Establishment of a Real-Time Monitoring System for the Flow Rate and Concentration of Process Gases for Calculating Tier 4 Emissions in the Semiconductor/Display Industry
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
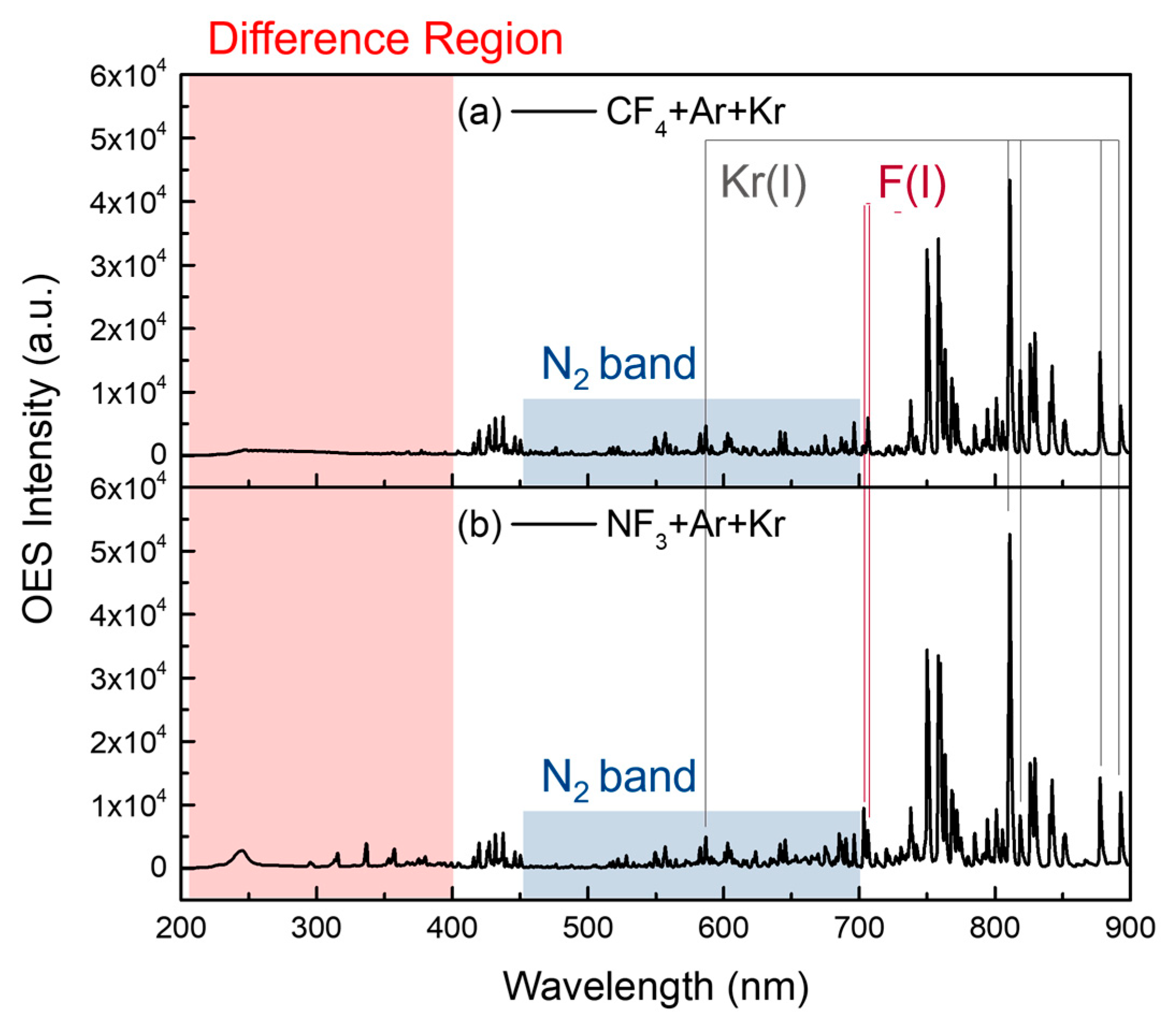
3.1. OES Spectra for Analysis
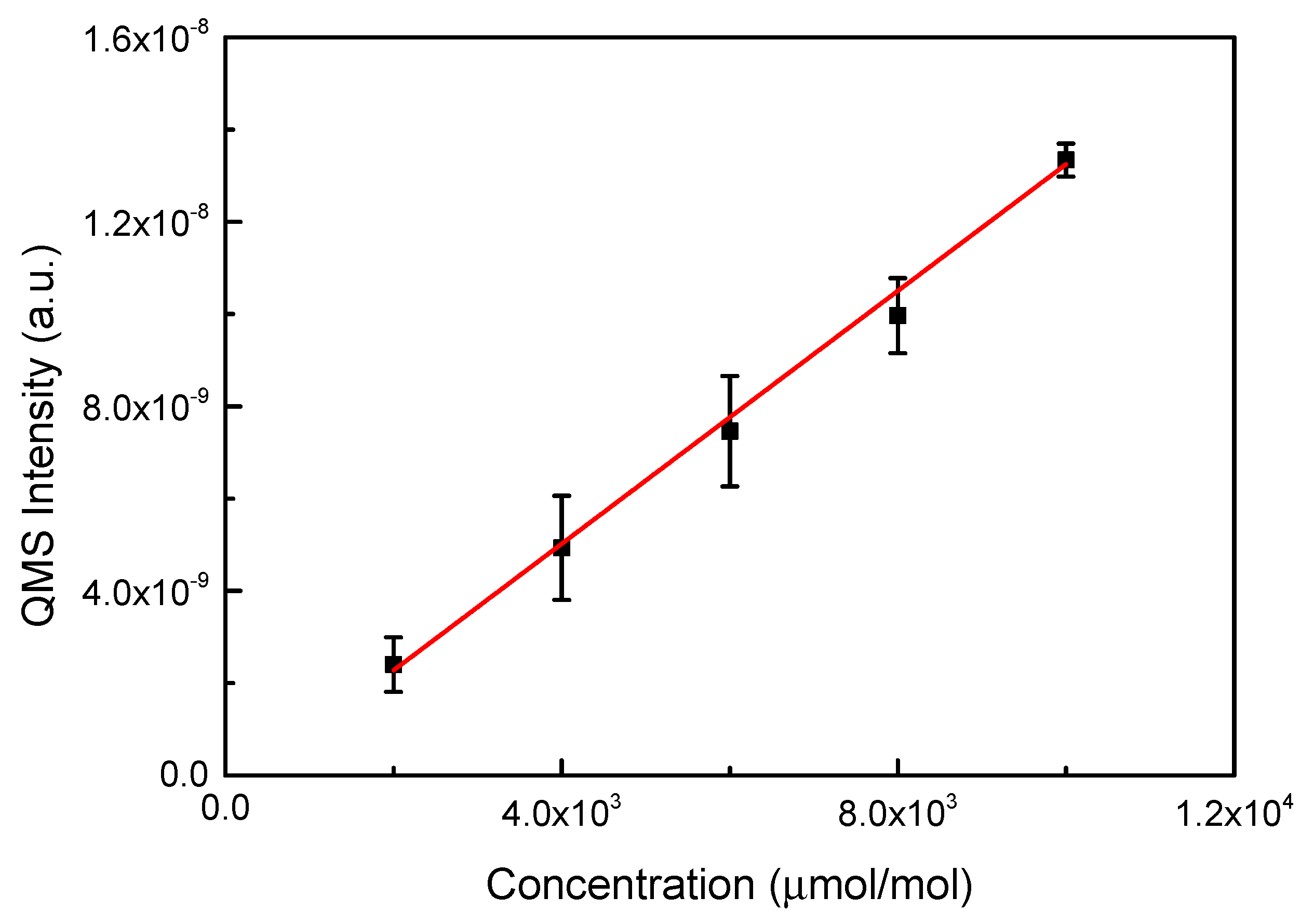
3.2. Calibration of QMS and RGA
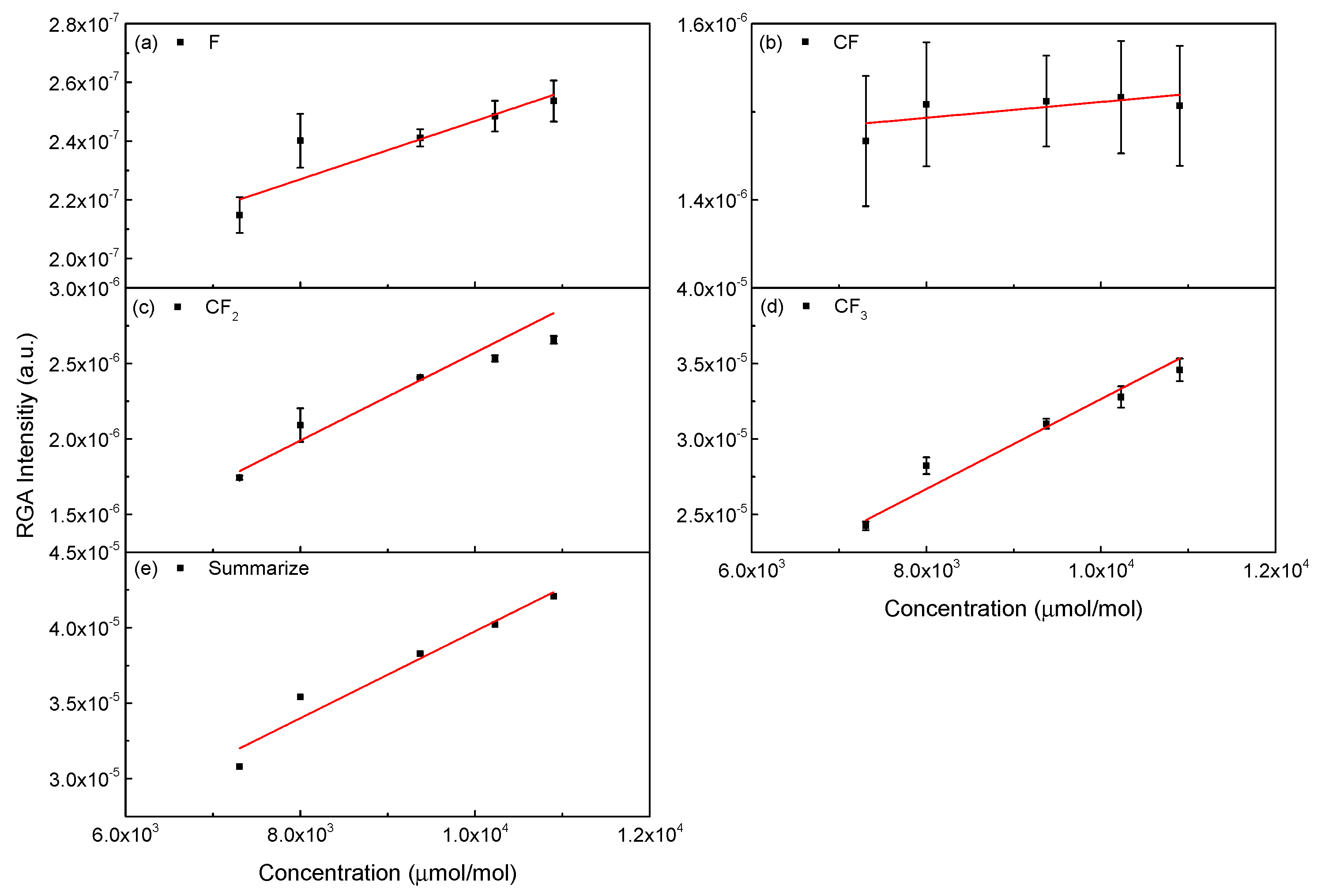
3.3. Analysis of CF4
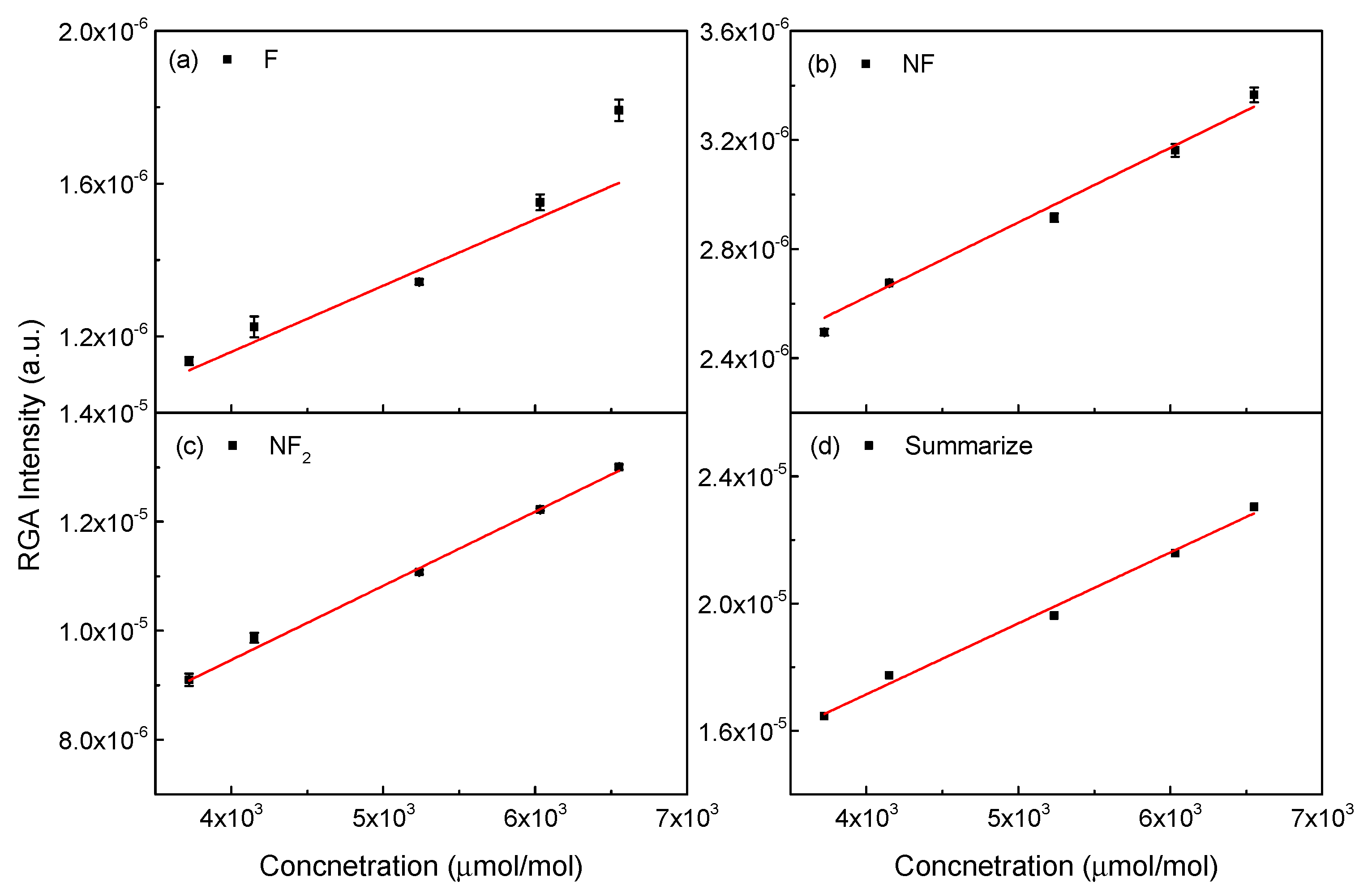
3.4. Analysis of NF3
3.5. Estimate Method for Concentraion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GWP | Global Warming Potential |
| RGA | Residual Gas Analyzer |
| OES | Optical Emission Spectroscopy |
| QMS | Quadrupole Mass Spectrometer |
| FT-IR | Fourier-Transform Infrared spectroscopy |
| EPA | U.S. Environmental Protection Agency |
| IPCC | Intergovernmental Panel on Climate Change |
| GHG | Greenhouse Gases |
| RIE | Reactive Ion Etching |
| TP | Turbomolecular Pump |
| CCD | Charge-Coupled Device Array Detector |
| DMP | Dry Mechanical Pump |
References
- Dorn, H.P.; Brandenburger, U.; Brauers, T.; Hausmann, M.; Ehhalt, D.H. In-Situ Detection of Tropospheric OH Radicals by Folded Long-Path Laser Absorption. Results from the POPCORN Field Campaign in August 1994. Geophys. Res. Lett. 1996, 23, 2537–2540. [Google Scholar] [CrossRef]
- Heard, D.E.; Pilling, M.J. Measurement of OH and HO2 in the Troposphere. Chem. Rev. 2003, 103, 5163–5198. [Google Scholar] [CrossRef]
- Holland, F.; Hessling, M.; Hofzumahaus, A. In Situ Measurement of Tropospheric OH Radicals by Laser-Induced Fluorescence. J. Atmos. Sci. 1995, 52, 3393–3401. [Google Scholar] [CrossRef]
