Frequent Menstrual Disturbance Post-COVID-19 Vaccination in Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size and Sampling Procedure
2.3. Data Collection Tool and Interpretations of Patients’ Responses
2.4. Ethics Approval
2.5. Statistical Analysis
3. Results
4. Discussion
- Self-Reported Data: Although it gives a good presumptive assessment, the reliance on anonymous self-reports introduces potential recall bias and limits the ability to build further interpretations.
- Due to the nature of the study, there was an absence of clinical and biomarker assessments: Stress, emotional challenges, and their perceived link to menstrual disturbances were based on participants’ perceptions rather than validated psychometric tools or biological measures. future studies should incorporate clinical and psychometric tools to objectively validate these findings, further understand the interplay between stress and menstrual health, and explore the role of other stressors beyond pandemic-related fears.
- Differentiation Between Infection and Vaccination Effects: This study could not distinguish whether menstrual disturbances were influenced by prior COVID-19 infection or vaccination due to the expected overlap between natural infection and vaccine titers. This can be delt with through further investigation.
- Sample Size: Larger sample sizes would enable more insights.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID-19 Cases|WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 29 February 2024).
- Saudagar, V.; Patil, S.; Goh, S.; Pothiawala, S. Vigilance Regarding Immune Thrombocytopenic Purpura after COVID-19 Vaccine. Ir. J. Med. Sci. 2022, 191, 919. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Quejada, L.; Toro Wills, M.F.; Martínez-Ávila, M.C.; Patiño-Aldana, A.F. Menstrual Cycle Disturbances after COVID-19 Vaccination. Womens Health 2022, 18, 17455057221109375. [Google Scholar] [CrossRef] [PubMed]
- Baena-García, L.; Aparicio, V.A.; Molina-López, A.; Aranda, P.; Cámara-Roca, L.; Ocón-Hernández, O. Premenstrual and Menstrual Changes Reported after COVID-19 Vaccination: The EVA Project. Womens Health 2022, 18, 17455057221112237. [Google Scholar] [CrossRef] [PubMed]
- Trogstad, L.; Laake, I.; Robertson, A.H.; Mjaaland, S.; Caspersen, I.H.; Juvet, L.K.; Magnus, P.; Blix, K.; Feiring, B. Heavy Bleeding and Other Menstrual Disturbances in Young Women after COVID-19 Vaccination. Vaccine 2023, 41, 5271–5282. [Google Scholar] [CrossRef]
- Farland, L.V.; Khan, S.M.; Shilen, A.; Heslin, K.M.; Ishimwe, P.; Allen, A.M.; Herbst-Kralovetz, M.M.; Mahnert, N.D.; Pogreba-Brown, K.; Ernst, K.C.; et al. COVID-19 Vaccination and Changes in the Menstrual Cycle among Vaccinated Persons. Fertil. Steril. 2023, 119, 392–400. [Google Scholar] [CrossRef]
