1. Introduction
Coronaviruses are positive-sense single-stranded RNA viruses that often cause a mild case of the common cold [
1,
2]. However, some coronaviruses can be deadly. The world is still affected by the coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [
3,
4,
5]. Nearly a billion people have become infected with SARS-CoV-2 worldwide, with millions of deaths, and COVID-19 has caused serious health, economic, and sociological problems. Although the pandemic of COVID-19 has officially ended, COVID-19 continues to affect the world population to this day. Many patients who had COVID-19 infection still experience consequences and complications of this disease, such as pulmonary hypertension. Therefore, studies of autopsy materials from patients who died of severe COVID-19 with pathological changes in the pulmonary arteries should provide important mechanistic insights that may contribute to the understanding of the pathogenesis of post-COVID-19 syndromes.
SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2), a physiological regulator of blood pressure that converts angiotensin II (Ang II) to angiotensin 1–7 (Ang 1–7), as a receptor to enter the host cells for infection [
6,
7,
8]. Lung cells are the primary targets of SARS-CoV-2, resulting in severe pneumonia and acute respiratory distress syndrome [
9,
10]. Certain populations of infected individuals are more susceptible to being severely affected by COVID-19. Elderly patients are particularly susceptible to developing severe and fatal conditions [
4,
11,
12], suggesting that this virus also affects organs other than the respiratory system.
We have previously reported that postmortem lung tissues collected from 10 patients who died of COVID-19 in Ukraine during the period of March to July 2020 exhibited thickened pulmonary vascular walls compared to the archival materials of lung tissues from 17 patients who died of influenza A virus subtype H1N1 during the epidemic in November and December of 2009 [
13]. In addition to these observations in postmortem tissues, we also found that the placental arteries of healthy women who gave birth to live full-term newborns who tested positive for COVID-19 during pregnancy without developing serious symptoms exhibited severe vascular wall thickening and occlusion of the vascular lumen [
14]. Thus, the vascular system is sensitive to COVID-19 even for those who are asymptomatic or only mildly symptomatic, and thus we need to consider the possibility of vascular problems post-pandemic. We previously communicated our concern that COVID-19 patients may become predisposed to pulmonary arterial hypertension with the scientific community [
15]. Thus, further investigation into the effects of COVID-19 on the pulmonary vessels are needed to clarify the pathogenetic mechanisms of pulmonary vascular remodeling, which is the aim of our study.
In the present study, using autopsy lung samples from patients who died of COVID-19 as well as H1N1 influenza (as a comparative group) in Ukraine, we performed a detailed histopathological characterization of the effects of COVID-19 on pulmonary vessels. Cases of patients who died of severe H1N1 influenza were chosen as a comparative group, as the structural changes detected in their lungs are similar to those found in COVID-19 patients, i.e., respiratory distress syndrome and pneumonia.
2. Materials and Methods
2.1. Patient Samples
Fifty-six postmortem autopsy lung samples from patients who died of COVID-19 with a severe fatal course of disease with subtotal lung damage were obtained in Ukraine during the period of March 2020 to January 2023. Samples were taken from the superior (S6), lateral basal (S9), and posterior basal (S10) segments of the inferior lobe of both right and left lungs. All cases were divided into three groups in accordance with the duration of the disease: Group 1—death on or before the 8th day of disease (18 cases); Group 2—death between the 9th and 13th day of disease (20 cases); Group 3—death after the 14th day of disease (18 cases); and a comparative group (Group 4)—patients who died of H1N1 influenza with a severe fatal course of disease with subtotal lung damage in 2009 between the 7th and 14th day of disease (17 cases). The onset of the disease was established by a clinical diagnosis of COVID-19 or H1N1 influenza. These viral infections were confirmed by PCR tests. Autopsy cases used in this study were randomly selected, and the time of autopsy did not exceed 12 h after death. In this study, the average age of COVID-19 and H1N1 patients at the time of death was 62.2 years of age (between the ages of 53 and 74). Of the COVID-19 patients, 49% were women.
All cases (patients who died of either COVID-19 or H1N1 influenza) were de-identified at the time of this study, so the researchers did not know which individual case belonged to which group. After the evaluation of histological changes and studied criteria, all cases were identified and categorized into COVID-19 groups (Group 1, Group 2, or Group 3 depending on the duration of the disease) and the comparative H1N1 group.