- Rigby, M.; Prinn, R.G.; O’doherty, S.; Montzka, S.A.; Mcculloch, A.; Harth, C.M.; Uhle, J.M.; Salameh, P.K.; Weiss, R.F.; Young, D.; et al. Re-Evaluation of the Lifetimes of the Major CFCs and CH3CCl3 Using Atmospheric Trends. Atmos. Chem. Phys. 2013, 13, 2691–2702. [Google Scholar] [CrossRef]
- Takahashi, K.; Matsumi, Y.; Wallington, T.J.; Hurley, M.D.; Takahashi, C.; Matsumi, Y.; Wallington, T.J.; Hurley, M.D. Atmospheric Chemistry of CF3CFHOCF3: Reaction with OH Radicals, Atmospheric Lifetime, and Global Warming Potential. J. Geophys. Res. Atmos. 2002, 107, 4574. [Google Scholar] [CrossRef]
- Kiefer, J.; Zhou, B.; Zetterberg, J.; Li, Z.; Alden, M. Laser-Induced Fluorescence Detection of Hot Molecular Oxygen in Flames Using an Alexandrite Laser. Appl. Spectrosc. 2014, 68, 1266–1273. [Google Scholar] [CrossRef]
- Orkin, V.L.; Huie, R.E.; Kurylo, M.J. Atmospheric Lifetimes of Hfc-143a and Hfc-245fa: Flash Photolysis Resonance Fluorescence Measurements of the Oh Reaction Rate Constants. J. Phys. Chem. 1996, 100, 8907–8912. [Google Scholar] [CrossRef]
- Prinn, R.G.; Rasmussen, R.A.; Simmonds, P.G.; Alyea, F.N.; Cunnold, D.M.; Lane, B.C.; Cardelino, C.A.; Crawford, A.J. The Atmospheric Lifetime Experiment: 5. Results for CH3CCl3 Based on Three Years of Data. J. Geophys. Res. Ocean. 1983, 88, 8415–8426. [Google Scholar] [CrossRef]
- Kurylo, M.J.; Orkin, V.L. Determination of Atmospheric Lifetimes via the Measurement of OH Radical Kinetics. Chem. Rev. 2003, 103, 5049–5076. [Google Scholar] [CrossRef]
- Vaghjiani, G.L.; Ravishankara, A.R. Kinetics and Mechanism of OH Reaction with CH3OOH. J. Phys. Chem. 1989, 93, 1948–1959. [Google Scholar] [CrossRef]
- Fisher, D.A.; Hales, C.H.; Wang, W.C.; Ko, M.K.W.; Sze, N.D. Model Calculations of the Relative Effects of CFCs and Their Replacements on Global Warming. Nature 1990, 344, 513–516. [Google Scholar] [CrossRef]
