Abstract
Background: Limited data exist on postoperative acute kidney injury (AKI) in patients who have undergone esophageal cancer surgery. The purpose of this study was to evaluate the incidence, risk factors, and consequences of postoperative acute kidney after esophagectomy. Methods: This was a retrospective cohort study. The study was conducted in a tertiary specialized cancer center in Italy. All patients undergoing elective esophageal cancer surgery between 2016 and 2021 were included in the study. AKI was defined according to Kidney Disease Improving Global Outcomes criteria (both serum creatinine and urine output), within 48 h after surgery. Preoperative and intraoperative data were registered. We also collected data concerning progression of AKI, need for renal replacement therapy, mortality, and medical (pulmonary, cardiovascular, septic) and surgical complications within 30 days from surgery, as well as length of hospital stay. Results: Incidence of postoperative AKI was 32%. The independent risk factors were body mass index and the use of an invasive surgical approach. Persistent AKI accounted for 15% of the cases and it was associated with increased risk of major cardiovascular events (odds ratio 4.14, 95% CI 1.05–15.8, p-value 0.036), pulmonary complications (OR 3.67, 95% CI 1.04–14.9, p-value 0.050), and increased length of hospital stay (AME 7.2, 0.5–13.9, p-value 0.035). Conclusions: Postoperative AKI is common after esophageal cancer surgery. BMI and a totally invasive surgical approach are independent risk factors. Persistent AKI lasting more than 48 h increased the risk for any cardiovascular or pulmonary complications, with prolonged length of hospital stay.
1. Introduction
Esophagectomy is considered to be the cornerstone treatment for locally advanced esophageal cancer [1]. Esophagectomy is a complex major intervention, with well-known risk of pulmonary, cardiac, anastomotic, and septic complications. The presence of postoperative complications after esophagectomies for cancer is associated with reduced long-term survival [2]. There is a paucity of data in the literature concerning postoperative renal outcomes after esophageal surgery, with a wide range of incidence [3,4,5,6,7]. Perioperative acute kidney injury (AKI) and the ways in which it develops is multifaceted and complex. Hypoperfusion, inflammation, and neuroendocrine response to surgery are common mechanisms affecting renal function and structure [8]. Moreover, AKI may be considered a sentinel complication which engenders distant organ dysfunctions, resulting in more postoperative complications with increased length of hospital stay [9].
The primary aim of this study was to evaluate the incidence of postoperative AKI in patients undergoing elective esophageal cancer surgery. As a secondary aim, we assessed the progression of the acute kidney injury and the association with adverse pulmonary, cardiovascular, anastomotic, and septic events, as well as mortality. We also identified the risk factors associated with AKI incidence.
2. Materials and Methods
2.1. Study Population
This is a retrospective observational study conducted at the University Hospital of Padua in patients who underwent elective esophagectomy from January 2016 to January 2021. Exclusion criteria were age < 18 years, end-stage renal disease, missing data for AKI definition, second surgery (for patients having multiple surgeries performed during a 30-day period, only the first in each period was included).
2.2. Objectives
Our primary objective was to evaluate the incidence of postoperative AKI according KDIGO criteria within 48 h after surgery. Our secondary objectives were the distinction between transient and persistent AKI, occurrence of recovery of AKI or progression at 7 days, 30 days, and 3 months, the association between AKI and pulmonary complications, major adverse cardiovascular events, surgical or septic complications, or death, i.e., the identification of risk factors associated with AKI occurrence.
2.3. Statistical Analysis
Descriptive statistics were reported as median (I quartile–III quartile) for continuous variables and as absolute numbers (percentages) for categorical variables. Univariable and multivariable logistic regression models were employed to identify factors associated with postoperative AKI. Results were reported as odds ratio, 95% Confidence Interval (CI), and p-value. Univariable logistic models were employed to evaluate the association between AKI and postoperative binary outcomes. Univariable gamma models were employed for continuous variables, given the non-normal distribution of all the continuous variables considered (normality of numerical data was assessed using the Shapiro–Wilk test). Regarding the gamma models, the marginal effect was computed considering the partial derivatives of the marginal expectation. Results were reported as average marginal effect (AME), 95% CI, and p-value. Analyses were performed using the R software version 4.1.2.
