar] [CrossRef]
- Debien, I.C.N.; Vardanega, R.; Santos, D.T.; Meireles, M.A.A. Optimization of pressurized liquid extraction of ecdysteroids from Brazilian Ginseng (Pfaffia glomerata) roots. In Proceedings of the III Iberoamerican Conference on Supercritical Fluids, Cartagena de Indias, Columbia, 1–5 April 2013; p. 9. [Google Scholar]
- Debien, C.N.; Meireles, M.A.A. Supercritical fluid extraction of beta-ecdysone from Brazilian ginseng (Pfaffia glomerata) roots. Food Public Health 2014, 4, 67–73. [Google Scholar] [CrossRef][Green Version]
- Bajkacz, S.; Rusin, K.; Wolny, A.; Adamek, J.; Erfurt, K.; Chorbok, A. Highly efficient extraction procedures based on natural deep eutectic solvents of ionic liquids for determination of 20-hydroxyecdysone in spinach. Molecules 2020, 25, 4736. [Google Scholar] [CrossRef]
- Lafont, R.; Pennetier, J.-L.; Andrianjafintino, M.; Claret, J. Sample processing for high-performance liquid chromatography of ecdysteroids. J. Chromatogr. 1982, 236, 137–149. [Google Scholar] [CrossRef]
- Wilson, I.D.; Morgan, E.D.; Murphy, S.J. Sample preparation for the chromatographic determination of ecdysteroids using solid-phase extraction methods. Anal. Chim. Acta 1990, 236, 145–155. [Google Scholar] [CrossRef]
- Lafont, R.; Blais, C.; Harmatha, J.; Wilson, I.D. Ecdysteroids: Chromatography. Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013; p. 15. [Google Scholar] [CrossRef]
- Dinan, L.; Harmatha, J.; Lafont, R. TLC of sterols, steroids and related triterpenoids. In Thin Layer Chromatography in Phytochemistry; Waksmundska-Hajnos, M., Sherma, J., Kowalska, T., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 575–603. [Google Scholar]
- Báthori, M.; Szendrei, K.; Herke, I. Application of combined chromatographic techniques in the screening and purification of ecdysteroids. Chromatographia 1986, 21, 234–238. [Google Scholar] [CrossRef]
- Báthori, M.; Pongrácz, Z.; Tóth, G.; Simon, A.; Kandra, L.; Kele, Z.; Ohmacht, R. Isolation of a new member of the ecdysteroid glycoside family: 2-deoxy-20-hydroxyecdysone 22-O-β-D-glucopyranoside. J. Chromatogr. Sci. 2002, 40, 409–415. [Google Scholar] [CrossRef]
- Báthori, M.; Pongrácz, Z.; Ohmacht, R.; Mathé, I. Preparative scale purification of shidasterone, 2-deoxy-polypodine B and 9α,20-dihydroxyecdysone from Silene italica ssp. nemoralis. J. Chromatogr. Sci. 2004, 42, 275–279. [Google Scholar] [CrossRef][Green Version]
- Kubo, I.; Klocke, J.A.; Ganjian, I.; Ichikawa, N.; Matsumoto, T. Efficient isolation of ecdysteroids from Ajuga plants by high-performance liquid chromatography and droplet counter-current chromatography. J. Chromatogr. 1983, 257, 157–161. [Google Scholar] [CrossRef]
- Fang, L.; Li, J.; Zhou, J.; Wang, X.; Guo, L. Isolation and purification of three ecdysteroids from the stems of Diploclisia glaucescens by high-speed countercurrent chromatography and their anti-inflammatory activities in vitro. Molecules 2017, 22, 1310. [Google Scholar] [CrossRef]
- Issaadi, H.M.; Tsai, Y.-C.; Chang, F.-R.; Hunyadi, A. Centrifugal partition chromatography in the isolation of minor ecdysteroids from Cyanotis arachnoidea. J. Chromatogr. B 2017, 1054, 44–49. [Google Scholar] [CrossRef]
- Lafont, R.; Martin-Sommé, G.; Chambet, J.-C. Separation of ecdysteroids by using high-pressure liquid chromatography on microparticulate supports. J. Chromatogr. 1979, 170, 185–194. [Google Scholar] [CrossRef]
- Lafont, R.; Sommé-Martin, G.; Mauchamp, B.; Maume, B.F.; Delbecque, J.-P. Analysis of ecdysteroids by high-peformance liquid chromatography and coupled gas-liquid chromatography-mass spectrometry. In Progress in Ecdysone Research; Hofmann, J.A., Ed.; Elsevier/North Holland Biomedical Press: Amsterdam, The Netherlands, 1980; pp. 45–68. [Google Scholar]
- Lafont, R.; Kaouadji, N.; Morgan, E.D.; Wilson, I.D. Selectivity in the high-performance liquid chromatography of ecdysteroids. J. Chromatogr. A 1994, 658, 55–67. [Google Scholar] [CrossRef]
- Dinan, L.; Harmatha, J.; Lafont, R. HPLC of steroids. In High Performance Liquid Chromatography in Phytochemical Analysis; Waksmundska-Hajnos, M., Sherma, J., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 679–708. [Google Scholar]
- Raynor, M.W.; Kithini, J.P.; Bartle, K.D.; Games, D.E.; Mylchreest, I.C.; Lafont, R.; Morgan, E.D.; Wilson, I.D. Packed column suercritical-fluid chromatography and linked supercritical-fluid chromatography-mass spectrometry for the analysis of phytoecdysteroids from Silene nutans and Silene otites. J. Chromatogr. 1989, 467, 292–298. [Google Scholar] [CrossRef]