- Roehl, C.M.; Boglu, D.; Brühl, C.; Moortgat, G.K. Infrared Band Intensities and Global Warming Potentials of CF4, C2F6, C3F8, C4F10, C5F12, and C6F14. Geophys. Res. Lett. 1995, 22, 815–818. [Google Scholar] [CrossRef]
- Wallington, T.J.; Schneider, W.F.; Sehested, J.; Bilde, M.; Platz, J.; Nielsen, O.J.; Christensen, L.K.; Molina, M.J.; Molina, L.T.; Wooldridge, P.W. Atmospheric Chemistry of HFE-7100 (C4F9OCH3): Reaction with OH Radicals, UV Spectra and Kinetic Data for C4F9OCH2 and C4F9OCH2O2 Radicals, and the Atmospheric Fate of C4F9OCH2O Radicals. J. Phys. Chem. A 1997, 101, 8264–8274. [Google Scholar] [CrossRef]
- Orkin, V.L.; Guschin, A.G.; Larin, I.K.; Huie, R.E.; Kurylo, M.J. Measurements of the Infrared Absorption Cross-Sections of Haloalkanes and Their Use in a Simplified Calculational Approach for Estimating Direct Global Warming Potentials. J. Photochem. Photobiol. A Chem. 2003, 157, 211–222. [Google Scholar] [CrossRef]
- Climate Change: The IPCC 1990 and 1992 Assessments—IPCC. Available online: https://www.ipcc.ch/report/climate-change-the-ipcc-1990-and-1992-assessments/ (accessed on 24 January 2025).
- Godal, O.; Fuglestvedt, J. Testing 100-Year Global Warming Potentials: Impacts on Compliance Costs and Abatement Profile. Clim. Change 2002, 52, 93–127. [Google Scholar] [CrossRef]
- Prather, M.J. Time Scales in Atmospheric Chemistry: Theory, GWPs for CH4 and CO, and Runaway Growth. Geophys. Res. Lett. 1996, 23, 2597–2600. [Google Scholar] [CrossRef]
- SAR Climate Change 1995: Synthesis Report—IPCC. Available online: https://www.ipcc.ch/report/ar2/syr/ (accessed on 24 January 2025).
- TAR Climate Change 2001: Synthesis Report—IPCC. Available online: https://www.ipcc.ch/report/ar3/syr/ (accessed on 24 January 2025).