2.4. Data Collection
A qualified team collected patients’ data in a CRF (Case Report Form). An electronic copy of the CRF was retained in Microsoft Excel format. We collected preoperative data (age, gender, body mass index (BMI), level of dependency (using the Karnofsky index), American Society of Anesthesiology (ASA) status, comorbidities, medications, laboratory tests, such as serum creatinine (SCr), urea, blood cells count, estimation of glomerular filtration rate (eGFR) and length of hospital stay according to the National Surgical Quality Improvement Program (NSQIP)), intraoperative data (type of surgery, type of anesthesia, duration of anesthesia, type of ventilation, lower systolic arterial pressure, lower ratio of oxygen saturation and fractional inspired oxygen content, amount of fluids, use of colloids or blood, use of vasopressors, inotropes or antiarrhythmics, blood loss, hemoglobin and lactate at the end of surgery, diuresis at the end of surgery and use of non-steroidal anti-inflammatory drugs), and postoperative data (serum creatinine, diuresis, fluid balance, medical and surgical complications). All collected data were identified and stored in such a way as to protect patient confidentiality. The staff involved in the collection and management of data did not use or disseminate such information for purposes other than those provided for the realization of the study. All data were collected and processed in a completely anonymous way, in compliance with the guaranteed terms of privacy and as approved by Comitato Etico per la Sperimentazione Clinica della Provincia di Padova.
2.5. Assessment of Renal Function
AKI was defined according to Kidney Disease Improving Global Outcomes (KDIGO) 2012 consensus guidelines [10] as an increase in SCr by ≥0.3 mg/dL, or an increase in SCr level 1.5–1.9 times the baseline value or urine volume <0.5mL/kg/h for 6–12 h. AKI was staged as followed: stage 1 as an increase in SCr level by ≥0.3 mg/dL, or an increase in SCr level 1.5–1.9 times the baseline value or urine volume <0.5mL/kg/h for 6–12 h; stage 2 as an increase in SCr level 2.0–2.9 times the baseline value or urine volume <0.5 mL/kg/h for ≥12 h; stage 3 as an increase in SCr level 3.0 times baseline or ≥4.0 mg/dL, or initiation of kidney replacement therapy. For those who did not have available baseline SCr levels, we expanded the screening criteria to an increase or decrease in SCr by 0.3 mg/dL during hospital stay. Every patient with suspected AKI was reviewed on a case-by-case basis to confirm or to rule out the diagnosis. Positive fluid balance and hemodilution were considered in the diagnosis and staging of AKI using the following formula [11,12]:
where the correction factor = weight (kg) upon hospital admission × 0.6 + Σ (daily cumulative fluid balance (L))/hospital admission weight × 0.6. The eGFR was determined using SCr [13]. The following definitions according to the Acute Dialysis Quality Initiative Consensus [14] were applied:
adjusted SCr level = SCr × correction factor
Persistent AKI: the protraction of AKI by serum creatinine or urine output criteria (according KDIGO criteria) beyond 48 h from AKI onset. Transient AKI: complete reversal of AKI by KDIGO criteria within 48 h of AKI onset. Acute kidney disease (AKD): a condition wherein criteria for AKI stage 1 or greater persists ≥7 days after surgery.
2.6. Assessment of Complications and Mortality
Postoperative pulmonary complications were identified and classified based on European Perioperative Clinical Outcome definitions from the European Society of Anesthesiology–European Society of Intensive Care Medicine taskforce on perioperative outcome measures [15]. These include respiratory infection, respiratory failure, pleural effusion, atelectasis, pneumothorax, bronchospasm, and aspiration pneumonitis. Major adverse cardiac and cerebrovascular events (MACCE) were a composite outcome of non-fatal cardiac arrest, acute myocardial infarction, congestive heart failure, new cardiac arrhythmia, angina, and ischemic stroke [15]. Sepsis was defined according to the Sepsis-3 definition [16]. We performed analyses of complications apparently related to surgical technique (classified according to the Esophagectomy Complications Consensus Group [17]) and medical complications.