- Shim, J.-H.; Wilson, I.D.; Morgan, E.D. Boronic esters as derivatives for supercritical fluid chromatography of ecdysteroids. J. Chromatogr. A 1993, 639, 281–285. [Google Scholar] [CrossRef]
- Isaac, R.E.; Rees, H.H. Isolation and identification of ecdysteroid phosphates and acetylecdysteroid phosphates from developing eggs of the locust, Schistocerca gregaria. Biochem. J. 1984, 221, 459–464. [Google Scholar] [CrossRef]
- Evershed, R.P.; Prescott, M.C.; Kabbouh, M.; Rees, H.H. High-performance liquid chromatography/mass spectrometry with thermospray ionization of free ecdysteroids. Rapid Commun. Mass Spectrom. 1993, 7, 477–481. [Google Scholar] [CrossRef]
- Girault, J.-P.; Lafont, R. The complete 1H-NMR assignment of ecdysone and 20-hydroxyecdysone. J. Insect Physiol. 1988, 7, 701–706. [Google Scholar] [CrossRef]
- Hikino, H.; Okuyama, T.; Konno, C.; Takemoto, T. Carbon-13 nuclear magnetic resonance spectra of phytoecdysones. Chem. Pharm. Bull. 1975, 23, 125–132. [Google Scholar] [CrossRef]
- Filho, J.G.S.; Duringer, J.; Mala, G.L.A.; Tavares, J.F.; Xavier, H.S.; da Silva, M.S.; da-Cunha, E.V.; Barbosa-Filho, J.M. Ecdysteroids from Vitex species: Distribution and compilation of their 13C-NMR spectral data. Chem. Biodivers. 2008, 5, 707–713. [Google Scholar] [CrossRef]
- Girault, J.-P. Determination of ecdysteroids structure by 1D and 2D NMR. Russ. J. Plant Physiol. 1998, 45, 306–309. [Google Scholar]
- Wilson, I.D.; Morgan, E.D.; Lafont, R.; Wright, B. High-performance liquid chromatography coupled to nuclear magnetic resonance spectroscopy. Application to the ecdysteroids of Silene otites. J. Chromatogr. A 1998, 799, 333–336. [Google Scholar] [CrossRef]
- Wilson, I.D.; Morgan, E.D.; Lafont, R.; Shockcor, J.P.; Lindon, J.C.; Nicholson, J.K.; Wright, B. High performance liquid chromatography coupled to nuclear magnetic resonance spectroscopy and mass spectrometry applied to plant products: Identification of ecdysteroids from Silene otites. Chromatographia 1999, 49, 374–378. [Google Scholar] [CrossRef]
- Wilson, I.D. Multiple hyphenation of liquid chromatography with nuclear magnetic resonance spectroscopy, mass spectrometry and beyond. J. Chromatogr. A 2000, 892, 315–327. [Google Scholar] [CrossRef]
- Louden, D.; Handley, A.; Taylor, S.; Lenz, E.; Miller, S.; Wilson, I.D.; Sage, A.; Lafont, R. Spectroscopic characterisation and identification of ecdysteroids using high-performance liquid chromatography combined with on-line UV-diode array, FT-infrared and 1H-nuclear magnetic resonance spectroscopy and time of flight mass spectrometry. J. Chromatogr. A 2001, 910, 237–246. [Google Scholar] [CrossRef]
- Louden, D.; Handley, A.; Lafont, R.; Taylor, S.; Sinclair, I.; Lenz, E.; Orton, T.; Wilson, I.D. HPLC analysis of ecdysteroids in plant extracts using superheated deuterium oxide with multiple on-line spectroscopic analysis (UV, IR, 1H NMR, and MS). Anal. Chem. 2002, 74, 288–294. [Google Scholar] [CrossRef]
- Claude, E.; Tower, M.; Lafont, R.; Wilson, I.D.; Plumb, R.S. High performance thin-layer chromatography of plant ecdysteroids coupled with desorption electrospray ionisation-ion mobility-time of flight high resolution mass spectrometry (HPTLC/DESI/IM/ToFMS). Chromatographia 2020, 83, 1029–1035. [Google Scholar] [CrossRef]
- Jaroszewski, J.W. Hyphenated NMR methods in natural products research, part 1: Direct hyphenation. Planta Med. 2005, 71, 691–700. [Google Scholar] [CrossRef]
- Borst, D.W.; O’Connor, J.D. Trace analysis of ecdysones by gas-liquid chromatography, radioimmunoassay and bioassay. Steroids 1974, 24, 637–656. [Google Scholar] [CrossRef]
- Borst, D.W.; O’Connor, J.D. Arthropod molting hormone: Radioimmune assay. Science 1972, 178, 418–419. [Google Scholar] [CrossRef]
- De Reggi, M.L.; Hirn, M.H.; Delaage, M.A. Radioimmunoassay of ecdysone an application to Drosophila larvae and pupae. Biochem. Biophys. Res. Comm. 1975, 66, 1307–1315. [Google Scholar] [CrossRef]
- Porcheron, P.; Foucrier, J.; Gros, C.; Pradelles, P.; Cassier, P.; Dray, F. Radioimmunoassay of arthropod moulting hormone: β-ecdysone antibodies production and 125I-iodinated tracer preparation. FEBS Lett. 1976, 61, 159–162. [Google Scholar] [CrossRef]
