How Can We Improve the Appetite of Older Patients on Dialysis in Japan?
Abstract
1. Introduction
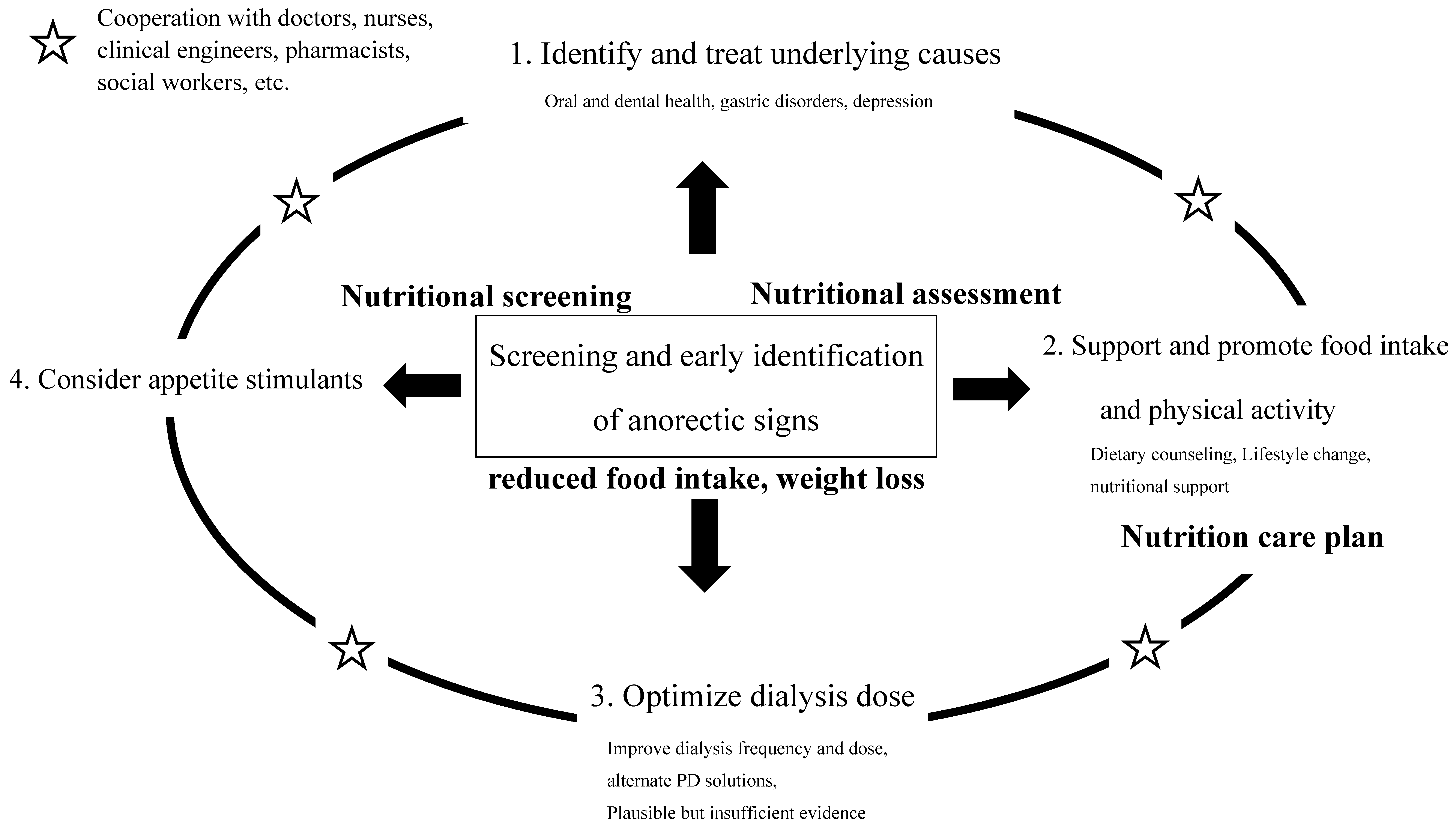
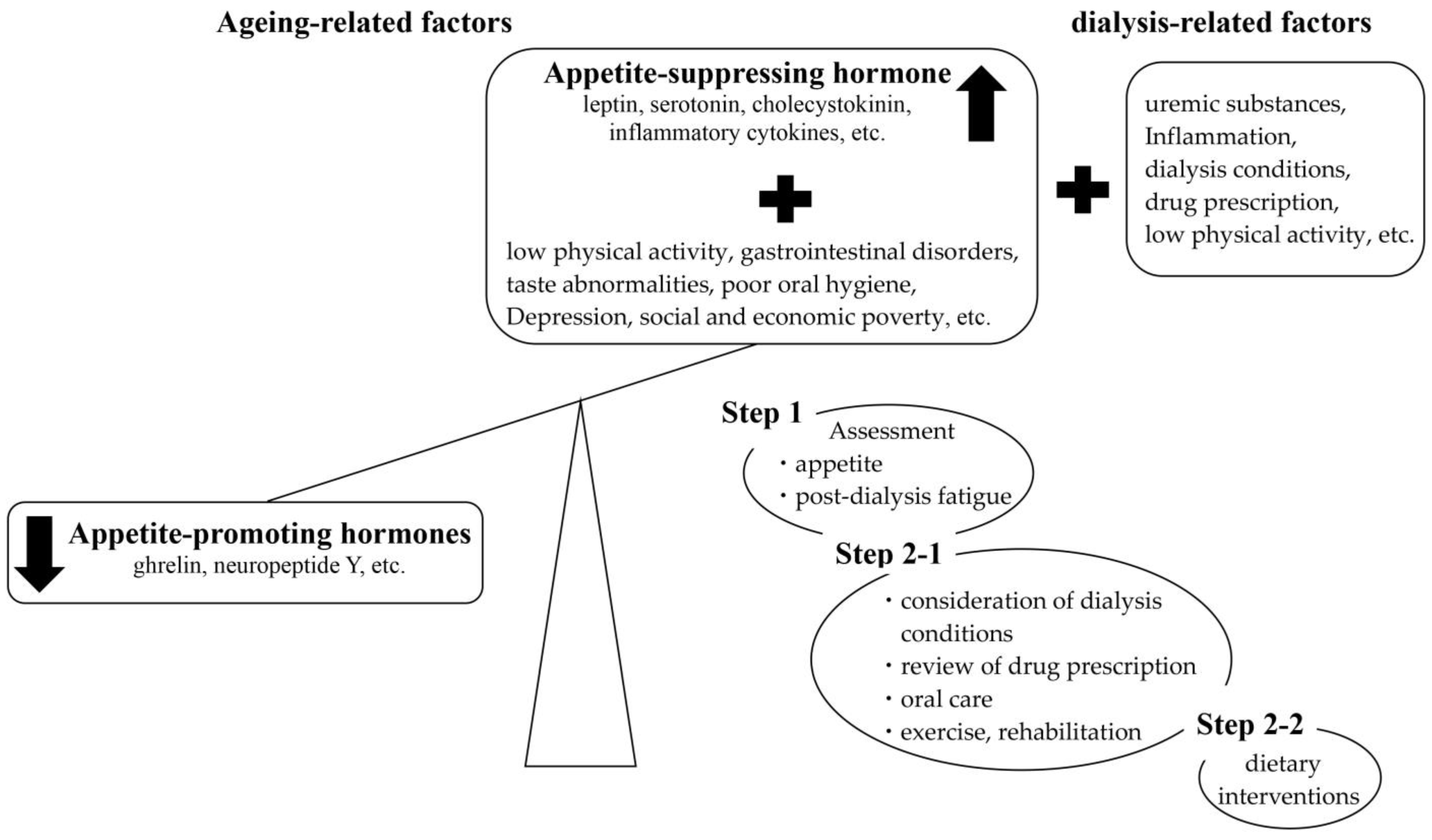
2. Loss of Appetite in Dialysis Patients
3. Appetite Assessment and Appetite-Related Factors
3.1. Appetite Assessment
3.2. Consideration of Dialysis Conditions
3.3. Review of Drug Prescription
3.4. Oral Care
3.5. Post-Dialysis Fatigue (PDF)
3.6. Physical Activity
4. Nutritional Intervention during Anorexia and Decreased Food Intake
4.1. Relaxation or Removal of Diet Restriction or Diet Therapy
4.2. Supplement Energy and Protein with Snacks and Frequent Meals
4.3. Use of ONS
4.4. Intradialytic Parenteral Nutrition (IDPN) Study
5. Specific Nutritional Counseling
6. Support from Conservative Phase to Dialysis Phase (Dialysis Transition Phase)
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanafusa, N.; Abe, M.; Joki, N.; Ogawa, T.; Kanda, E.; Kikuchi, K.; Goto, S.; Taniguchi, M.; Nakai, S.; Naganuma, T.; et al. Annual Dialysis Data Report 2019, JSDT Renal Data Registry. Ren. Replace. Ther. 2023, 9, 47. [Google Scholar] [CrossRef]
- United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020.
- Wysokiński, A.; Sobów, T.; Kłoszewska, I.; Kostka, T. Mechanisms of the anorexia of aging—A review. Age 2015, 37, 9821. [Google Scholar] [CrossRef]
- Merchant, R.A.; Vathsala, A. Healthy aging and chronic kidney disease. Kidney Res. Clin. Pract. 2022, 41, 644–656. [Google Scholar] [CrossRef]
- Bossola, M.; Muscaritoli, M.; Tazza, L.; Panocchia, N.; Liberatori, M.; Giungi, S.; Tortorelli, A.; Rossi Fanelli, F.; Luciani, G. Variables associated with reduced dietary intake in HD patients. J. Renal Nutr. 2005, 15, 244–252. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Block, G.; McAllister, C.J.; Humphreys, M.H.; Kopple, J.D. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am. J. Clin. Nutr. 2004, 80, 299–307. [Google Scholar] [CrossRef]
- Bossola, M.; Muscaritoli, M.; Tazza, L.; Giungi, S.; Tortorelli, A.; Rossi Fanelli, F.; Luciani, G. Malnutrition in HD patients: What therapy. Am. J. Kidney Dis. 2005, 46, 371–386. [Google Scholar] [CrossRef]
- Wright, M.; Woodrow, G.; O’Brien, S.; King, N.; Dye, L.; Blundell, J.; Brownjohn, A.; Turney, J. Disturbed appetite patterns and nutrient intake in peritoneal dialysis patients. Perit. Dial. Int. 2003, 23, 550–556. [Google Scholar] [CrossRef]