All complications were evaluated at 30 days after surgery. Only in-hospital mortality was considered. This was more reliably quantified than 30-day mortality.
3. Results
3.1. Participants
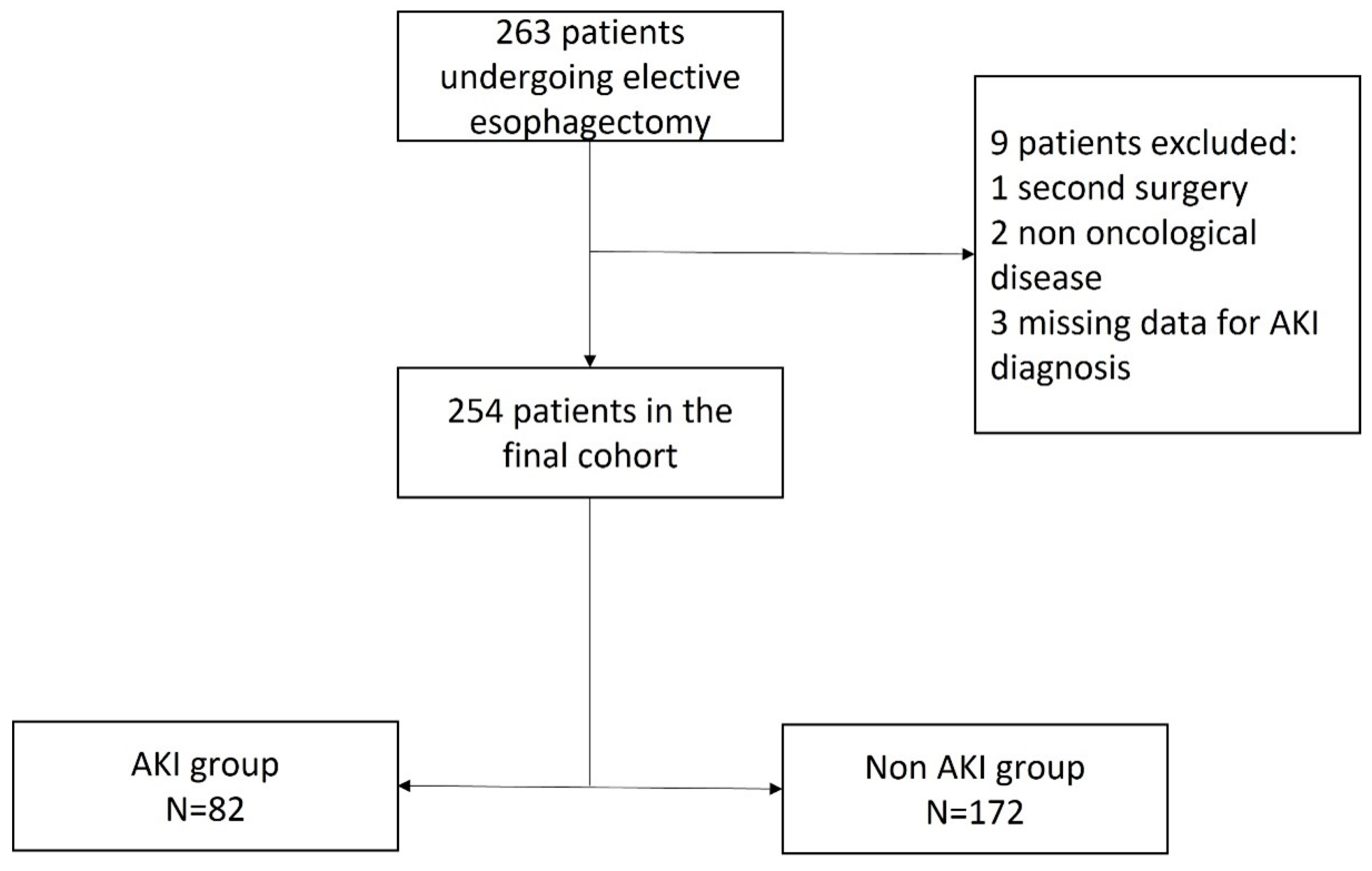
A total of 263 patients underwent elective esophagectomy during the 60-month study period. According to the study protocol, nine patients were excluded, while 254 patients were eligible for analysis, as shown in Figure 1.