These studies were approved by the regional committee for medical research ethics in Kyiv, Ukraine, and performed in line with the Helsinki Declaration of 1975 revised in 2013 or comparable ethical standards.
2.2. Histological Examinations
Autopsy sample material approximately 10 mm thick was fixed overnight in 10% buffered formalin at room temperature. Fixed tissues were embedded in paraffin. From paraffin blocks, 5 µm thick sections were made using a microtome. The sections were subjected to hematoxylin and eosin, Masson’s trichrome, and Verhoeff Van Gieson (EVG) stainings. Immunohistochemistry was also performed using anti-AXL (Abcam Limited, Cambridge, UK), anti-spike protein (MyBioSource, Inc., San Diego, CA, USA), anti-α-smooth muscle actin (Thermo Fisher Scientific, Waltham, MA, USA), anti-CD34 (VITRO S.A., Alcalá de Guadaíra, Spain), and anti-CD105 (Abcam) antibodies. Histological specimens were examined using an Axiolab 5 microscope with an Axiocam 305 color digital camera, and the Labscope v. 2.7 software. Morphometric investigations included the assessment of pulmonary arterial wall thickness, measurement of arterial lumen area, and total arterial area (lumen + wall). The ratio of the luminal area to the total area of the artery is expressed both in hundredth (area index) and percent (lumen area%). Six (three small-sized and three medium-sized) vessels were analyzed for each patient.