- AR4 Climate Change 2007: Synthesis Report—IPCC. Available online: https://www.ipcc.ch/report/ar4/syr/ (accessed on 24 January 2025).
- AR5 Synthesis Report: Climate Change 2014—IPCC. Available online: https://www.ipcc.ch/report/ar5/syr/ (accessed on 24 January 2025).
- AR6 Synthesis Report: Climate Change 2023—IPCC. Available online: https://www.ipcc.ch/report/sixth-assessment-report-cycle/ (accessed on 24 January 2025).
- Lee, B.J.; Yun, S.Y.; Jeong, I.K.; Hwang, Y.; Park, J.H.; Kim, J. Improving the Measurement of Characteristic Parameters for the Determination of GHG Emissions in the Semiconductor and Display Industries in Korea. Appl. Sci. 2023, 13, 8834. [Google Scholar] [CrossRef]
- Lee, B.J.; Hwang, Y.; Jo, D.K.; Jeong, J.; Li, P.; Wang, L.; Lu, J.; Lee, B.J.; Hwang, Y.; Jo, D.K.; et al. A Study on Greenhouse Gas (PFCs) Reduction in Plasma Scrubbers to Realize Carbon Neutrality of Semiconductors and Displays. Atmosphere 2023, 14, 1220. [Google Scholar] [CrossRef]
- Kwon, H.T.; Kim, W.J.; Shin, G.W.; Lee, H.H.; Lee, T.H.; Kang, M.H.; Kwon, G.C. Plasma Etching of Silicon at a High Flow and a High Pressure of NF3 in Reactive Ion Etching. J. Korean Phys. Soc. 2019, 74, 1135–1139. [Google Scholar] [CrossRef]
- Barnes, R.M.; Jacksier, T. Atomic Emission Spectra of Xenon, Krypton, and Neon: Spectra from 200 to 900 Nm by Sealed Inductively Coupled Plasma/Atomic Emission Spectroscopy. Appl. Spectrosc. 1994, 48, 65–71. [Google Scholar] [CrossRef]
- Shabushnig, J.G.; Demko, P.R.; Savage, R.N. Applications of optical emission spectroscopy to semiconductor processing. Mater. Res. Soc. Symp. Proc. 1985, 38, 77–84. [Google Scholar] [CrossRef]