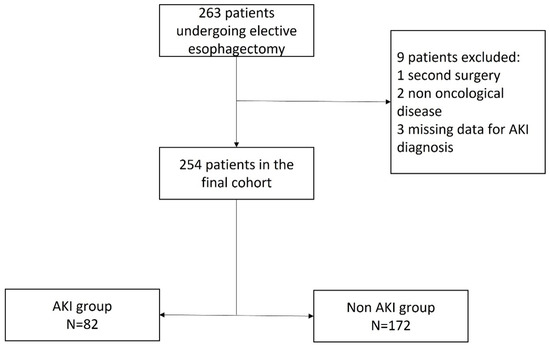
Figure 1.
Flowchart. AKI, acute kidney injury.
The baseline perioperative characteristics of these 254 patients are shown in Table 1.

Table 1.
Preoperative and intraoperative characteristics. BMI, body mass index; ASA, American Society of Anesthesiology; COPD, chronic obstructive pulmonary disease; NSQIP, National Surgical Quality Improvement Program; SpO2/FiO2, ratio between peripheral saturation of oxygen and fractional inspired oxygen content.
3.2. Incidence and Risk Factors of Postoperative AKI
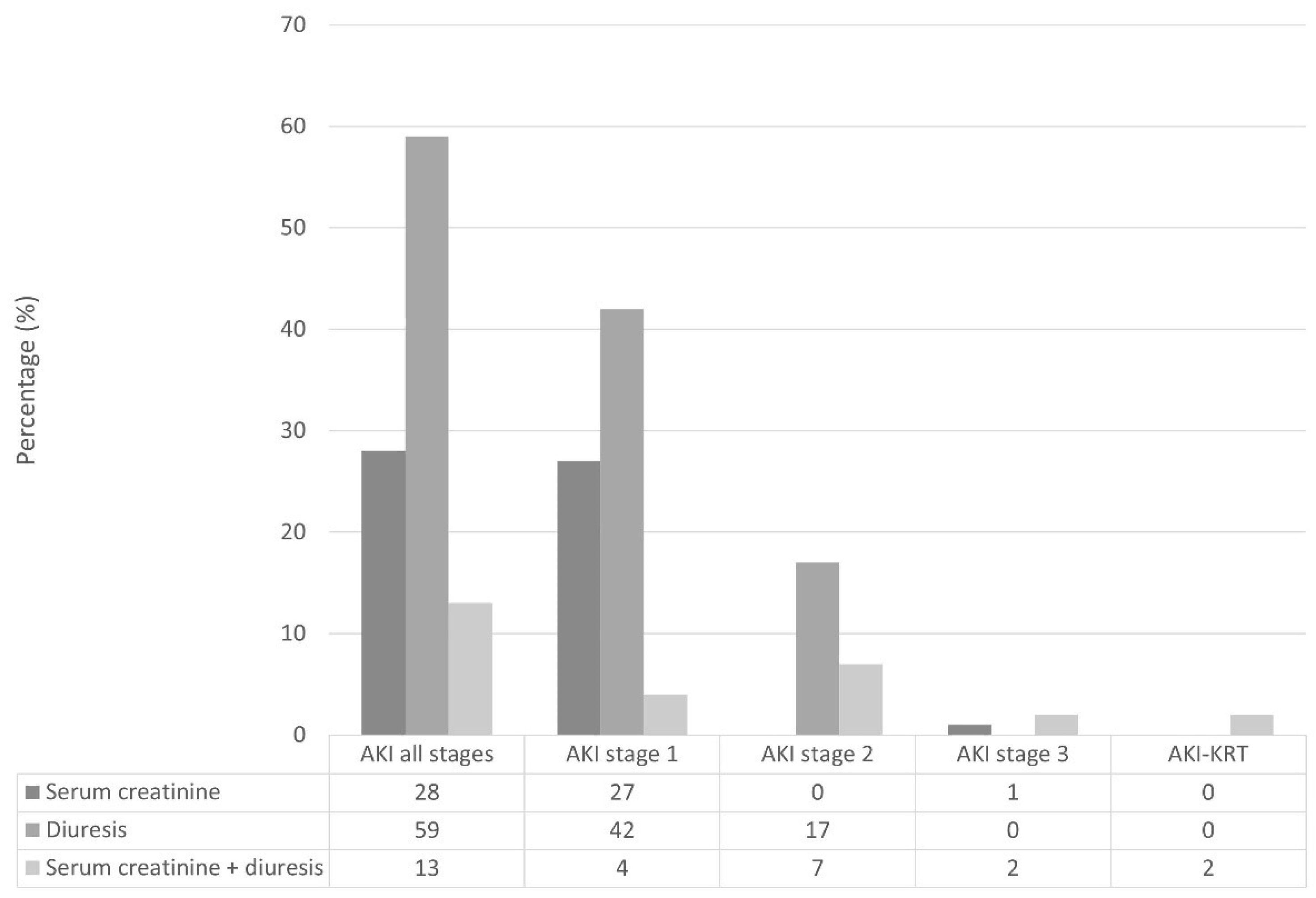
The incidence of postoperative AKI within 48 h from esophageal cancer surgery was 32%. Figure 2 shows the distribution of AKI KDIGO criteria within different stages of postoperative AKI.
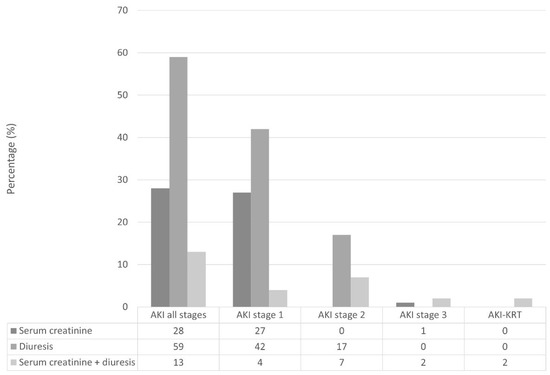
Figure 2.
Distribution of AKI according to Kidney Disease Improving Global Outcomes criteria and stages. AKI, acute kidney injury; AKI-KRT, acute kidney injury requiring kidney replacement therapy.
Only two patients required renal replacement therapy after surgery, for 8 and 9 days. In univariable model regression, eight factors were associated with postoperative AKI: BMI, history of hypertension, smoking habit, NSQIP-calculated length of hospital