2.3. Statistical Analysis
GraphPad Prism version 10.3.1 program (GraphPad Software, La Jolla, CA, USA) was used for statistical analysis of the data. The results were statistically analyzed using one-way ANOVA followed by a Tukey’s test for multiple comparisons. A p value less than or equal to 0.05 was considered significant. Results are expressed as the mean ± SEM of n experiments. Significance was tested using the two-tailed Mann–Whitney test and the Kruskal–Wallis test (non-parametric one-way ANOVA) for multiple-group comparison.
3. Results
Histological and immunohistochemical examination of autopsy materials of patients who died of COVID-19 with a severe fatal course of the disease demonstrated signs of SARS-CoV-2-mediated damage to the lung structures, in particular the pulmonary arteries.
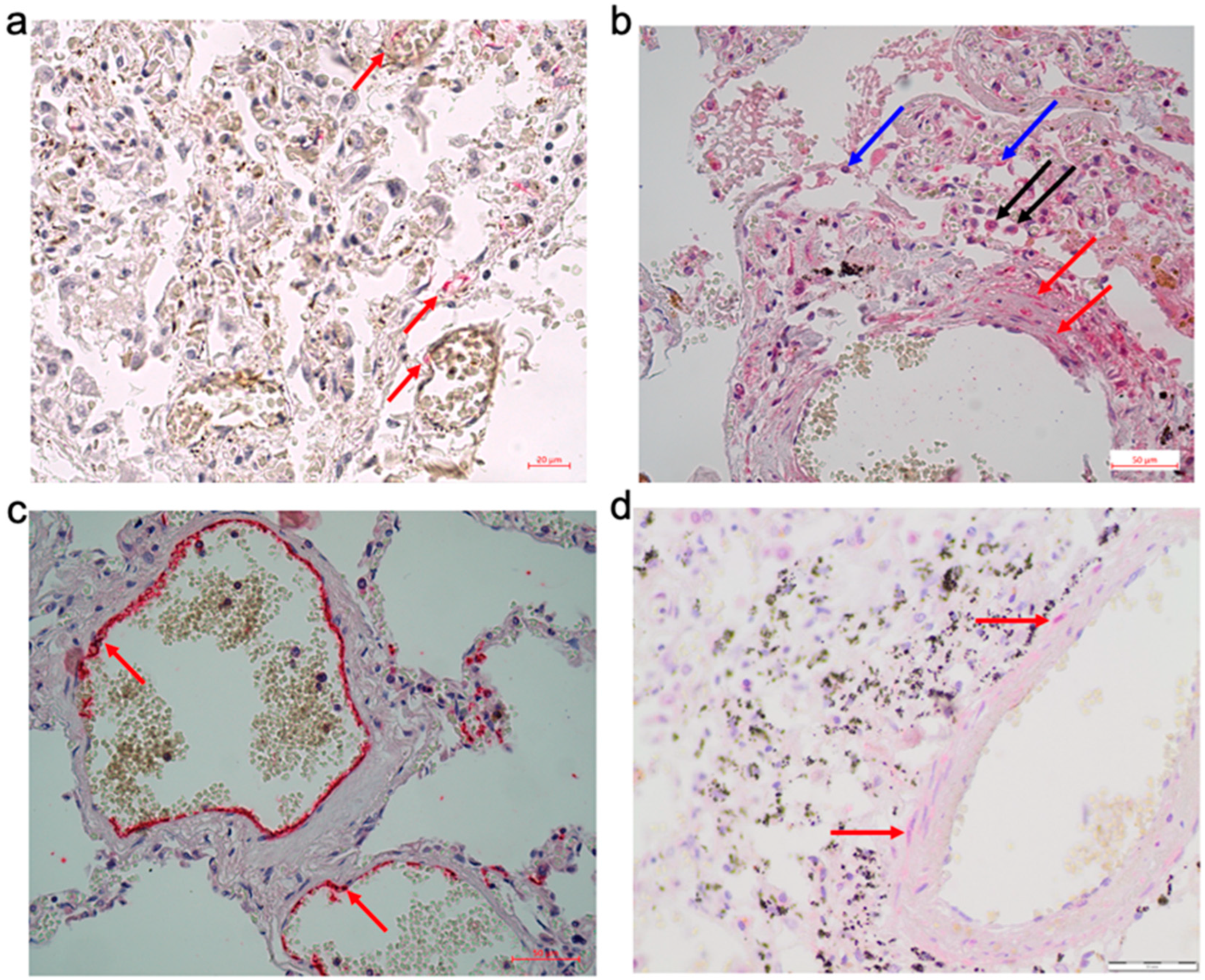
Expression of ACE2 was detected in the endothelial cells, in particular in vasa vasorum (
Figure 1a). In the pulmonary arterial walls, the expression of AXL, which has been shown to be an alternative cell entry receptor for SARS-CoV-2 [
16], was detected. The expression of AXL was observed, not only in alveolocytes and alveolar macrophages but also in smooth muscle cells of the pulmonary arterial walls (