- Motohashi, K.; Takahashi, T.; Takahashi, N.; Tsurubuchi, S. Emission Cross Sections in Low-Energy Collisions between He+, Ne+ Ions and CF4 Molecule. J. Phys. B At. Mol. Opt. Phys. 2005, 38, 3339–3348. [Google Scholar] [CrossRef]
- Jin, H.; Tang, C.; Zheng, N.; Den, W.; Chen, X. Investigation of the Chemical Composition and Atomic Emission Spectroscopy of Fused Silica in CF4 Plasma Processing. In Selected Papers of the Chinese Society for Optical Engineering; SPIE: Bellingham, WA, USA, 2017; Volume 10255, p. 102551. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, A.; Li, X.; Zhao, G.; Lu, W.; Xu, Y.; Wang, Y. Determination of Plasma Parameters in a Dual-Frequency Capacitively Coupled Cf4 Plasma Using Optical Emission Spectroscopy. Plasma Sci. Technol. 2013, 15, 885–890. [Google Scholar] [CrossRef]
- An, S.; Hong, S.J. Spectroscopic Analysis of NF3 Plasmas with Oxygen Additive for PECVD Chamber Cleaning. Coatings 2023, 13, 91. [Google Scholar] [CrossRef]




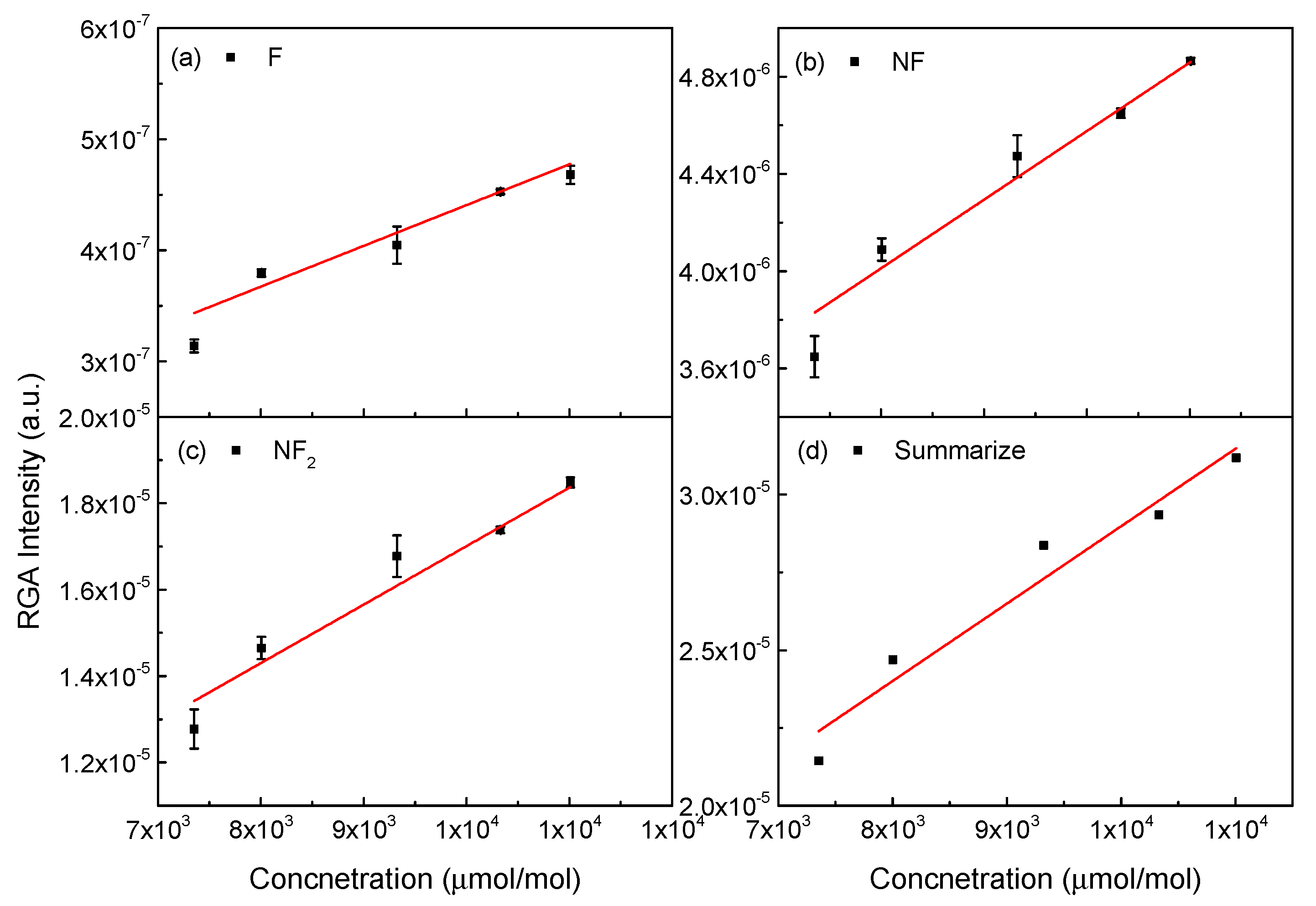
| RGA Intensity (a.u.) | Estimated Concentration (μmol/mol) | Calculated Flow Rate (slm) | DMP Flow Rate (slm) | Total Flow Rate (slm) | QMS Flow Rate (slm) | Error (%) |
|---|---|---|---|---|---|---|
| 3.00 × 10−5 | 3.9 × 105 | 0.415 | 25.4 | 25.8 | 25.6 | 0.78 |
| 3.13 × 10−5 | 4.0 × 105 | 0.395 | 25.4 | 25.8 | 26.0 | 0.77 |
| 3.02 × 10−5 | 3.9 × 105 | 0.412 | 25.4 | 25.8 | 26.6 | 3.00 |
| 2.94 × 10−5 | 3.8 × 105 | 0.424 | 25.3 | 25.7 | 25.7 | 0.00 |
| 2.83 × 10−5 | 3.6 × 105 | 0.443 | 25.3 | 25.7 | 27.4 | 6.20 |
| 2.79 × 10−5 | 3.6 × 105 | 0.450 | 25.3 | 25.8 | 26.1 | 1.10 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 3.49 × 10−7 | 9.36 × 103 | 8.42 × 103 | 10.0 |
| 3.44 × 10−7 | 8.99 × 103 | 8.43 × 103 | 6.37 |
| 3.41 × 10−7 | 8.80 × 103 | 8.43 × 103 | 4.26 |