stay, angiotensin-receptor blocker use, preoperative eGFR, totally invasive surgery, and intraoperative urinary output. In multivariable model regression, the only independent predictors for postoperative AKI were BMI (OR 1.1, 95% CI 1.03–1.18, p-value < 0.01) and totally invasive surgery (OR 3.5, 95% CI 1.3–9.2, p-value < 0.05). The complete analysis is presented in Supplementary File S1.
3.3. Difference between Transient and Persistent Postoperative AKI
The vast majority of AKI patients (85%) had transient postoperative AKI, while only 12 AKI patients (15%) had persistent postoperative AKI lasting more than 48 h. Within the AKI group, eight patients (9.8%) didn’t recover within 7 days from surgery, and were therefore diagnosed as having acute kidney disease. When comparing patients with transient AKI, patients with persistent AKI had less preoperative eGFR (86 ± 18 vs. 77 ± 18, p-value 0.02; OR 0.97, 95% CI 0.94–0.99, p-value 0.024), had greater AKI stage (stage 1 74% vs. 67%, stage 2 26% vs. 8%, stage 3 0% vs. 25%, p-value < 0.001; OR 5.7, 95% CI 2.7–11.9, p-value < 0.001), and were more likely to be diagnosed according to both serum creatinine and diuresis KDIGO criteria (10% vs. 33%, p-value 0.02; OR 2.9, 95% CI 1.3–6.7, p-value 0.009).