- Horn, D.H.; Wilkie, J.S.; Sage, B.A.; O’Connor, J.D. A high affinity antiserum specific for the ecdysone nucleus. J. Insect Physiol. 1976, 22, 901–905. [Google Scholar] [CrossRef]
- Spindler, K.D.; Beckers, C.; Gröschel-Stewart, U.; Emmerich, H. A radioimmunoassay for arthropod moulting hormones, introducing a novel method of immunogen coupling. Hoppe Seylers Z Physiol Chem. 1978, 359, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Reum, L.; Koolman, J. Analysis of ecdysteroids by radioimmunoassay: Comparison of three different antisera. Insect Biochem. 1979, 9, 135–142. [Google Scholar] [CrossRef]
- Soumoff, C.; Horn, D.H.; O’Connor, J.D. Production of a new antiserum to arthropod molting hormone and comparison with two other antisera. J. Steroid Biochem. 1981, 14, 429–435. [Google Scholar] [CrossRef]
- Reum, L.; Klinger, W.; Koolman, J. A New Immunoassay for Ecdysteroids Based on Chemiluminescence; Kricka, L.J., Ed.; Academic Press: London, UK, 1984; pp. 249–252. [Google Scholar]
- Reum, L.; Koolman, J. Radioimmunoassay of ecdysteroids. In Ecdysone; Koolman, J., Ed.; Georg Thieme Verlag: Stuttgart, Germany, 1989; pp. 131–143. [Google Scholar]
- Warren, J.T.; Smith, W.; Gilbert, L.I. Simplification of the ecdysteroid radioimmunoassay by the use of protein A from Staphylococcus aureus. Experientia 1984, 40, 393–394. [Google Scholar] [CrossRef]
- Porcheron, P.; Moriniere, M.; Grassi, J.; Pradelles, P. Development of an enzyme immunoassay for ecdysteroids using acetylcholinesterase as label. Insect Biochem. 1989, 19, 117–122. [Google Scholar] [CrossRef]
- Royer, C.; Porcheron, P.; Pradelles, P.; Mauchamp, B. Development and use of an enzymatic tracer for an enzyme immunoassay of makisterone A. Insect Biochem. Mol. Biol. 1993, 23, 193–197. [Google Scholar] [CrossRef]
- Von Glyscinski, U.; Delbecque, J.-P.; Böcking, D.; Sedlmeier, D.; Dirksen, H.; Lafont, R. Three new antisera with high sensitivity to ecdysone, 3-dehydroecdysone and other A-ring derivatives: Production and characterization. Eur. J. Entomol. 1995, 92, 75–79. [Google Scholar]
- Pascual, N.; Bellés, X.; Delbecque, J.-P.; Hua, Y.-J.; Koolman, J. Quantification of ecdysteroids by immunoassay: Comparison of enzyme immunoassay and radioimmunoassay. Z. Naturforsch. 1995, 50c, 862–867. [Google Scholar] [CrossRef]
- McKinney, D.A.; Strand, M.R.; Brown, M.R. Evaluation of ecdysteroid antisera for a competitive enzyme immunoassay and extraction procedures for the measurement of mosquito ecdysteroids. Gen. Comp. Endocrinol. 2017, 253, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Cymborowski, B. Bioassays for ecdysteroids. In Ecdysone: From Chemistry to Mode of Action; Koolman, J., Ed.; Thieme Verlag: Stuttgart, Germany, 1989; pp. 144–149. [Google Scholar]
- Dinan, L. Ecdysteroid structure and hormonal activity. In Ecdysone: From Chemistry to Mode of Action; Koolman, J., Ed.; Thieme Verlag: Stuttgart, Germany, 1989; pp. 345–354. [Google Scholar]
- Cherbas, L.; Yonge, C.D.; Cherbas, P.; Williams, C.M. The morphological response of Kc-H cells to ecdysteroids: Hormonal specificity. Wilhelm Roux’ Arch. 1980, 189, 1–15. [Google Scholar] [CrossRef]
- Clément, C.Y.; Bradbrook, D.A.; Lafont, R.; Dinan, L. Assessment of a microplate-based bioassay for the detection of ecdysteroid-like or antiecdysteroid activities. Insect Biochem. Molec. Biol. 1993, 23, 187–193. [Google Scholar] [CrossRef]
- Dinan, L.; Hormann, R.E. Ecdysteroid agonists and antagonists. In Comprehensive Molecular Insect Science, Vol. 3, Endocrinology; Gilbert, L.I., Iatrou, K., Gill, S.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 197–242. [Google Scholar]
- Wang, J.-L.; Ruan, D.-C.; Cheng, Z.-Y.; Yang, C.-R. The dynamic variations of 20-hydroxyecdysone in Cyanotis arachnoidea. Acta Bot. Yunnanica 1996, 18, 459–464. [Google Scholar]
- Bandara, B.M.R.; Jayasinghe, L.; Karunaratne, V.; Wanningama, G.P.; Bokel, M.; Kraus, W. Ecdysterone from stem of Diploclisia glaucescens. Phytochemistry 1989, 28, 1073–1075. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Ohyama, K.; Nomura, K.; Hyodo, R.; Takahashi, K.; Yamada, J.; Morisaki, M. Biosynthesis of sterols and ecdysteroids in Ajuga hairy roots. Lipids 2000, 35, 279–288. [Google Scholar] [CrossRef]