- Rastegar, T.; Feryduni, L.; Fakhraei, M. COVID-19 Vaccine Side Effects on Menstrual Disturbances among Iranian Women. New Microbes New Infect. 2023, 53, 101114. [Google Scholar] [CrossRef]
- Kareem, R.; Sethi, M.R.; Inayat, S.; Irfan, M. The Effect of COVID-19 Vaccination on the Menstrual Pattern and Mental Health of the Medical Students: A Mixed-Methods Study from a Low and Middle-Income Country. PLoS ONE 2022, 17, e0277288. [Google Scholar] [CrossRef]
- Alghamdi, A.N.; Alotaibi, M.I.; Alqahtani, A.S.; Al Aboud, D.; Abdel-Moneim, A.S. BNT162b2 and ChAdOx1 SARS-CoV-2 Post-Vaccination Side-Effects Among Saudi Vaccinees. Front. Med. 2021, 8, 760047. [Google Scholar] [CrossRef]
- Shabu, A.; Nishtala, P.S. Safety Outcomes Associated with the Moderna COVID-19 Vaccine (MRNA-1273): A Literature Review. Expert Rev. Vaccines 2023, 22, 393–409. [Google Scholar] [CrossRef]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 Vaccines: Comparison of Biological, Pharmacological Characteristics and Adverse Effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1679. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and Cellular Immune Memory to Four COVID-19 Vaccines. Cell 2022, 185, 2434–2451.e17. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Kumar, S.; Mishra, G.; Saxena, S.K. Tracking the COVID-19 Vaccines: The Global Landscape. Hum. Vaccin. Immunother. 2023, 19, 2191577. [Google Scholar] [CrossRef] [PubMed]
- Benner, M.; Ferwerda, G.; Joosten, I.; van der Molen, R.G. How Uterine Microbiota Might Be Responsible for a Receptive, Fertile Endometrium. Hum. Reprod. Update 2018, 24, 393–415. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.D.; Romero, R.; Gervasi, M.T.; Gomez-Lopez, N.; Tran, M.R.; Garcia-Flores, V.; Pacora, P.; Jung, E.; Hassan, S.S.; Hsu, C.D.; et al. Does the Endometrial Cavity Have a Molecular Microbial Signature? Sci. Rep. 2019, 9, 9905. [Google Scholar] [CrossRef]
- Pelzer, E.S.; Willner, D.; Buttini, M.; Huygens, F. A Role for the Endometrial Microbiome in Dysfunctional Menstrual Bleeding. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2018, 111, 933–943. [Google Scholar] [CrossRef]
- Maher, M.; O’Keeffe, A.; Phelan, N.; Behan, L.A.; Collier, S.; Hevey, D.; Owens, L. Female Reproductive Health Disturbance Experienced During the COVID-19 Pandemic Correlates with Mental Health Disturbance and Sleep Quality. Front. Endocrinol. 2022, 13, 838886. [Google Scholar] [CrossRef]
- Phelan, N.; Behan, L.A.; Owens, L. The Impact of the COVID-19 Pandemic on Women’s Reproductive Health. Front. Endocrinol. 2021, 12, 642755. [Google Scholar] [CrossRef]
- Nazzal, W.; Al-Maqati, T.N.; Almulhim, M.A.; Alsulmi, E.S.; Alotaibi, J.F.; AlBahrani, S.; Alsuhaibani, O.; Alenezi, E.H.; Albusaili, S.; Alharbi, A.; et al. Saudi Women’s Perception of the Effect of COVID-19 Infection and Vaccination on Menstrual Cycle Length. Womens Health Rep. 2024, 5, 495–502. [Google Scholar] [CrossRef]