| 3.39 × 10−7 | 8.69 × 103 | 8.43 × 103 | 2.95 |
| 3.38 × 10−7 | 8.63 × 103 | 8.43 × 103 | 2.29 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 8.12 × 10−7 | 8.84 × 103 | 8.42 × 103 | 4.72 |
| 7.97 × 10−7 | 8.59 × 103 | 8.43 × 103 | 1.86 |
| 7.91 × 10−7 | 8.48 × 103 | 8.43 × 103 | 0.63 |
| 7.79 × 10−7 | 8.29 × 103 | 8.43 × 103 | 1.76 |
| 7.77 × 10−7 | 8.24 × 103 | 8.43 × 103 | 2.25 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 2.77 × 10−7 | 8.52 × 103 | 8.42 × 103 | 1.21 |
| 2.77 × 10−7 | 8.47 × 103 | 8.43 × 103 | 0.54 |
| 2.77 × 10−7 | 8.51 × 103 | 8.43 × 103 | 0.97 |
| 2.77 × 10−7 | 8.52 × 103 | 8.43 × 103 | 0.98 |
| 2.78 × 10−7 | 8.56 × 103 | 8.43 × 103 | 1.50 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 3.41 × 10−7 | 8.43 × 103 | 8.42 × 103 | 0.09 |
| 3.44 × 10−7 | 8.51 × 103 | 8.43 × 103 | 0.94 |
| 3.42 × 10−7 | 8.46 × 103 | 8.43 × 103 | 0.37 |
| 3.41 × 10−7 | 8.44 × 103 | 8.43 × 103 | 0.09 |
| 3.41 × 10−7 | 8.43 × 103 | 8.43 × 103 | 0.02 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 2.47 × 10−7 | 1.01 × 104 | 8.69 × 103 | 13.5 |
| 2.43 × 10−7 | 9.58 × 103 | 8.68 × 103 | 9.32 |
| 2.39 × 10−7 | 9.20 × 103 | 8.69 × 103 | 5.60 |
| 2.39 × 10−7 | 9.19 × 103 | 8.68 × 103 | 5.51 |
| 2.39 × 10−7 | 9.23 × 103 | 8.69 × 103 | 5.92 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 1.58 × 10−6 | 1.79 × 104 | 8.69 × 103 | 51.4 |
| 1.52 × 10−6 | 1.14 × 104 | 8.68 × 103 | 23.7 |
| 1.50 × 10−6 | 8.81 × 103 | 8.69 × 103 | 1.35 |
| 1.48 × 10−6 | 6.22 × 103 | 8.68 × 103 | 39.6 |
| 1.46 × 10−6 | 4.44 × 103 | 8.69 × 103 | 95.7 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 2.35 × 10−6 | 9.25 × 103 | 8.69 × 103 | 6.04 |
| 2.32 × 10−6 | 9.14 × 103 | 8.68 × 103 | 4.95 |
| 2.32 × 10−6 | 9.13 × 103 | 8.69 × 103 | 4.84 |