- Carrero, J.J. Identification of patients with eating disorders: Clinical and biochemical signs of appetite loss in dialysis patients. J. Ren. Nutr. 2009, 19, 10–15. [Google Scholar] [CrossRef]
- Burrowes, J.D.; Larive, B.; Chertow, G.M.; Cockram, D.B.; Dwyer, J.T.; Greene, T.; Kusek, J.W.; Leung, J.; Rocco, M.V.; Hemodialysis (HEMO) Study Group. Self-reported appetite, hospitalization and death in HD patients: Findings from the HD (HEMO) Study. Nephrol. Dial. Transplant. 2005, 20, 2765–2774. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.J.; Woodrow, G.; O’Brien, S.; King, N.A.; Dye, L.; Blundell, J.E.; Brownjohn, A.M.; Turney, J.H. A novel technique to demonstrate disturbed appetite profiles in haemodialysis patients. Nephrol. Dial. Transplant. 2001, 16, 1424–1429. [Google Scholar] [CrossRef][Green Version]
- Notomi, S.; Kitamura, M.; Yamaguchi, K.; Harada, T.; Nishino, T.; Funakoshi, S.; Kuno, K. Impact of Cafeteria Service Discontinuation at a Dialysis Facility on Medium-Term Nutritional Status of Elderly Patients Undergoing Hemodialysis. Nutrients 2022, 14, 1628. [Google Scholar] [CrossRef]
- Bossola, M.; Tazza, L. Appetite is associated with the time of recovery after the dialytic session in patients on chronic hemodialysis. Nephron Clin. Pract. 2013, 123, 129–133. [Google Scholar] [CrossRef]
- Kim, J.C.; Kalantar-Zadeh, K.; Kopple, J.D. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J. Am. Soc. Nephrol. 2013, 24, 337–351. [Google Scholar] [CrossRef]
- Cox, N.J.; Ibrahim, K.; Sayer, A.A.; Robinson, S.M.; Roberts, H.C. Assessment and treatment of the anorexia of aging: A systematic review. Nutrients 2019, 11, 144. [Google Scholar] [CrossRef]
- Wazny, L.D.; Nadurak, S.; Orsulak, C.; Giles-Smith, L.; Tangri, N. The Efficacy and Safety of Megestrol Acetate in Protein-Energy Wasting due to Chronic Kidney Disease: A Systematic Review. J. Ren. Nutr. 2016, 26, 168–176. [Google Scholar] [CrossRef]
- Carrero, J.J.; Qureshi, A.R.; Axelsson, J. Anorexia and appetite stimulats in chronic kidney disease. In Nutrition Management of Renal Disease, 4th ed.; Kopple, J., Massry, S., Kalantar-Zadeh, K., Fouque, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 893–906. [Google Scholar]
- Carrero, J.J.; Qureshi, A.R.; Axelsson, J.; Avesani, C.M.; Suliman, M.E.; Kato, S.; Bárány, P.; Snaedal-Jonsdottir, S.; Alvestrand, A.; Heimbürger, O.; et al. Comparison of nutritional and inflammatory markers in dialysis patients with reduced appetite. Am. J. Clin. Nutr. 2007, 85, 695–701. [Google Scholar] [CrossRef]