- Duport, C.; Spagnoli, R.; Degryse, E.; Pompon, D. Self-sufficient biosynthesis of pregnenolone and progesterone in engineered yeast. Nat. Biotechnol. 1998, 16, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Szczerbara, F.M.; Chandelier, C.; Villeret, C.; Masurel, A.; Bourot, S.; Duport, C.; Blanchard, S.; Groisillier, A.; Testet, E.; Costaglioli, P.; et al. Total biosynthesis of hydrocortisone from a simple carbon source in yeast. Nat. Biotechnol. 2003, 21, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Dinan, L.; Dioh, W.; Veillet, S.; Lafont, R. 20-Hydroxyecdysone, from plant extracts to clinical use: Therapeutic potential for the treatment of neuromuscular, cardio-metabolic and respiratory diseases. Biomedicines 2021, 9, 492. [Google Scholar] [CrossRef]
- Kametani, T.; Tsubuki, M. Strategies for the synthesis of ecdysteroids. In Ecdysone: From Chemistry to Mode of Action; Koolman, J., Ed.; Thieme Verlag: Stuttgart, Germany, 1989; pp. 74–96. [Google Scholar]
- Thiem, B.; Kikowska, M.; Malinski, M.P.; Kruszka, D.; Napierala, M.; Florek, E. Ecdysteroids: Production in plant in vitro cultures. Phytochem. Rev. 2017, 16, 603–622. [Google Scholar] [CrossRef]
- John, R.; Shajitha, P.P.; Devassy, A.; Mathew, L. Effect of elicitation and precursor feeding on accumulation of 20-hydroxyecdysone in Achyranthes aspera Linn. cell suspension cultures. Physiol. Molec. Biol. Plants 2018, 24, 275–284. [Google Scholar] [CrossRef]
- Malinski, M.P.; Kikowska, M.; Kruszka, D.; Napierala, M.; Florek, E.; Sliwinska, E.; Thiem, B. Various in vitro systems of Ragged Robin (Lychnis flos-cuculi L.): A new potential source of phytoecdysteroids? Plant Cell Tissue Organ Cult. 2019, 139, 39–52. [Google Scholar] [CrossRef]
- Corio-Costet, M.-F.; Chapuis, L.; Delbecque, J.-P.; Ustache, K. Genetic transformation of Serratula tinctoria (Dyer’s Savory) for ecdysteroid production. In Biotechnology in Agriculture and Forestry 45: Transgenic Medicinal Plants; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 284–297. [Google Scholar]
- Skala, E.; Grabkowska, R.; Sitarek, P.; Kuzma, L.; Blauz, A.; Wysokinska, H. Rhaponticum carthamoides regeneration through direct and indirect organogenesis, molecular profiles and secondary metabolite production. Plant Cell Tiss. Organ Cult. 2015, 123, 83–98. [Google Scholar] [CrossRef]
- Chajra, H.; Salwinski, A.; Guillaumin, A.; Mignard, B.; Hannewald, P.; Duriot, L.; Warnault, P.; Guillet-Claude, C.; Fréchet, M.; Bourgaud, F. Plant Milking Technology—An Innovative and Sustainable Process to Produce Highly Active Extracts from Plant Roots. Molecules 2020, 25, 4162. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, Y. Yeast as a promising heterologous host for steroid bioproduction. J. Indust. Microbiol. Biotechnol. 2020, 47, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, J.; Yongyan, X.; Zhubo, D.; Bi, C.; Xi, C.; Fan, F.; Zhang, X. Production of 14α-hydroxysteroids by a recombinant Saccharomyces cerevisiae biocatalyst expressing of a fungal steroid 14α-hydroxylation system. Appl. Microbiol. Biotechnol. 2019, 103, 8363–8374. [Google Scholar] [CrossRef]
- Findeisen, E. Ecdysteroide in Menschlicher Nahrung. Ph.D. Thesis, University of Marburg, Marburg, Germany, 2004. [Google Scholar]
- Grebenok, R.J.; Ripa, P.V.; Adler, J.H. Occurrence and levels of ecdysteroids in spinach. Lipids 1991, 26, 666–668. [Google Scholar] [CrossRef]
- Kumpun, S.; Maria, A.; Crouzet, S.; Evrard-Todeschi, N.; Girault, J.-P.; Lafont, R. Ecdysteroids from Chenopodium quinoa Willd., an ancient Andean crop of high nutritional value. Food Chem. 2011, 125, 1226–1234. [Google Scholar] [CrossRef]
- Sautour, M.; Canon, F.; Miyamoto, T.; Dongmo, A.; Lacaille-Dubois, M.A. A new ecdysteroid and other constituents from two Dioscorea species. Biochem. Syst. Ecol. 2008, 36, 559–563. [Google Scholar] [CrossRef]
- Lafont, R.; Dinan, L. Practical uses for ecdysteroids in mammals including humans: An update. J. Insect Sci. 2003, 3, 7. [Google Scholar] [CrossRef]
- Ambrosio, G.; Wirth, D.; Joseph, J.F.; Mazzarino, M.; de la Torre, X.; Botrè, F.; Parr, M.K. How reliable is dietary supplement labelling?—Experiences from the analysis of ecdysterone supplements. J. Pharm. Biomed. Anal. 2020, 177, 112877. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi, A.; Herke, I.; Lenglyel, K.; Báthori, M.; Kele, Z.; Simon, A.; Tóth, G.; Szendrei, K. Ecdysteroid-containing food supplements from Cyanotis arachnoidea on the European market: Evidence for spinach product counterfeiting. Sci. Rep. 2016, 6, 37322. [Google Scholar] [CrossRef]