- Ozimek, N.; Velez, K.; Anvari, H.; Butler, L.; Goldman, K.N.; Woitowich, N.C. Impact of Stress on Menstrual Cyclicity During the Coronavirus Disease 2019 Pandemic: A Survey Study. J. Womens Health 2022, 31, 84–90. [Google Scholar] [CrossRef]
- Tripathy, S.; Preethika, A.; Sajeetha Kumari, R.; Anuradha, M.; Mohapatra, S. The Potential Impact of COVID-19 on Women’s Reproductive and Mental Health: A Questionnaire Study. J. Obstet. Gynaecol. 2022, 42, 3328–3335. [Google Scholar] [CrossRef]
- Bruinvels, G.; Blagrove, R.C.; Goldsmith, E.; Shaw, L.; Martin, D.; Piasecki, J. How Lifestyle Changes during the COVID-19 Global Pandemic Affected the Pattern and Symptoms of the Menstrual Cycle. Int. J. Environ. Res. Public Health 2022, 19, 13622. [Google Scholar] [CrossRef] [PubMed]
- Barsom, S.H.; Mansfield, P.K.; Koch, P.B.; Gierach, G.; West, S.G. Association between Psychological Stress and Menstrual Cycle Characteristics in Perimenopausal Women. Womens Health Issues 2004, 14, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Haji Seyed Javadi, S.A.; Shafikhani, A.A. Anxiety and Depression in Patients with Gastroesophageal Reflux Disorder. Electron. Physician 2017, 9, 5107–5112. [Google Scholar] [CrossRef] [PubMed]
- Sibelli, A.; Chalder, T.; Everitt, H.; Workman, P.; Windgassen, S.; Moss-Morris, R. A Systematic Review with Meta-Analysis of the Role of Anxiety and Depression in Irritable Bowel Syndrome Onset. Psychol. Med. 2016, 46, 3065–3080. [Google Scholar] [CrossRef]
- Duran-Pinedo, A.E.; Solbiati, J.; Frias-Lopez, J. The Effect of the Stress Hormone Cortisol on the Meta transcriptome of the Oral Microbiome. NPJ Biofilms Microbiomes 2018, 4, 25. [Google Scholar] [CrossRef]
- Karagiannis, A.; Harsoulis, F. Gonadal dysfunction in systemic diseases. Eur. J. Endocrinol. 2005, 152, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Hajjo, R.; Momani, E.; Sabbah, D.A.; Baker, N.; Tropsha, A. Identifying a causal link between prolactin signaling pathways and COVID-19 vaccine-induced menstrual changes. NPJ Vaccines 2023, 8, 129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bar-Joseph, H.; Raz, Y.; Eldar-Boock, A.; Michaan, N.; Angel, Y.; Saiag, E.; Nemerovsky, L.; Ben-Ami, I.; Shalgi, R.; Grisaru, D. The direct effect of SARS-CoV-2 virus vaccination on human ovarian granulosa cells explains menstrual irregularities. NPJ Vaccines 2024, 9, 117, Erratum in: NPJ Vaccines 2024, 9, 172.. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine Immunity and Microbiota: A Shifting Paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pollack, B.; von Saltza, E.; McCorkell, L.; Santos, L.; Hultman, A.; Cohen, A.K.; Soares, L. Female reproductive health impacts of Long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: A literature review. Front. Rehabil. Sci. 2023, 4, 1122673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amer, A.A.; Amer, S.A.; Alrufaidi, K.M.; Abd-Elatif, E.E.; Alafandi, B.Z.; Yousif, D.A.; Armi, N.T.; Alkhalaf, A.A.; Shah, J.; Ramadan, M.S. Menstrual changes after COVID-19 vaccination and/or SARS-CoV-2 infection and their demographic, mood, and lifestyle determinants in Arab women of childbearing age, 2021. Front. Reprod. Health 2022, 4, 927211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Homam Safiah, M.; Kalalib Al Ashabi, K.; Khalayli, N.; Hodaifa, Y.; Kudsi, M. The prevalence of menstrual changes in COVID-19 vaccinated women: A cross-sectional study. Prev. Med. Rep. 2024, 44, 102804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- AlRawi, H.Z.; AlQurashi, A.; AlDahan, D.; Alkhudhayri, M.; Alsharidah, A.R.; Wani, T.; AlJaroudi, D. Association between receiving COVID-19 vaccine and menstrual cycle patterns among childbearing women: A cross-sectional study. Health Sci. Rep. 2024, 7, e1934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yildiz, E.; Timur, B.; Guney, G.; Timur, H. Does the SARS-CoV-2 MRNA Vaccine Damage the Ovarian Reserve? Medicine 2023, 102, E33824. [Google Scholar] [CrossRef]
- Conti, P.; Younes, A. Coronavirus COV-19/SARS-CoV-2 Affects Women Less than Men: Clinical Response to Viral Infection. J. Biol. Regul. Homeost. Agents 2020, 34, 339–343. [Google Scholar] [CrossRef]
- Takmaz, T.; Gundogmus, I.; Okten, S.B.; Gunduz, A. The impact of COVID-19-related mental health issues on menstrual cycle characteristics of female healthcare providers. J. Obstet. Gynaecol. Res. 2021, 47, 3241–3249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Turke, P.W. Five Reasons COVID-19 Is Less Severe in Younger Age-Groups. Evol. Med. Public Health 2021, 9, 113. [Google Scholar] [CrossRef]
- Fish, E.N. The X-Files in Immunity: Sex-Based Differences Predispose Immune Responses. Nat. Rev. Immunol. 2008, 8, 737. [Google Scholar] [CrossRef]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine Microbiota: Residents, Tourists, or Invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef]
- Said, K.B.; Al-Otaibi, A.; Aljaloud, L.; Al-Anazi, B.; Alsolami, A.; Alreshidi, F.S. The Frequency and Patterns of Post-COVID-19 Vaccination Syndrome Reveal Initially Mild and Potentially Immunocytopenic Signs in Primarily Young Saudi Women. Vaccines 2022, 10, 1015. [Google Scholar] [CrossRef]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the Gut-Brain Axis: Regulation by the Microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Asghar, S.; Rathore, M.A.; Shahzad, A.; Shahid, A.; Ashraf Khan, A.; Malik, A.; Fakhar, T.; Kausar, H.; Malik, J. Menstrual Abnormalities after COVID-19 Vaccines: A Systematic Review. Vacunas 2022, 23, S77–S87. [Google Scholar] [CrossRef] [PubMed]