| 2.32 × 10−6 | 9.13 × 103 | 8.68 × 103 | 4.90 |
| 2.30 × 10−6 | 9.07 × 103 | 8.69 × 103 | 4.20 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 3.03 × 10−5 | 9.22 × 103 | 8.69 × 103 | 5.81 |
| 2.99 × 10−5 | 9.08 × 103 | 8.68 × 103 | 4.34 |
| 2.96 × 10−5 | 8.99 × 103 | 8.69 × 103 | 3.35 |
| 2.95 × 10−5 | 8.93 × 103 | 8.68 × 103 | 2.74 |
| 2.93 × 10−5 | 8.87 × 103 | 8.69 × 103 | 2.10 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 1.29 × 10−6 | 4.77 × 103 | 4.57 × 103 | 4.33 |
| 1.28 × 10−6 | 4.69 × 103 | 4.58 × 103 | 2.52 |
| 1.27 × 10−6 | 4.63 × 103 | 4.59 × 103 | 0.86 |
| 1.25 × 10−6 | 4.54 × 103 | 4.59 × 103 | 1.23 |
| 1.24 × 10−6 | 4.49 × 103 | 4.63 × 103 | 3.19 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 2.80 × 10−6 | 4.77 × 103 | 4.57 × 103 | 4.33 |
| 2.79 × 10−6 | 4.69 × 103 | 4.58 × 103 | 2.52 |
| 2.79 × 10−6 | 4.63 × 103 | 4.59 × 103 | 0.86 |
| 2.79 × 10−6 | 4.54 × 103 | 4.59 × 103 | 1.23 |
| 2.78 × 10−6 | 4.49 × 103 | 4.63 × 103 | 3.19 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 1.03 × 10−5 | 4.63 × 103 | 4.57 × 103 | 1.45 |
| 1.05 × 10−5 | 4.77 × 103 | 4.58 × 103 | 3.94 |
| 1.04 × 10−5 | 4.72 × 103 | 4.59 × 103 | 2.78 |
| 1.04 × 10−5 | 4.68 × 103 | 4.59 × 103 | 1.82 |
| 1.04 × 10−5 | 4.67 × 103 | 4.63 × 103 | 0.85 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 4.00 × 10−7 | 8.89 × 103 | 8.67 × 103 | 2.53 |
| 4.10 × 10−7 | 9.16 × 103 | 8.66 × 103 | 5.52 |
| 4.21 × 10−7 | 9.46 × 103 | 8.66 × 103 | 8.51 |
| 4.24 × 10−7 | 9.55 × 103 | 8.66 × 103 | 9.26 |
| 4.27 × 10−7 | 9.63 × 103 | 8.67 × 103 | 9.99 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 4.35 × 10−6 | 9.20 × 103 | 8.67 × 103 | 5.54 |
| 4.33 × 10−6 | 9.13 × 103 | 8.66 × 103 | 4.86 |