- Sláma, K.; Lafont, R. Insect hormones—Ecdysteroids: Their presence and action in vertebrates. Eur. J. Entomol. 1995, 92, 355–377. [Google Scholar]
- Matsuda, T.; Kawaba, T.; Yamamoto, Y. Pharmacological studies of insect metamorphosing steroids from Achyranthis radix. Nippon. Yakurigaku Zasshi (Folia Pharmacol. Jpn.) 1970, 66, 551–563. [Google Scholar]
- Ogawa, S.; Nishimoto, N.; Matsuda, H. Pharmacology of ecdysones in Vertebrates. In Invertebrate Endocrinology and Hormonal Heterophylly; Burdette, W.J., Ed.; Springer: Berlin, Germany, 1974; pp. 341–344. [Google Scholar]
- Uchiyama, M.; Yoshida, T. Effect of ecdysterone on carbohydrate and lipid metabolism. In Invertebrate Endocrinology and Hormonal Heterophylly; Burdette, W.J., Ed.; Springer: Berlin, Germany, 1974; pp. 401–416. [Google Scholar]
- Uchiyama, M.; Otaka, T. Phytoecdysones and protein metabolism in mammalia. In Invertebrate Endocrinology and Hormonal Heterophylly; Burdette, W.J., Ed.; Springer: Berlin, Germany, 1974; pp. 375–400. [Google Scholar]
- Lafont, R.; Serova, M.; Didry-Barca, B.; Raynal, S.; Guibout, L.; Dinan, L.; Veillet, S.; Latil, M.; Dioh, W.; Dilda, P. 20-Hydroxyecdysone activates the protective arm of the RAAS via Mas receptor. BioRiv 2020. [Google Scholar] [CrossRef]
- Parr, M.K.; Zhao, P.; Haupt, O.; Tchoukouegno Ngueu, S.; Hengevoss, J.; Fritzemeier, K.-H.; Piechotta, M.; Schlörer, N.; Muhn, P.; Zheng, W.-Y.; et al. Estrogen receptor beta is involved in skeletal muscle hypertrophy induced by the phytoecdysteroid ecdysterone. Mol. Nutr. Food Res. 2014, 58, 1861–1872. [Google Scholar] [CrossRef]
- Gorelick-Feldman, J.; Cohick, W.; Raskin, I. Ecdysteroids elicit a rapid Ca2+ flux leading to Akt activation and increased protein synthesis in skeletal muscles. Steroids 2010, 75, 632–637. [Google Scholar] [CrossRef]
- Detmar, M.; Dumas, M.; Bonté, F.; Meybeck, A.; Orfanos, C.E. Effects of ecdysterone on the differentiation of normal human keratinocytes in vitro. Eur. J. Dermatol. 1994, 4, 558–562. [Google Scholar]
- Syrov, V.N.; Khushbaktova, Z.A. Wound-healing effect of ecdysteroids. Dokl Akad Nauk. Resp Uzb. 1996, 12, 47–50. [Google Scholar]
- Li, Y.L.; Wu, X.; Zhang, C.; Zhenggao, Y.; Wang, W. Effect of ecdysterone paste on wound healing in rabbits. J. Xinxiang Med Coll. 2008, 25, 116–118. [Google Scholar]
- Zhou, Y.; Wu, X.; Wu, C.; Zhang, Y.; Zhang, Z.; Wang, W. The healing promoting effect of ecdysterone on experimental skin wound in rabbit. J. Pract. Med. 2010, 14, 2494–2496. [Google Scholar]
- Ho, R.; Teai, T.; Meybeck, A.; Raharivelomanana, P. UV-protective effects of phytoecdysteroids from Microsorum grossum extracts on human dermal fibroblasts. Nat. Prod. Commun. 2015, 10, 33–36. [Google Scholar] [CrossRef]
- Napierała, M.; Nawrot, J.; Gornowicz-Porowska, J.; Florek, E.; Moroch, A.; Admaski, Z.; Kroma, A.; Miechowicz, I.; Nowak, G. Separation and HPLC characterization of active natural steroids in a standardized extract from the Serratula coronata herb with antiseborrheic dermatitis activity. Int. J. Environ. Res. Public Health 2020, 17, 6453. [Google Scholar] [CrossRef] [PubMed]
- WADA. Summary of Major Modifications and Explanatory Notes. 2020 Prohibited List. Available online: https://www.wada-ama.org/sites/default/files/wada_2020_english_summary_of_modifications_.pdf (accessed on 1 April 2021).
- Smetanin, B.Y. The Influence of Preparations of Plant Origin on Physical Work Capacity; The Russian Ministry of Public Health: Moscow, Russian, 1986. [Google Scholar]
- Simakin, S.Y.; Panyushkin, V.V.; Portugalov, S.N.; Kostina, L.V.; Martisorov, E.G. Combined application of preparation Ecdysten and product Bodrost during training in cyclic sports. Sports Sci. Bull. 1988, 2, 29–31. [Google Scholar]
- Gadzhieva, R.M.; Portugalov, S.N.; Paniushkin, V.V.; Kondrat’eva, I.I. A comparative study of the anabolic action of Ecdysten, Leveton and Prime Plus, preparations of plant origin. Eksp Klin Farmakol. 1995, 58, 46–48. [Google Scholar]
- Azizov, A.P.; Seifulla, R.D.; Ankudinova, I.A.; Kondrat’eva, I.I.; Borisova, I.G. Effect of the antioxidants elton and leveton on the physical work capacity of athletes. Eksp Klin Farmakol. 1998, 61, 60–62. [Google Scholar] [PubMed]
- Semenov, V. Trial of the Leuzea-containing preparation «Rus-Olympic» under extreme conditions. In Leuzea and Your Health; Antoshechkin, A., Ed.; Ceptima Publishing Inc.: Clearwater, FL, USA, 2000. [Google Scholar]