- Wilson, M.M.; Thomas, D.R.; Rubenstein, L.Z.; Chibnall, J.T.; Anderson, S.; Baxi, A.; Diebold, M.R.; Morley, J.E. Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am. J. Clin. Nutr. 2005, 82, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Burrowes, J.D.; Powers, S.N.; Cockram, D.B.; Cunniff, P.J.; Paranandi, L.; Kusek, J.W. Use of an appetite and diet assessment tool in the pilot phase of a hemodialysis clinical trial: Mortality and morbidity in hemodialysis study. J. Ren. Nutr. 1996, 6, 229–232. [Google Scholar] [CrossRef]
- Molfino, A.; Kaysen, G.A.; Chertow, G.M.; Doyle, J.; Delgado, C.; Dwyer, T.; Laviano, A.; Rossi Fanelli, F.; Johansen, K.L. Validating appetite assessment tools among patients receiving hemodialysis. J. Ren. Nutr. 2016, 26, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Zabel, R.; Ash, S.; Bauer, J.; King, N. Assessment of subjective appetite sensations in hemodialysis patients. Agreement and feasibility between traditional paper and pen and a novel electronic appetite rating system. Appetite 2009, 52, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Ribaudo, J.M.; Cella, D.; Hahn, E.A.; Lloyd, S.R.; Tchekmedyian, N.S.; Von Roenn, J.; Leslie, W.T. Re-validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual. Life Res. 2000, 9, 1137–1146. [Google Scholar] [CrossRef]
- Molina, P.; Vizcaíno, B.; Molina, M.D.; Beltrán, S.; González-Moya, M.; Mora, A.; Castro-Alonso, C.; Kanter, J.; Ávila, A.I.; Górriz, J.L.; et al. The effect of high-volume online haemodiafiltration on nutritional status and body composition: The ProtEin Stores prEservaTion (PESET) study. Nephrol. Dial. Transplant. 2018, 33, 1223–1235. [Google Scholar] [CrossRef]
- Sakurai, K. Biomarkers for evaluation of clinical outcomes of hemodiafiltration. Blood Purif. 2013, 35, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Cano, N.J.; Aparicio, M.; Brunori, G.; Carrero, J.J.; Cianciaruso, B.; Fiaccadori, E.; Lindholm, B.; Teplan, V.; Fouque, D.; Guarnieri, G. ESPEN Guidelines on Parenteral Nutrition: Adult renal failure. Clin. Nutr. 2009, 28, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Tentori, F.; Karaboyas, A.; Robinson, B.M.; Morgenstern, H.; Zhang, J.; Sen, A.; Ikizler, T.A.; Rayner, H.; Fissell, R.B.; Vanholder, R.; et al. Association of dialysate bicarbonate concentration with