Figure 1b), suggesting the possible direct invasion of SARS-CoV-2 into smooth muscle cells. Indeed, while SARS-CoV-2 spike protein expression was largely observed in endothelial cells (
Figure 1c), in some cases, the spike protein was also detected in smooth muscle cells of pulmonary arterial walls (
Figure 1d). Thus, in severe courses of COVID-19, SARS-CoV-2 and its proteins are present in the structures of the pulmonary arterial walls.
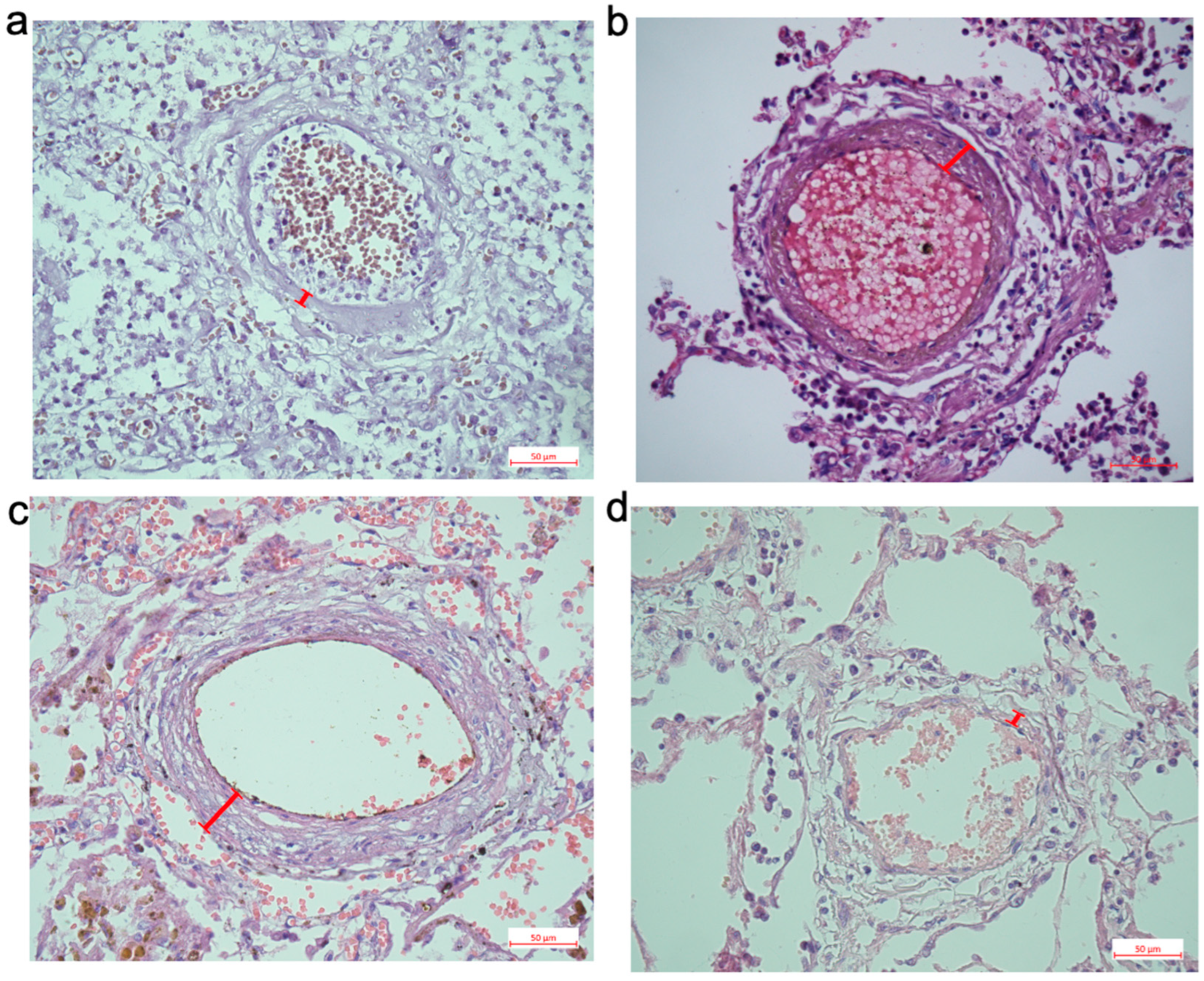
This study, with a large number of patient samples, determined that the severity of pulmonary artery thickening depends on the duration of the disease. Marked remodeling of the pulmonary arteries was detected in patients who died of COVID-19 at least 2 weeks after the onset of the disease (
Figure 2). One of the morphological features of the pulmonary arteries was the thickening (hypertrophy) of the smooth muscle layer of their walls. Immunohistochemical analysis of the pulmonary arteries of patients who died of COVID-19 indicated that the thickening is largely due to the hypertrophy of the smooth muscle layer in small pulmonary arteries (
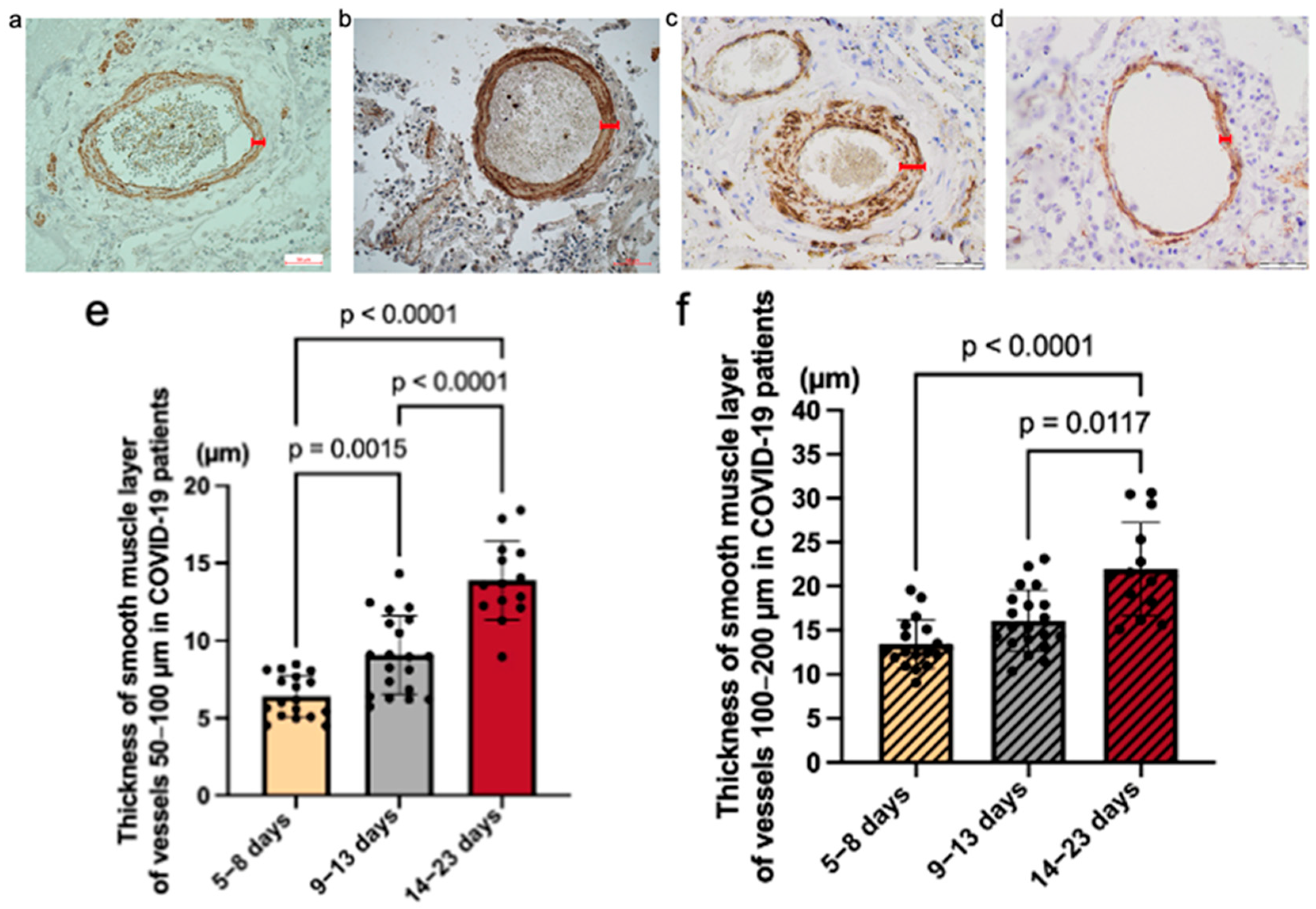