- Meringer, H.; Mehandru, S. Gastrointestinal Post-Acute COVID-19 Syndrome. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 345. [Google Scholar] [CrossRef] [PubMed]
- Creed, F. Risk Factors for Self-Reported Irritable Bowel Syndrome with Prior Psychiatric Disorder: The Lifelines Cohort Study. J. Neurogastroenterol. Motil. 2022, 28, 442–453. [Google Scholar] [CrossRef]
- Yarandi, S.S. Overlapping Gastroesophageal Reflux Disease and Irritable Bowel Syndrome: Increased Dysfunctional Symptoms. World J. Gastroenterol. 2010, 16, 1232. [Google Scholar] [CrossRef]
- Martinson, M.L.; Lapham, J.; Ercin-Swearinger, H.; Teitler, J.O.; Reichman, N.E. Generational Shifts in Young Adult Cardiovascular Health? Millennials and Generation X in the United States and England. J. Gerontol. B Psychol. Sci. Soc. Sci. 2022, 77 (Suppl. 2), S177. [Google Scholar] [CrossRef]
- Demir, O.; Sal, H.; Comba, C. Triangle of COVID, Anxiety and Menstrual Cycle. J. Obstet. Gynaecol. 2021, 41, 1257–1261. [Google Scholar] [CrossRef]
- Hunter, P.R. Thrombosis after COVID-19 Vaccination. BMJ 2021, 373, n958. [Google Scholar] [CrossRef]
| Characteristics (n = 1372) | Frequency | Percent | |
|---|---|---|---|
| Age (years) | <18 | 216 | 15.7 |
| 19–29 | 838 | 61.1 | |
| 30–39 | 175 | 12.8 | |
| 40–50 | 143 | 10.4 | |
| Nationality | Non-Saudi | 80 | 5.8 |
| Saudi | 1292 | 94.2 | |
| Region | Central Region | 303 | 22.1 |
| Eastern Region | 402 | 29.3 | |
| Southern area | 73 | 5.3 | |
| Northern area | 305 | 22.2 | |
| Western Region | 289 | 21.1 | |
| Educational | No primary education | 7 | 0.5 |
| Elementary or Intermediate | 70 | 5.1 | |
| Secondary | 331 | 24.1 | |
| Graduate | 911 | 66.4 | |
| Postgraduate | 53 | 3.9 | |
| Employment status | Employed | 182 | 13.3 |
| Housewife | 306 | 22.3 | |
| Retired | 25 | 1.8 | |
| Social status | Married | 407 | 29.7 |
| Single | 965 | 70.3 | |
| Chronic diseases | No | 1167 | 85.1 |
| Yes | 205 | 14.9 | |
| Type of Chronic diseases (n = 205) | Cardiovascular disease | 3 | 1.5 |
| Blood diseases | 28 | 13.7 | |
| Diabetes | 15 | 7.3 | |
| Hypertension | 20 | 9.8 | |
| Kidney disease | 2 | 1.0 | |
| Respiratory system diseases | 33 | 16.1 | |
| Rheumatic diseases | 10 | 4.9 | |
| Liver diseases | 1 | 0.5 | |
| Other diseases | 60 | 29.3 | |
| Multiple diseases | 33 | 16.1 | |
| Medications | No | 1177 | 85.8 |
| Yes | 195 | 14.2 | |
| Type of medications used (n = 195) | Anti-hypertensive medications | 24 | 12.3 |
| Insulin | 22 | 11.3 | |
| Cardiovascular medications | 4 | 2.1 | |
| Salbutamol | 11 | 5.6 | |
| Other medications | 107 | 54.9 | |
| Multiple medications | 27 | 13.8 | |