3.4. Postoperative Complications and Association with Kidney-Related Adverse Events
The most frequent postoperative complications were pulmonary (37%), followed by renal (32%), septic (18%), surgical (14%, with second surgery in 6%), and cardiovascular (11%). We had data on renal function at 3 months from surgery in only 47 patients; two patients (0.8% of the overall cohort and 2.4% of the AKI patients) were classified as having chronic kidney disease. The median lengths of stay were 1 day (IQR 1–2) and 12 days (IQR 10–14) in ICU and in hospital, respectively. Intra-hospital mortality accounted for 0.8% of the overall population (two patients); one patient (0.4%) died during ICU stay. Patients with postoperative AKI had greater risk of postoperative MACCE within 30 days from surgery (OR 2.51, 95 %CI 1.14–5.56, p-value 0.021). No significant association was found between postoperative AKI and other medical or surgical complications. Although patients who developed AKI did not stay longer in hospital, patients who experienced persistent AKI were more likely to have increased length of hospital stay (p-value 0.035).
Patients who had persistent postoperative AKI had a higher risk of medical complications, such as MACCE (OR 4.14, 95% CI 1.05–15.8, p-value 0.036) and pulmonary complications (OR 3.67, 95% CI 1.04–14.9, p-value 0.050), with a significant effect on hospital length of stay (AME 7.2, 0.5–13.9, p-value 0.035). The complete analysis is presented in Supplementary File S1.
4. Discussion
4.1. Major Findings
In this monocentric retrospective study, the incidence of postoperative AKI after esophageal cancer surgery, as defined by serum creatinine and diuresis KDIGO criteria, was 32%. The only independent risk factor associated with postoperative AKI was BMI. The AKI episode was transient in the majority of the cases and did not affect length of hospital stay; nonetheless, it was associated with a two-fold risk increase for MACCE within 30 days from surgery. Moreover, an AKI episode lasting more than 48 h was not only associated with a four-fold risk increase for MACCE and three-fold risk increase for pulmonary complications within 30 days from surgery, but it also increased the length of hospital stay by an average of 7 days.
4.2. Comparison with Previous Studies
There are few studies investigating the incidence of renal dysfunction specifically in patients undergoing elective esophageal cancer surgery. The incidence reported was variable, ranging from 1.3% to 35.3% [2,3,4,5,6,7]; some studies, however, lacked a sufficient definition of renal dysfunction [2,4], and others used Acute Kidney Injury Network SCr criterion [3,5,7], or Risk, Injury, Failure, Loss, and End-Stage kidney disease SCr criterion [6]. The population analyzed in our study is similar to former studies [3,4,5,6,7] in terms of preoperative characteristics. Our study differs from the previous studies [3,4,5,6,7] by using KDIGO criteria, urine output, and SCr for AKI diagnosis, and not only SCr. We also conducted the adjustment for SCr according to cumulative fluid balance (which reduced the possible underestimation of AKI incidence due to perioperative change in body water). This methodology of SCr adjustment has been previously tested and used for SCr correction [11,12]. Cumulative fluid balance is certainly subject to variation because it does not account for insensible losses; however, we had no information on body weight change, or other surrogate parameters of fluid status, and fluid balance is a widely used method of assessing changes in volume status.