| 4.32 × 10−6 | 9.09 × 103 | 8.66 × 103 | 4.43 |
| 4.34 × 10−6 | 9.16 × 103 | 8.66 × 103 | 5.23 |
| 4.36 × 10−6 | 9.23 × 103 | 8.67 × 103 | 5.92 |
| RGA Intensity (a.u.) | Calculated Concentration (μmol/mol) | Measured Calculation (μmol/mol) | Error (%) |
|---|---|---|---|
| 1.56 × 10−5 | 8.96 × 103 | 8.67 × 103 | 3.03 |
| 1.60 × 10−5 | 9.26 × 103 | 8.66 × 103 | 6.17 |
| 1.59 × 10−5 | 9.18 × 103 | 8.66 × 103 | 5.36 |
| 1.58 × 10−5 | 9.11 × 103 | 8.66 × 103 | 4.66 |
| 1.57 × 10−5 | 9.03 × 103 | 8.67 × 103 | 3.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, B.G.; Park, S.-H.; Goh, D.-H.; Lee, B.-J. Establishment of a Real-Time Monitoring System for the Flow Rate and Concentration of Process Gases for Calculating Tier 4 Emissions in the Semiconductor/Display Industry. Metrology 2025, 5, 60. https://doi.org/10.3390/metrology5040060
Jeong BG, Park S-H, Goh D-H, Lee B-J. Establishment of a Real-Time Monitoring System for the Flow Rate and Concentration of Process Gases for Calculating Tier 4 Emissions in the Semiconductor/Display Industry. Metrology. 2025; 5(4):60. https://doi.org/10.3390/metrology5040060
Chicago/Turabian StyleJeong, Bong Gyu, Sang-Hoon Park, Deuk-Hoon Goh, and Bong-Jae Lee. 2025. "Establishment of a Real-Time Monitoring System for the Flow Rate and Concentration of Process Gases for Calculating Tier 4 Emissions in the Semiconductor/Display Industry" Metrology 5, no. 4: 60. https://doi.org/10.3390/metrology5040060
APA StyleJeong, B. G., Park, S.-H., Goh, D.-H., & Lee, B.-J. (2025). Establishment of a Real-Time Monitoring System for the Flow Rate and Concentration of Process Gases for Calculating Tier 4 Emissions in the Semiconductor/Display Industry. Metrology, 5(4), 60. https://doi.org/10.3390/metrology5040060