| Questions | Responses | Number | Percent |
|---|---|---|---|
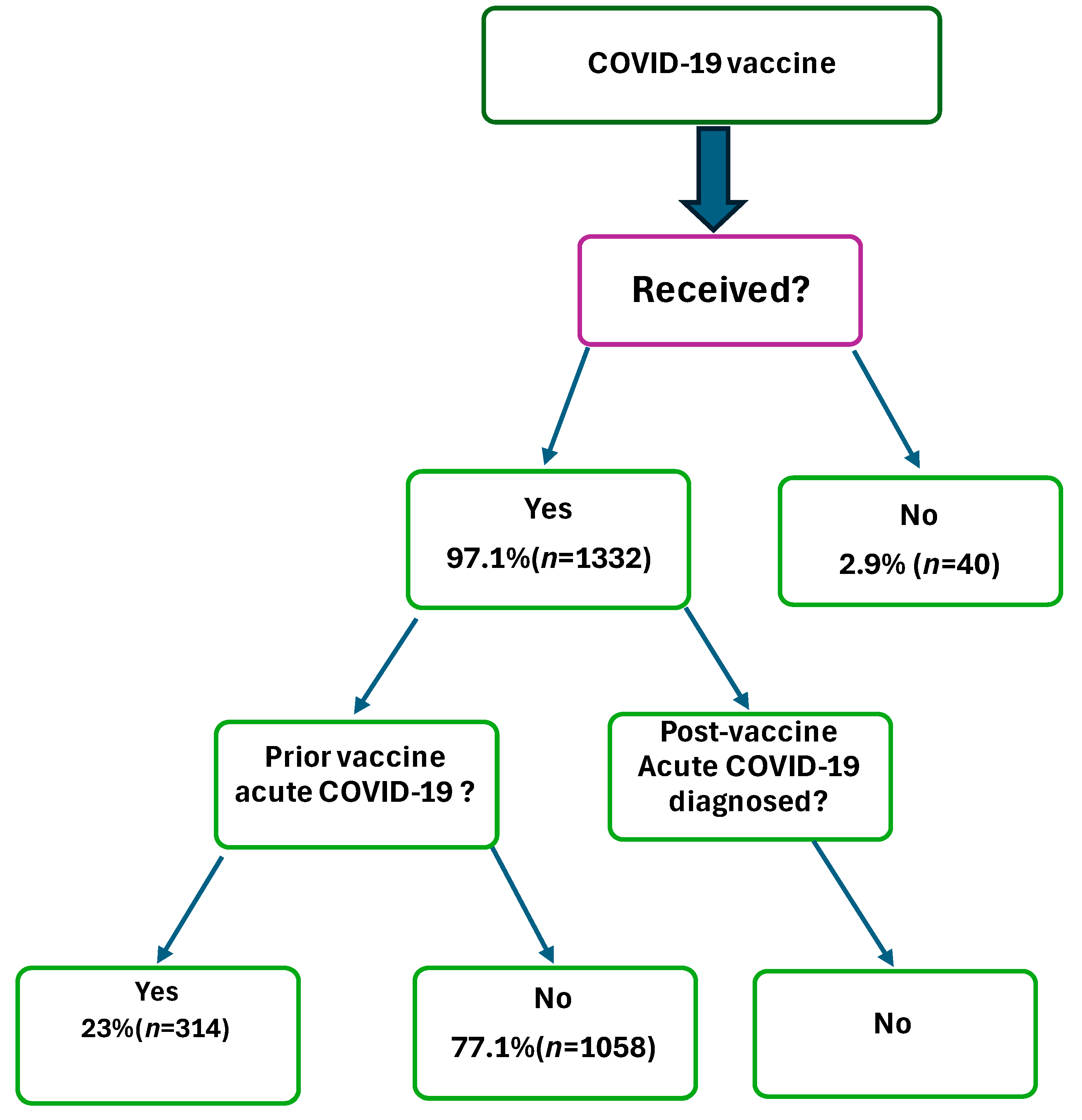
| Infected with SARS-CoV-2, prior vaccination | No | 1058 | 77.1 |
| Yes | 314 | 22.9 | |
| Received COVID-19 vaccine | No | 40 | 2.9 |
| Yes | 1332 | 97.1 | |
| Doses taken (n = 1332) | One dose | 47 | 3.5 |
| Two doses | 1285 | 96.5 | |
| First dose taken (n = 1332) | Pfizer | 1091 | 81.9 |
| Oxford | 209 | 15.7 | |
| Moderna | 12 | 0.9 | |
| Don’t know | 20 | 1.5 | |
| Second dose taken (n = 1285) | Pfizer | 1006 | 78.3 |
| Oxford | 187 | 14.6 | |
| Moderna | 61 | 4.7 | |
| Don’t know | 31 | 2.4 |
| Questions. | Responses | Number | Percent |
|---|---|---|---|
| 1.Have you suffered from any menstrual problems before taking the vaccine (n = 1332) | No | 1057 | 77.0 |
| Yes | 315 | 23.0 | |
| 1.1 If yes, got worsened after taking the vaccine | No | 180 | 57.1 |
| Yes | 135 | 42.9 | |
| 1.2 If not, did you have any menstrual problems after taking the vaccine (n = 1332) | No | 703 | 52.8 |
| Yes | 629 | 47.2 | |
| 2. Time of menstrual problems (of n = 629 who responded Yes to issues after vaccination) | After the first dose | 259 | 41.2 |
| After the second dose | 370 | 58.8 | |
| 2.1. Types of menstrual problems reported by participants (n = 629) | Decrease the time between the two menstrual cycles | 12 | 1.9 |
| 2.1.1 Decrease amount of blood | 18 | 2.9 | |
| 2.1.2 Increase amount of blood | 23 | 3.7 | |
| 2.1.3 Decreased number of days of bleeding | 7 | 1.1 | |
| 2.1.4 Increase in numbers of days of bleeding | 12 | 1.9 | |
| 2.1.5 Irregular menstruation | 53 | 8.4 | |
| 2.1.6 Missed periods after taking vaccine | 26 | 4.1 | |
| 2.1.7 Severe pain than usual | 7 | 1.1 | |
| 2.1.8 Simultaneous multiple menstrual problems | 471 | 74.9 | |
| 3. Perception that menstrual problems were linked to pandemic vaccine-related emotional challenges (n = 1332) | Yes | 674 | 50.6 |
| No | 658 | 49.4 | |
| 4. Worried about pandemic/vaccine (n = 1332) | Yes | 701 | 52.6 |
| No | 631 | 47.4 |
| Menstrual Problems After Taking the Vaccine for Each Character = (Yes + No) | p Value | |||
|---|---|---|---|---|
| No | Yes | |||
| Age | 12–18 | 109 | 95 | 0.05 |
| 53.4% | 46.6% | |||
| 19–29 | 439 | 380 | ||
| 53.6% | 46.4% | |||
| 30–39 | 85 | 88 | ||
| 49.1% | 50.9% | |||
| 40–50 | 70 | 66 | ||
| 51.5% | 48.5% | |||
| Nationality | Non-Saudi | 41 | 35 | 0.833 |
| 53.9% | 46.1% | |||
| Saudi | 662 | 594 | ||
| 52.7% | 47.3% | |||
| Social status | Married | 214 | 183 | 0.592 |
| 53.9% | 46.1% | |||
| Single | 489 | 446 | ||
| 52.3% | 47.7% | |||
| Chronic disease | No | 593 | 542 | 0.351 |
| 52.2% | 47.8% | |||
| Yes | 110 | 87 | ||
| 55.8% | 44.2% | |||
| Taking medication | No | 605 | 540 | 0.913 |
| 52.8% | 47.2% | |||
| Yes | 98 | 89 | ||
| 52.4% | 47.6% | |||