mortality in the dialysis ouncomes and practice patterns study (DOPPS). Am. J. Kidney Dis. 2013, 62, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Vashistha, T.; Kalantar–Zadeh, K.; Molnar, M.Z.; Torlén, K.; Mehrotra, R. Dialysis modality and correction of uremic metabolic acidosis: Relationship with all– cause and cause–specific mortality. Clin. J. Am. Nephrol. 2013, 8, 254–264. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. K/DOQI clinical practice guideline for bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003, 42, S1–S201. [Google Scholar] [CrossRef]
- Bommer, J.; Locatelli, F.; Satayathum, S.; Keen, M.L.; Goodkin, D.A.; Saito, A.; Akiba, T.; Port, F.K.; Young, E.W. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2004, 44, 661–671. [Google Scholar] [CrossRef]
- Movilli, E.; Camerini, C.; Zein, H.; D’Avolio, G.; Sandrini, M.; Strada, A.; Maiorca, R. A prospective comparison of bicarbonate dialysis, hemodiafiltration, and acetate-free biofiltration in the elderly. Am. J. Kidney Dis. 1996, 27, 541–547. [Google Scholar] [CrossRef]
- Notomi, S.; Kitamura, M.; Yamaguchi, K.; Komine, M.; Sawase, K.; Nishino, T.; Funakoshi, S. Anorexia Assessed by Simplified Nutritional Appetite Questionnaire and Association with Medication in Older Patients Undergoing Hemodialysis. Biol. Pharm. Bull. 2003, 46, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.E.; Lynch, R.; Curhan, G.C.; Brunelli, S.M. Altered taste perception and nutritional status among hemodialysis patients. J. Ren. Nutr. 2013, 23, 288–295.e1. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.K.; Bowersox, E.M.; Rye, D.L.; Abu-Hamdan, D.K.; Prasad, A.S.; McDonald, F.D.; Biersack, K.L. Factors underlying abnormal zinc metabolism in uremia. Kidney Int. Suppl. 1989, 27, S269–S273. [Google Scholar] [PubMed]
- Wang, L.J.; Wang, M.Q.; Hu, R.; Hu, R.; Yang, Y.; Huang, Y.S.; Xian, S.X.; Lu, L. Effect of Zinc Supplementation on Maintenance Hemodialysis Patients: A Systematic Review and Meta-Analysis of 15 Randomized Controlled Trials. Biomed. Res. Int. 2017, 2017, 1024769. [Google Scholar] [CrossRef] [PubMed]