- Emirova, L.R.; Rozhkova, E.A.; Paniushkin, V.V.; Mirzolan, R.S.; Seifulla, N.R. The effects of cytamines and their combinations with Ecdysten, Apilak, Vitamax and Essentiale on the work capacity of athletes. Eksp Klin Farmakol. 2004, 67, 66–68. [Google Scholar]
- Wilborn, C.D.; Taylor, L.W.; Campbell, B.I.; Kerksick, C.; Rasmussen, C.J.; Greenwood, M.; Kreider, R.B. Effects of methoxyisoflavone, ecdysterone, and sulfo-polysaccharide supplementation on training adaptations in resistance-trained males. J. Int. Soc. Sports Nutr. 2006, 3, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Safarova, D.D.; Tursunov, N.B. Aspects of sports medicine: The effect of Ekdisten. Sci. Sports Curr. Trends 2016, 12, 52–57. [Google Scholar]
- Isenmann, E.; Ambrosio, G.; Joseph, J.F.; Mazzarino, M.; de la Torre, X.; Zimmer, P.; Kazlauskas, R.; Goebel, C.; Botrè, F.; Diel, P.; et al. Ecdysteroids as non-conventional anabolic agent: Performance enhancement by ecdysterone supplementation in humans. Arch. Toxicol. 2019, 93, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Dioh, W.; Chabane, M.; Tourette, C.; Azbekyan, A.; Morelot-Panzini, C.; Hajjar, L.A.; Lins, M.; Nair, G.B.; Whitehouse, T.; Mariani, J.; et al. Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): A structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Sláma, K. Ecdysteroids: Insect hormones, plant defensive factors or human medicine? Phytoparasitica 1993, 20, 3–8. [Google Scholar] [CrossRef]
- Esposito, D.; Rathisanabapathy, T.; Poulev, A.; Komarnytsky, S.; Raskin, I. Akt-dependent anabolic activity of natural and synthetic brassinosteroids in rat skeletal muscle cells. J. Med. Chem. 2011, 54, 4057–4066. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Kizelsztein, P.; Komarnytski, S.; Raskin, I. Hypoglycemic effects of brassinosteroid in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E652–E658. [Google Scholar] [CrossRef]
- Esposito, D.; Rathisanabapathy, T.; Schmidt, B.; Shakarjian, M.P.; Komarnytsky, S.; Raskin, I. Acceleration of cutaneous wound healing by brassinosteroids. Wound Repair Regen. 2013, 21, 688–696. [Google Scholar] [CrossRef]
- Abu Bakar, M.H.; Azmi, M.N.; Shariff, K.A.; Tan, J.S. Withaferin A protects against high-fat diet-induced obesity via attenuation of oxidative stress, inflammation, and insulin resistance. Appl. Biochem. 2019, 188, 241–259. [Google Scholar] [CrossRef]






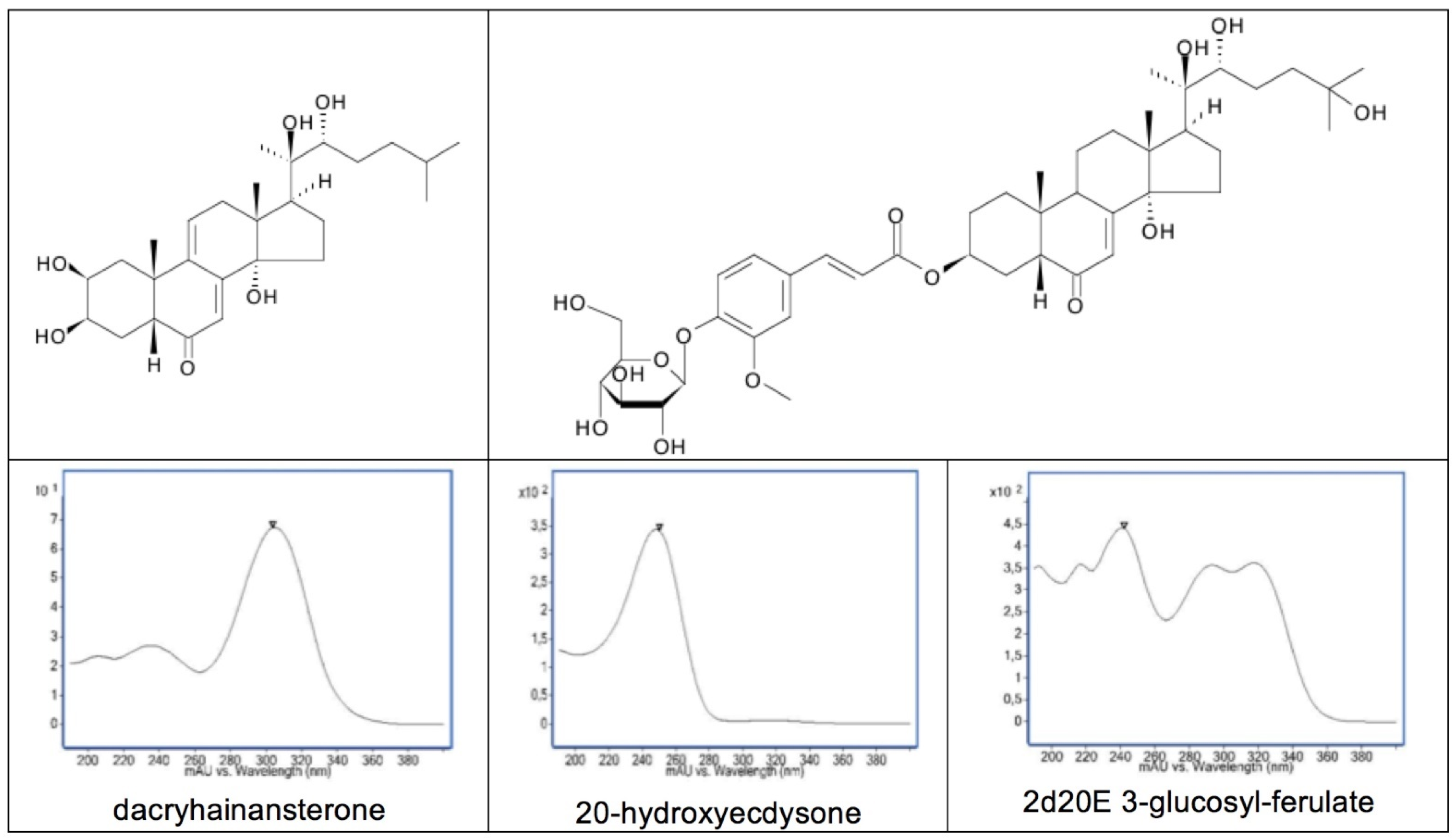



| Species | Family | Plant Part | Amount (mg/g) |
|---|---|---|---|
| Achyranthes japonica | Amaranthaceae | dry leaves | 2 |
| Ajuga chamæpitys | Labiatae | dry whole plant | 1.6 |
| Ajuga decumbens | Labiatae | whole plant | 1.3 |
| Ajuga japonica | Labiatae | dry leaves | 2 |
| Cyanotis arachnoidea | Commelinaceae | dry whole plant | 12 |
| dry roots | 23 | ||
| Cyanotis vaga | Commelinaceae | dry leaves | 7 |
| Dacrydium intermedium | Podocarpaceae | dry bark | 10 |
| Diploclisia glaucescens | Menispermaceae | dry stem | 32 |
| Helleborus abchasicus | Ranunculaceae | dry aerial parts | 2.4 |
| Helleborus atrorubens | Ranunculaceae | dry aerial parts | 2.5 |
| Helleborus bocconei | Ranunculaceae | dry aerial parts | 2.6 |
| Helleborus cyclophyllus | Ranunculaceae | dry aerial parts | 2.7 |
| Helleborus dumentorum | Ranunculaceae | dry aerial parts | 2.9 |
| Helleborus guttatus | Ranunculaceae | dry aerial parts | 2.7 |
| Helleborus multifidus | Ranunculaceae | dry aerial parts | 3.6 |
| Helleborus niger | Ranuculaceae | dry plant or roots | 2.1 |
| Helleborus orientalis | Ranunculaceae | dry aerial parts | 4.5 |
| Helleborus viridis | Ranunculaceae | dry aerial parts | 2.1 |
| Ipomoea calonyction | Convolvulaceae | seeds | 1.5 |
| Lychnis chalcedonica | Caryophyllaceae | dry aerial parts | 7.9 |
| Lychnis flos-coculi | Caryophyllaceae | dry plant | 1.7 |
| Microsorum membranifolium | Polypodiaceae | dry fronds | 2.1 |
| dry rhizomes | 0.6 | ||
| Microsorum scolopendria | Polypodiaceae | dry fronds | 2.2 |
| dry rhizomes | 6.8 | ||
| Pandiaka involucra | Amaranthaceae | dry aerial parts | 3.0 |
| Podocarpus elatus | Podocarpaceae | dry bark | 4.5 |
| Polypodium vulgare | Polypodiaceae | dry rhizomes | 10 |
| Rhaponticum carthamoides | Asteraceae | dry fruits | 15 |
| Rhaponticum integrifolium | Asteraceae | dry flowers | 1.5 |
| Rhaponticum scariosum | Asteraceae | dry fruits | 13 |
| Serratula inermis | Asteraceae | dry fruits/flowers | 20 |
| dry leaves | 2.5 | ||