| First dose | Pfizer (n = 1091) | 561 | 530 | 0.047 |
| 51.4% | 48.6% | |||
| Oxford (n = 209) | 121 | 88 | ||
| 57.9% | 42.1% | |||
| Moderna (n = 12) | 6 | 6 | ||
| 50.0% | 50.0% | |||
| I do not know (n = 20) | 15 | 5 | ||
| 75.0% | 25.0% | |||
| Second dose | Pfizer | 515 | 491 | 0.049 |
| 51.2% | 48.8% | |||
| Oxford | 106 | 81 | ||
| 56.7% | 43.3% | |||
| Moderna | 32 | 29 | ||
| 52.5% | 47.5% | |||
| I do not know | 23 | 8 | ||
| 74.2% | 25.8% | |||
| Dependent Variable = Menorrhea Disturbances | Odd’s Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|---|
| Age (=19–29 years) | 2.07 | 0.66 | 6.50 | 0.209 |
| Nationality | 0.57 | 0.25 | 1.29 | 0.181 |
| Region | 1.17 | 0.62 | 2.19 | 0.624 |
| Education = Postgraduate | 2.11 | 0.98 | 4.72 | 0.015 |
| Employment | 2.18 | 0.95 | 4.86 | 0.049 |
| Social status | 1.76 | 0.94 | 3.31 | 0.076 |
| Chronic diseases | 0.91 | 0.47 | 1.74 | 0.781 |
| Medications | 0.95 | 0.65 | 1.39 | 0.775 |
| Type of vaccine taken = Pfizer | 2.09 | 0.96 | 4.10 | 0.029 |
| Doses taken = 2 doses | 1.59 | 0.74 | 3.29 | 0.014 |
| Suffered from any menstrual problems before taking vaccine | 0.91 | 0.45 | 2.21 | 0.412 |
| Menstrual problems occurred after the second dose | 3.21 | 1.23 | 5.21 | 0.030 |
| Perception that menstrual problems changes were linked to pandemic-related emotional challenges | 1.78 | 0.76 | 3.21 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, K.F.; Said, K.B.; Aljadani, A.; Alotaibi, A.A.; Alshammary, F.M.; Ahmed, R.M.E.; Alshammari, A.T.; Al-shammari, T.A.; Alkwai, H.; Shahin, M.M.; et al. Frequent Menstrual Disturbance Post-COVID-19 Vaccination in Saudi Arabia. COVID 2025, 5, 95. https://doi.org/10.3390/covid5070095
Alshammari KF, Said KB, Aljadani A, Alotaibi AA, Alshammary FM, Ahmed RME, Alshammari AT, Al-shammari TA, Alkwai H, Shahin MM, et al. Frequent Menstrual Disturbance Post-COVID-19 Vaccination in Saudi Arabia. COVID. 2025; 5(7):95. https://doi.org/10.3390/covid5070095
Chicago/Turabian StyleAlshammari, Khalid F., Kamaleldin B. Said, Ahmed Aljadani, Arwa A. Alotaibi, Fahad M. Alshammary, Ruba M. Elsaid Ahmed, Abdulrahman T. Alshammari, Turki A. Al-shammari, Hend Alkwai, Mona M. Shahin, and et al. 2025. "Frequent Menstrual Disturbance Post-COVID-19 Vaccination in Saudi Arabia" COVID 5, no. 7: 95. https://doi.org/10.3390/covid5070095
APA StyleAlshammari, K. F., Said, K. B., Aljadani, A., Alotaibi, A. A., Alshammary, F. M., Ahmed, R. M. E., Alshammari, A. T., Al-shammari, T. A., Alkwai, H., Shahin, M. M., Elhussein, G. E. M. O., Ibrahim, S., Alfouzan, F. R., Mahmoud, T., Abdalla, R. A. H., Mohamed, A. A. A., Albayih, Z. A., & Osman, A. A. A. (2025). Frequent Menstrual Disturbance Post-COVID-19 Vaccination in Saudi Arabia. COVID, 5(7), 95. https://doi.org/10.3390/covid5070095