Figure 3a–d). We performed morphometric analysis to assess the differences between the four groups in two indicators: the thickness of the muscular layer of arteries measuring 50–100 μm (
Figure 3e,g) and 100–200 μm (
Figure 3f,h) and the lumen area and area index of both small-sized arteries (50–100 μm;
Figure 3i,k) and medium-sized arteries (100–200 μm;
Figure 3j,l).
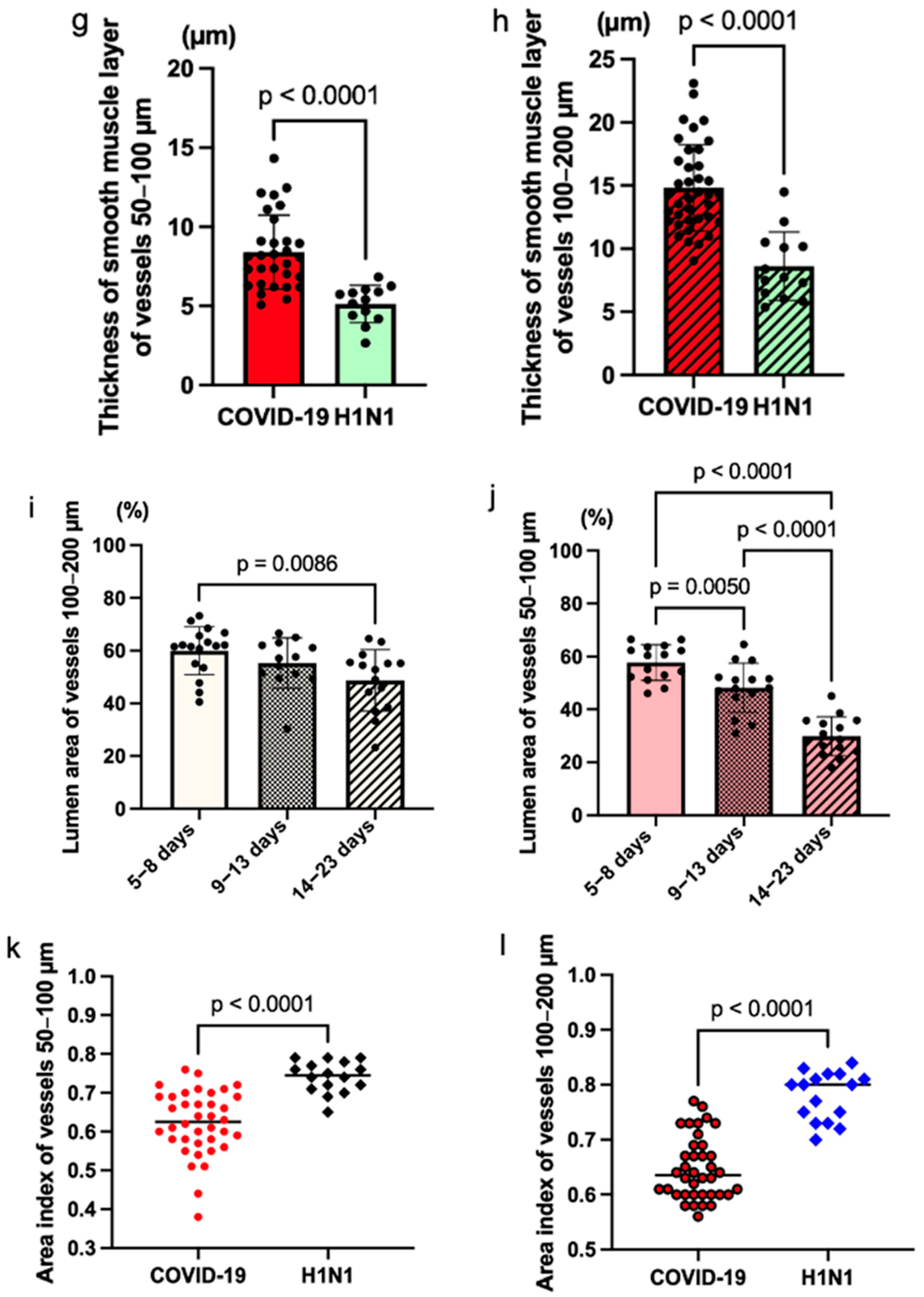
A statistically significant difference was detected in the smooth muscle layer thickness of the small arteries between COVID-19 groups. The mean values are as follows: Group 1 = 6.41 (StDev = 1.37; CV = 21.43%), Group 2 = 9.07 (StDev = 2.52; CV = 27.75%), Group 3 = 13.49 (StDev = 2.52; CV = 18.68%). A significant difference was detected in the muscular layer thickness of the medium-sized arteries between COVID-19 groups. The mean values are as follows: Group 1 = 13.42 (StDev = 2.78; CV = 20.72%), Group 2 = 16.08 (StDev = 3.51; CV = 21.81%), Group 3 = 23.49 (StDev = 5.83; CV = 24.84%). A significant difference was detected in the muscular layer thickness of the small arteries between all cases of the COVID-19 group and the H1N1 influenza comparative group. The mean values are as follows: Group COVID-19 = 8.41 (StDev = 2.34; CV = 27.84%), H1N1 group = 5.13 (StDev = 1.17; CV = 22.76%). A significant difference was detected in the distribution of the muscular layer thickness of the medium-sized arteries between all cases of the COVID-19 group and the H1N1 influenza comparative group. The averages for groups are as follows: Group COVID-19 = 14.83 (StDev = 3.46; CV = 23.32%), H1N1 group = 8.62 (StDev = 2.72; CV = 31.51%).
A statistically significant difference was detected in the distribution of the lumen area (ratio of the luminal area to the total area of the artery, %) of the small and medium-sized arteries between COVID-19 groups (
Figure 3i,j). The percentage of the lumen area of the pulmonary arteries inversely correlated with the duration of the disease. The smallest lumen area of both small and medium-sized vessels was observed in Group 3. For small vessels, the statistical significance (
p) was 0.0050 between Group 1 and Group 2,
p < 0.0001 between Group 2 and Group 3, and
p < 0.0001 between Group 1 and Group 3. Among medium-sized vessels, there was a significant difference between Group 1 and Group 3 (
p = 0.0086). The highest wall thickness and the smallest lumen area index were observed in the group after the 14th day.
Figure 3k,l demonstrate a significant difference in the distribution of the area index of small and medium-sized vessels among all patients who died of COVID-19 compared with patients who died of H1N1 influenza (
p < 0.0001).