| Serratula tinctoria | Asteraceae | fresh plant | 2 |
| Serratula xeranthemoides | Asteraceae | dry flower buds | 1.6 |
| dry inflorescences | 3.3 | ||
| fruit formation | 2.8 | ||
| seeds | 1.1 | ||
| Sesuvium portulacastrum | Aizoaceae | dry whole plant | 3.5 |
| Silene jenisseensis | Caryophyllaceae | dry whole plant | 35 |
| Silene nutans | Caryophyllaceae | dry whole plant | 2.7 |
| Silene otites | Caryophyllaceae | dry whole plant | 9.8 |
| dry inflorescences | 32.6 | ||
| dry stems/leaves | 5.9 | ||
| Silene praemixta | Caryophyllaceae | dry leaves | 25 |
| dry roots | 3.4 | ||
| dry inflorescences | 17 | ||
| Silene repens | Caryophyllaceae | dry whole plant | 12 |
| Tinospora cordifolia | Menispermaceae | dry stems | 0.25 |
| Vitex glabrata | Verbenaceae | dry stem bark | 18 |
| Species | Order | Common Name | Mono-/Oligo-/Polyphagous | Effects of Feeding 20E | Resistant or Susceptible | Fate of Ingested 20E | Refs. |
|---|---|---|---|---|---|---|---|
| INSECTS | |||||||
| Achaea janata | Castor semi-looper | Polyphagous | Inhibition of adult emergence | Susceptible | [68] | ||
| Acherontia atropos | Lepidoptera | Death’s head hawkmoth | Polyphagous (some host plants ecdysteroid-positive) | None at 400 ppm | Resistant | Excretes 20E unmetabolised | [63] |
| Acrolepiopsis assectella | Lepidoptera | Leek moth | Oligophagous on the Alliaceae (ecdysteroid-negative) | Developmental disruption and death | Susceptible | [69] | |
| Aglais urticae | Lepidoptera | Small tortoiseshell butterfly | Monophagous (on nettles—ecdysteroid negative) | Initial antifeedant effect, followed by reduced feeding and developmental disruption | Susceptible | Excretion of 20E and polar metabolites (no FA conjugates) | [64] |
| Agrius convolvulae | Lepidoptera | Sweet potato hornworm | Polyphagous | None at 1600 ppm (but effects of E at 400 ppm) | Resistant | [70] | |
| Bombyx mori | Lepidoptera | Domestic silkworm | Monphagous (on mulberry—ecdysteroid-negative) | Enhanced developmental synchrony at very low concentrations. Feeding deterrence, developmental defects and death at higher concentrations | Susceptible | E is converted to 3-epiE and a sulphate conjugate | [71,72,73,74,75] |
| Bradysia impatiens | Diptera | Darkwinged fungus gnat | Soil-dwelling mycophage | Reduced survival | Susceptible | [76] | |
| Chloridea (Heliothis/ Helicoverpa) virescens | Lepidoptera | Tobacco budworm | Polyphagous | None at 1000 ppm | Resistant | Excretion of 20E 22-FA esters | [73,77] |
| Cynthia cardui | Lepidoptera | Painted lady butterfly | Polyphagous (some host plants ecdysteroid-positive) | Tolerates low levels, but increasing antifeedant effect at higher concentrations | Intermediate | Excretion of 20E, polar metabolites and minor apolar metabolites (no FA conjugates) | 64 |
| Gryllus bimaculatus | Orthoptera | Two-spotted cricket | Polyphagous | Conversion to 14-deoxy20E and 20E 22-FA esters | [78,79] | ||
| Heliothis (Helicoverpa) armigera | Lepidoptera | Cotton bollworm | Polyphagous | None at 50 μg E per insect | Resistant | E is excreted unchanged, mainly as E 22-palmitate and as 2/3-acetate | [80] |
| Inachis io | Lepidoptera | Peacock butterfly | Oligophagous (host plants ecdysteroid-negative) | Strong antifeedant effect, resulting in starvation | Susceptible | Excretion of 20E and polar metabolites (no FA conjugates) | [64] |
| Lacanobia oleracea | Lepidoptera | Tomato moth | Polyphagous (some host plants ecdysteroid-positive) | None at 400 ppm | Resistant | Conjugation to long-chain FAs and rapid excretion | [62,63] |
| Lobesia botrana | Lepidoptera | European grapevine moth | Oligophagous (on ecdysteroid-negative plants) | Enhanced mortality | Susceptible | [81] | |
| Locusta migratoria | Orthoptera | Migratory locust | Polyphagous | None at 400 ppm | Resistant | Conversion to 20E 2-phosphate, 3-acetyl 20E 2-phosphate and 20E 3Ac | [82,83,84] |
| Mamestra brassicae | Lepidoptera | Cabbage armyworm | Polyphagous | None at 800 ppm | Resistant | [70] | |
| Manduca sexta | Lepidoptera | Tobacco hornworm | Oligophagous on plants of the Solanaceae (some are ecdysteroid-positive) | None at 800 ppm | Resistant | Conversion to 2- and 22-phosphates and 3-epi-20E | [85,86] |
| Myzus persicae | Hemiptera | Peach-potato aphid | Polyphagous | Converted to 22-glucoside | [87] | ||
| Ostrinia nubilalis | Lepidoptera | European corn borer | Polyphagous | Antifeedant effect | Susceptible | Excreted as FA conjugates | [57,75,87] |