Considering urine output as a diagnostic criterion, we certainly increased the estimated incidence of AKI, also including those patients (substantially, AKI stage 1 by urine output criterion only) who may have responded to multiple physiological perioperative factors, including volume status, relative, or absolute hypotension and neurohormonal response to surgery. Similar to other previous studies [3,4,5,6,7], we found that BMI was a modifiable factor that was found to be associated with increased risk for AKI occurrence [3,7]. Unlike the results of our study, previous studies also showed that older age and elevated ASA status were associated with AKI development [3,6], as well as some intraoperative factors, like colloid use [3], fluid management [5], or duration of surgery [2]. Interestingly, the choice of a totally invasive surgery was associated with increased risk for AKI, possibly because of more traumatic body injury with more inflammation or more difficult management of perioperative status, including fluid or pain management. We investigated the relevance of duration of renal insult, by distinguishing between transient and persistent postoperative AKI event. In line with Murphy et al. [7], in our population AKI was mostly transient and did not increase length of hospital stay, even though it was associated with a two-fold risk increase for major cardiovascular events within 30 days from surgery. Persistent AKI was a less frequent but frightening postoperative complication, which appeared to be associated with increased risk for MACCE and increased incidence of pulmonary complications with significant impact on length of hospital stay. It is now well-established that AKI is associated with increased morbidity and mortality, but in the perioperative settings, data on short- and long-term outcomes are still limited. Few studies have shown a strong relationship between AKI and cardiovascular, pulmonary complications or mortality in abdominal surgery [18,19,20], but none have pointed out the relationship between these complications and the duration of AKI event. From a pathophysiological perspective, in the perioperative period, the factors determining kidney dysfunction may be multiple and simultaneous, sustaining the perpetuation of AKI event. We should also keep in mind that major thoraco-abdominal surgery, like esophagectomy, stresses many organs at the same time. In this scenario, the renal insult may initiate or result from another organ dysfunction. The link between kidney dysfunction and other organs’ dysfunction is conceptualized as organ crosstalk and may be at the basis of persistent postoperative AKI. This, for example, explains the relationship found between kidney dysfunction and cardio-pulmonary complications.
4.3. Limitations
Our results should be generalized carefully because the study was conducted in a single tertiary specialized cancer hospital. The retrospective nature of the study is the major limitation: the study lacks information on several variables of clinical relevance (notably neoadjuvant treatment, preoperative dysphagia, previous AKI history, cancer staging, or strategies to mitigate AKI). Moreover, the authors acknowledge that the use of preoperative eGFR to unmask possible preoperative chronic kidney dysfunction might be disputable, but it took into account the fact that there were few cases of patients with known history of CKD. The study shows an association between persistent AKI and other postoperative complications, but the cause–effect relationship cannot be determined due to the retrospective analysis. Moreover, the limited number of events for mortality limit the evaluation of association between AKI and in-hospital death from surgery. Unfortunately, the follow-up at 90 days for determining CKD association was not available. We used the timeframe of 48 h from surgery to diagnose postoperative AKI, instead of 7 days as suggested by the more recent Acute Dialysis Quality Initiative consensus published by Prowle et al. [21]; on the other hand, the authors believed that using such a time limitation could also limit the error of including AKI events not necessarily associated with surgery.
5. Conclusions
In our study, 32% of patients who underwent esophagectomy experienced postoperative AKI, which was transitory in the majority of cases. However, a postoperative AKI event lasting more than 48 h was associated with an increased risk of MACCE and pulmonary complications and increased the length of hospital stay. The factors found to be associated with increased risk for AKI development were BMI and the use of a totally invasive surgical approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/kidneydial4020007/s1, File S1.
Author Contributions
I.G. contributed to the design of the work, analysis, and interpretation of data. P.F., I.T. and M.V. contributed to the design of the work and interpretation of data. A.A. and R.S. contributed to data collection. G.L. and D.G. contributed to the analysis of data. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by Comitato Etico per la Sperimentazione Clinica della Provincia di Padova (protocol number 3073). The study is identified with the registration number NCT04773080.