- Takehara, S.; Hirani, V.; Wright, F.A.C.; Naganathan, V.; Blyth, F.M.; Le Couteur, D.G.; Waite, L.M.; Seibel, M.J.; Handelsman, D.J.; Cumming, R.G. Appetite, oral health and weight loss in community-dwelling older men: An observational study from the Concord Health and Ageing in Men Project (CHAMP). BMC Geriatr. 2021, 16, 255. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Tazza, L. Postdialysis fatigue: A frequent and debilitating symptom. Semin. Dial. 2016, 29, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.L.; Doyle, J.W.; Johansen, K.L. Postdialysis fatigue is associated with sedentary behavior. Clin. Nephrol. 2011, 75, 426–433. [Google Scholar] [PubMed]
- Artom, M.; Moss-Morris, R.; Caskey, F.; Chilcot, J. Fatigue in advanced kidney disease. Kidney Int. 2014, 86, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Jhamb, M.; Weisbord, S.D.; Steel, J.L.; Unruh, M. Fatigue in patients receiving maintenance dialysis: A review of definitions, measures, and contributing factors. Am. J. Kidney Dis. 2008, 52, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.M.; Heidenheim, P.A.; Nesrallah, G.; Garg, A.X.; Suri, R. Minutes to recovery after a hemodialysis session: A simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin. J. Am. Soc. Nephrol. 2006, 1, 952–959. [Google Scholar] [CrossRef]
- Kodama, H.; Togari, T.; Konno, Y.; Tsuji, A.; Fujinoki, A.; Kuwabara, S.; Inoue, T. A new assessment scale for post-dialysis fatigue in hemodialysis patients. Renal Replacement Therapy 2020, 6, 1. [Google Scholar] [CrossRef]
- Cox, N.J.; Howson, F.; Ibrahim, K.; Morrison, L.; Sayer, A.A.; Roberts, H.C.; Robinson, S.M. Mood and physical activity are associated with appetite in hospitalised older men and women. Age Ageing 2022, 51, afac297. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, L.; He, L.; Zhang, Y.; Chen, H.; Liu, Y.; Tang, S.; Zheng, H. Study on the relationship between sarcopenia and its components and anorexia in elderly maintenance haemodialysis patients. Nurs. Open 2022, 9, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Sahathevan, S.; Khor, B.H.; Ng, H.M.; Gafor, A.H.A.; Mat Daud, Z.A.; Mafra, D.; Karupaiah, T. Understanding development of malnu trition in hemodialysis patients: A narrative review. Nutrients 2020, 12, 3147. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Shimazu, S.; Shiraishi, A.; Nagano, F.; Tominaga, S.; Hamada, T.; Kudo, M.; Yamasaki, Y.; Noda, S.; Bise, T. Ghrelin active- tion by ingestion of medium-chain triglycerides in healthy adults: A pilot trial. J. Aging Res. Clin. Pract. 2018, 7, 42–46. [Google Scholar]