| Pectinophora gossypiella | Lepidoptera | Pink bollworm | Oligophagous (on ecdysteroid-negative plants) | Growth inhibition and developmental disruption | Susceptible | [71] | |
| Plodia interpunctella | Lepidoptera | Indian meal moth | Polyphagous (on grains) | Effects at 200 ppm | Resistant to low to moderate concentrations of 20E | Converted to 3-oxo- and 3-epi-derivatives, excreted in free form and conjugated to FAs | [87,88,89] |
| Spodoptera littoralis | Lepidoptera | Egyptian cotton leafworm | Polyphagous (some host plants ecdysteroid-positive) | None at 100 ppm; feeding deterrent for 1st instar larvae | Resistant | Excretion of 20E and 22-FA conjugates | [60,75] |
| Tyria jacobae | Lepidoptera | Cinnabar moth | Oligophagous (some host plants ecdysteroid-positive) | Dose-dependent effects, tolerating low levels, but higher concentrations bring about developmental defects and death | Intermediate | Rapid excretion of 20E and polar and apolar metabolites (no FA conjugates) | [64] |
| Nematodes | |||||||
| Heterodera avenae | Nematoda | Cereal cyst nematode | Oligophagous on cereal crops (ecdysteroid-negative) | Abnormal moulting, immobility and impaired development | Susceptible | [67] | |
| Heterodera schlachtii | Nematoda | Sugarbeet cyst nematode | Polyphagous | Abnormal moulting, immobility and impaired development | Susceptible | [67] | |
| Meloidogyne javanica | Nematoda | Root-knot nematode/sugar cane eelworm | Polyphagous | Abnormal moulting, immobility and impaired development | Susceptible | [67] | |
| Pratylenchus neglectus | Nematoda | Root lesion nematode | Oligophagous (on ecdysteroid-negative plants) | Abnormal moulting, immobility and impaired development | Susceptible | [67] | |
| Major Effects Described for 20-Hydroxyecdysone or Related Molecules | |
|---|---|
| Anabolic (muscle) | Cardioprotective |
| Fat-reducing/Hypolipidaemic | Neuromuscular protective |
| Anti-diabetic | Neuroprotective |
| Anti-fibrotic | Memory protective |
| Anti-inflammatory | Liver protective |
| Antioxidant | Lung protective |
| Anti-thrombotic | Kidney protective |
| Vasorelaxant | Gastric protective |
| Hematopoiesis stimulation | Bone and cartilage protective |
| Angiogenic | Skin protective/repairing |
| Aim | Age | N | Dose | Duration | Output | Reference |
|---|---|---|---|---|---|---|
| Physical capacity | Athletes 18–28 | 117 | ? | ? | Ecdysterone treatment improves oxygenation and decreases recovery time after exercise | [202] |
| Anabolic effect | Young adults | 10 M, 10 F /arm | 25 mg 3 x/day | 10 days | Ecdysten and/or protein supplement + physical training evoke a reduction of fat mass (−7% M, −14% F) and an increase in muscle mass (+6% M, 7% F) | [203] |
| Anabolic effect | Runners 15–25 | 20 (4 arms) | ? | 21 days | Reduction of subcutaneous fat, muscle mass increase | [204] |
| Physical capacity | Athletes | 44 | ? | 20 days | Increase of working capacity by 10–15% | [205] |
| Endurance | Athletes | ? | ? | ? | Enhancement of endurance performances and of immune system response | [206] |
| Physical capacity | Athletes | 10/arm | ? | 3 weeks | A combination of ecdysten and cytamins increases bench press performance and endurance | [207] |
| Physical capacity | 20.5 ± 3 | 45 M | 30 mg/day * | 8 weeks | No observed effects on body composition, anabolic/catabolic hormonal status, or physical performance | [208] |
| Physical capacity | Athletes | 64 M | 5, 10, then 15 mg/day | 10 days each | Muscle mass increase (+5%), fat mass decrease, strength increase (+12%) | [209] |
| Anabolic effect | Athletes 25.6 ± 3.7 | 46 | 12/48 mg/day ** | 10 weeks | Increase in body weight (ca. 3 kg), muscle hypertrophy, and improved performance at bench press | [210] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lafont, R.; Balducci, C.; Dinan, L. Ecdysteroids. Encyclopedia 2021, 1, 1267-1302. https://doi.org/10.3390/encyclopedia1040096
Lafont R, Balducci C, Dinan L. Ecdysteroids. Encyclopedia. 2021; 1(4):1267-1302. https://doi.org/10.3390/encyclopedia1040096
Chicago/Turabian StyleLafont, René, Christine Balducci, and Laurence Dinan. 2021. "Ecdysteroids" Encyclopedia 1, no. 4: 1267-1302. https://doi.org/10.3390/encyclopedia1040096
APA StyleLafont, R., Balducci, C., & Dinan, L. (2021). Ecdysteroids. Encyclopedia, 1(4), 1267-1302. https://doi.org/10.3390/encyclopedia1040096