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The datasets analyzed during the study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Van Hagen, P.; Hulshof, M.C.C.M.; Van Lanschot, J.J.B.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, P.; Ward, J.; Tekkis, P.P. Mortality and morbidity in gastro-oesophageal cancer surgery: Initial results of ASCOT multicentre prospective cohort study. BMJ 2003, 327, 1192–1197. [Google Scholar] [CrossRef]
- Lee, E.-H.; Kim, H.R.; Baek, S.-H.; Kim, K.-M.; Chin, J.-H.; Choi, D.-K.; Kim, W.-J.; Choi, I.-C. Risk factors of postoperative acute kidney injury in patients undergoing esophageal cancer surgery. J. Cardiothorac. Vasc. Anesth. 2014, 28, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.M.; Hooker, C.M.; Molena, D.; Mungo, B.; Brock, M.V.; Battafarano, R.J.; Yang, S.C. Complex Esophageal Reconstruction Procedures Have Acceptable Outcomes Compared with Routine Esophagectomy. Ann. Thorac. Surg. 2016, 102, 215–222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Konda, P.; Ai, D.; Guerra, C.E.; Rodriguez-Restrepo, A.; Mehran, R.J.; Rice, D.; Hofstetter, W.; Heir, J.; Kwater, P.; Gottumukkala, V.; et al. Identification of Risk Factors Associated With Postoperative Acute Kidney Injury After Esophagectomy for Esophageal Cancer. J. Cardiothorac. Vasc. Anesth. 2017, 31, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, T.; Feng, X.; Sun, L. Incidence and risk factors of acute kidney injury after esophageal cancer surgery: A nested case-control study. Int. J. Surg. 2017, 39, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.F.M.; Dunne, T.; Elliott, J.A.P.; Kamarajah, S.K.M.; Leighton, J.M.; Evans, R.P.T.M.; Bundred, J.M.; King, S.B.; Ravi, N.M.; Donohoe, C.L.M.; et al. Acute Kidney Injury After Esophageal Cancer Surgery: Incidence, Risk Factors, and Impact on Oncologic Outcomes. Ann. Surg. 2020, 275, e683–e689. [Google Scholar] [CrossRef] [PubMed]
- Gumbert, S.D.; Kork, F.; Jackson, M.L.; Vanga, N.; Ghebremichael, S.J.; Wang, C.Y.; Eltzschig, H.K. Perioperative Acute Kidney Injury. Anesthesiology 2020, 132, 180–204. [Google Scholar] [CrossRef] [PubMed]
- Bihorac, A.; Yavas, S.; Subbiah, S.B.; Hobson, C.E.; Schold, J.D.; Gabrielli, A.; Layon, A.J.; Segal, M.S. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann. Surg. 2009, 249, 851–858. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Macedo, E.; Bouchard, J.; Soroko, S.H.; Chertow, G.M.; Himmelfarb, J.; Ikizker, T.A.; Paganini, E.P.; Mehta, R.L. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit. Care 2010, 14, R82. [Google Scholar] [CrossRef]
- Husain-Syed, F.; Wilhelm, J.; Kassoumeh, S.; Birk, H.-W.; Herold, S.; Vadász, I.; Walmrath, H.-D.; A Kellum, J.; Ronco, C.; Seeger, W. Acute kidney injury and urinary biomarkers in hospitalized patients with coronavirus disease-2019. Nephrol. Dial. Transplant. 2020, 35, 1271–1274. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef]
- Jammer, I.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: A statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur. J. Anaesthesiol. 2015, 32, 88–105. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Low, D.E.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.E.; D’Journo, X.B.; Griffin, S.M.; Hölscher, A.H.; Hofstetter, W.L.; Jobe, B.A.; et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg. 2015, 262, 286–294. [Google Scholar] [CrossRef]
- Long, T.E.; Helgason, D.; Helgadottir, S.; Palsson, R.; Gudbjartsson, T.; Sigurdsson, G.H.; Indridason, O.S.; Sigurdsson, M.I. Acute Kidney Injury After Abdominal Surgery: Incidence, Risk Factors, and Outcome. Anesth. Analg. 2016, 122, 1912–1920. [Google Scholar] [CrossRef]
- Biteker, M.; Dayan, A.; Tekkeşin, A.İ.; Can, M.M.; Taycı, İ.; Ilhan, E.; Şahin, G. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am. J. Surg. 2014, 207, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, J.; Neves, J.B.; Rodrigues, N.; Bekerman, C.; Melo, M.J.; Pereira, M.; Teixeira, C.; Mendes, I.; Jorge, S.; Rosa, R.; et al. Acute kidney injury, long-term renal function and mortality in patients undergoing major abdominal surgery: A cohort analysis. Clin. Kidney J. 2016, 9, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Prowle, J.R.; Forni, L.G.; Bell, M.; Chew, M.S.; Edwards, M.; Grams, M.E.; Grocott, M.P.W.; Liu, K.D.; McIlroy, D.; Murray, P.T.; et al. Postoperative acute kidney injury in adult non-cardiac surgery: Joint consensus report of the Acute Disease Quality Initiative and PeriOperative Quality Initiative. Nat. Rev. Nephrol. 2021, 17, 605–618. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).