- Hiroshige, K.; Sonta, T.; Suda, T.; Kanegae, K.; Ohtani, A. Oral supplementation of branched-chain amino acid improves nutritional status in elderly patients on chronic haemodialysis. Nephrol. Dial. Transplant. 2001, 16, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Boudville, N.; Rangan, A.; Moody, H. Oral nutritional supplementation increases caloric and protein intake in peritoneal dialysis patients. Am. J. Kidney Dis. 2003, 41, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.E.; Tighiouart, H.; Ladik, V.; Meyer, K.B.; Zager, P.G.; Johnson, D.S. Oral intradialytic nutritional supplement use and mortality in hemodialysis patients. Am. J. Kidney Dis. 2014, 63, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Leonberg-Yoo, A.K.; Wang, W.; Weiner, D.E.; Lacson, E., Jr. Oral nutritional supplements and 30-day readmission rate in hypoalbuminemic maintenance hemodialysis patients. Hemodial. Int. 2019, 23, 93–100. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Pupim, L.B.; Brouillette, J.R.; Levenhagen, D.K.; Farmer, K.; Hakim, R.M.; Flakoll, P.J. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E107–E116. [Google Scholar] [CrossRef]
- Pace, R.C.; Kirk, J. Academy of Nutrition and Dietetics and National Kidney Foundation: Revised 2020 Standards of Practice and Standards of Professional Performance for Registered Dietitian Nutritionists (Competent, Proficient, and Expert) in Nephrology Nutrition. J. Ren. Nutr. 2021, 31, 100–115.e41. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kogirima, M.; Hayashi, F.; Kitajima, Y. Standards of professional practice and the qualities or abilities required for registered dietitians: Findings from a surgery of practicing registered dietitians. Jpn. J. Nutr. Diet. 2019, 77, 44–56. [Google Scholar] [CrossRef]
- Tentori, F.; Elder, S.J.; Thumma, J.; Pisoni, R.L.; Bommer, J.; Fissell, R.B.; Fukuhara, S.; Jadoul, M.; Keen, M.L.; Saran, R.; et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): Correlates and associated outcomes. Nephrol. Dial. Transplant. 2010, 25, 3050–3062. [Google Scholar] [CrossRef]
- Krebs, P.; Norcross, J.C.; Nicholson, J.M.; Prochaska, J.O. Stages of change and psychotherapy outcomes: A review and meta-analysis. J. Clin. Psychol. 2018, 74, 1964–1979. [Google Scholar] [CrossRef]
| Appetite Assessment Tool | Time Frame of Assessment | Description |
|---|---|---|
| numerical rating scale (NRS) [18] | Dietary habits for a week | Simple and easy to quantify. Determines present appetite indicated with a line on a scale (each end of the scale: ‘Appetite’ or ‘No appetite’). |
| Council on Nutrition appetite questionnaire (CNAQ) [19] | Dietary habits for a month | Contains 8 questions related to appetite, food intake, satiety, and number of meals consumed per day derived from the Appetite, Hunger and Sensory Perception Questionnaire (AHSPQ). |
| Simplified Nutrition Assessment Questionnaire (SNAQ) [19] | Dietary habits for a month | Contains four questions related to appetite, food intake, satiety, and number of meals consumed per day. |
| Appetite and Diet Assessment Tool (ADAT) [20] | everyday | 44-Item self-administered questionnaire divided into three sections about appetite and eating habits in general, on dialysis, and on non-dialysis days, respectively. |
| self-assessment of appetite changes [21] | Last month (30 days) | Compares present appetite vs. appetite over the last month (increased, decreased, or unchanged). |
| subjective assessment of appetite [6,10] | Last week (7 days) | Compares present appetite vs. appetite last week (increased, decreased, or unchanged). |
| Visual analogue scale (VAS) [22] | At that point | Simple and easy to quantify. Determines present appetite indicated with a line on a scale (scale extremities: 0 mm, ‘no hunger’; 100 mm, ‘hunger’). |
| Functional Assessment of Anorexia/Cachexia Therapy (FAACT) score [23] | At that point | 12 questions related to appetite and food intake. Each question allows for 5 answers (i.e., not at all, a little bit, somewhat, quite a bit, very much.) |
| Anorexia questionnaire (AQ) [21] | At that point | 4 questions on the presence of early satiety, taste/smell alterations, meat aversion, and nausea/vomiting. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitajima, Y. How Can We Improve the Appetite of Older Patients on Dialysis in Japan? Kidney Dial. 2024, 4, 105-115. https://doi.org/10.3390/kidneydial4020008
Kitajima Y. How Can We Improve the Appetite of Older Patients on Dialysis in Japan? Kidney and Dialysis. 2024; 4(2):105-115. https://doi.org/10.3390/kidneydial4020008
Chicago/Turabian StyleKitajima, Yukie. 2024. "How Can We Improve the Appetite of Older Patients on Dialysis in Japan?" Kidney and Dialysis 4, no. 2: 105-115. https://doi.org/10.3390/kidneydial4020008
APA StyleKitajima, Y. (2024). How Can We Improve the Appetite of Older Patients on Dialysis in Japan? Kidney and Dialysis, 4(2), 105-115. https://doi.org/10.3390/kidneydial4020008