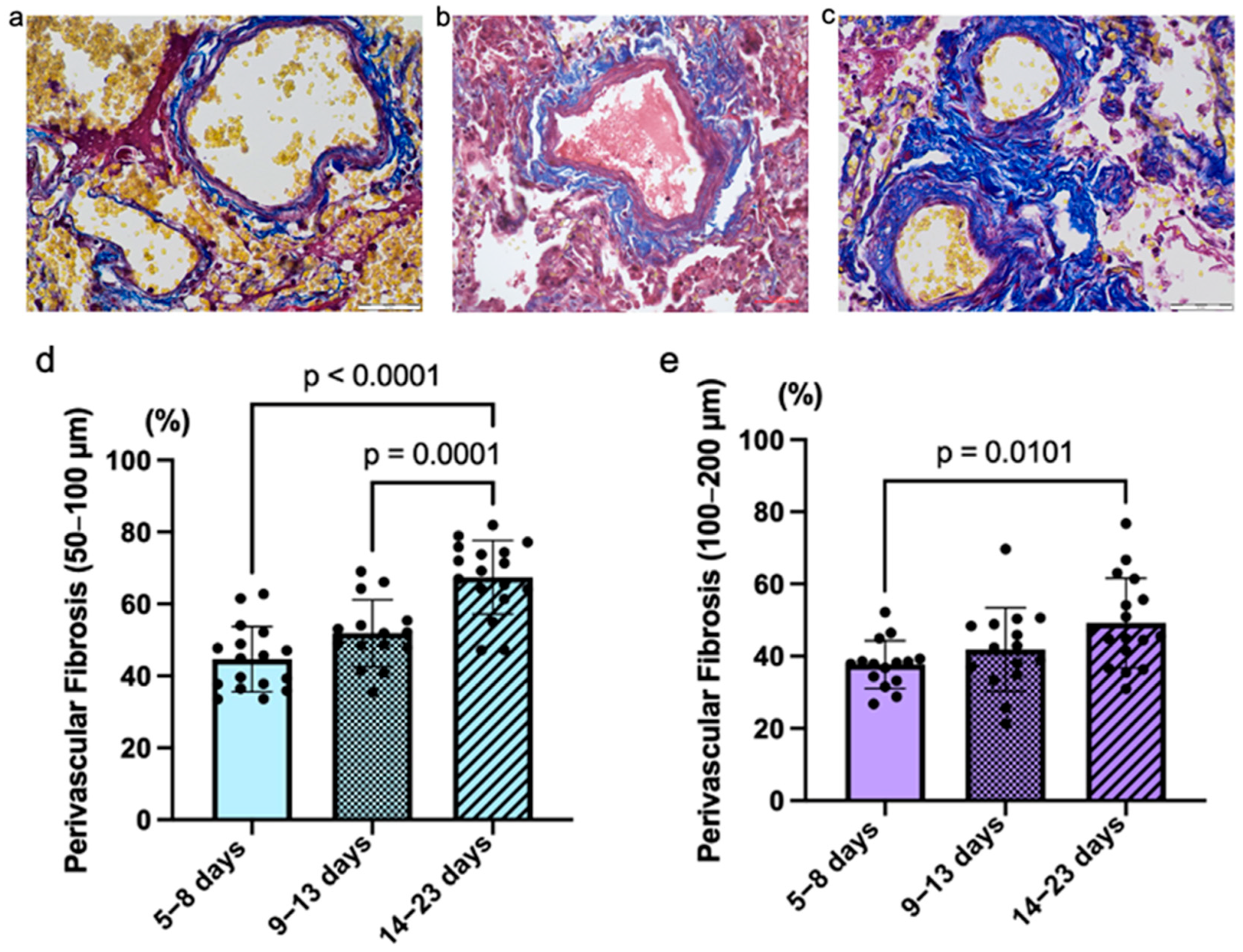
All the studied fatal COVID-19 cases were associated with the development of perivascular fibrosis in the pulmonary arteries of different grades—low, medium, and high—which depend on the duration of the disease (
Figure 4). A statistically significant difference was detected in the area of perivascular fibrosis of the small arteries between the COVID-19 groups. Mean values are as follows: Group 1 = 44.62% (StDev = 0.09; CV = 20.43%), Group 2 = 51.85% (StDev = 0.09; CV = 17.96%), Group 3 = 67.42% (StDev = 0.10; CV = 15.20%). Also, a significant difference was detected in the distribution of perivascular fibrosis of the medium-sized arteries between COVID-19 groups. The average for groups were as follows: Group 1 = 40.04% (StDev = 9.09%; CV = 22.72%), Group 2 = 41.89% (StDev = 11.60%; CV = 27.69%), Group 3 = 49.22% (StDev = 12.40%; CV = 25.20%).
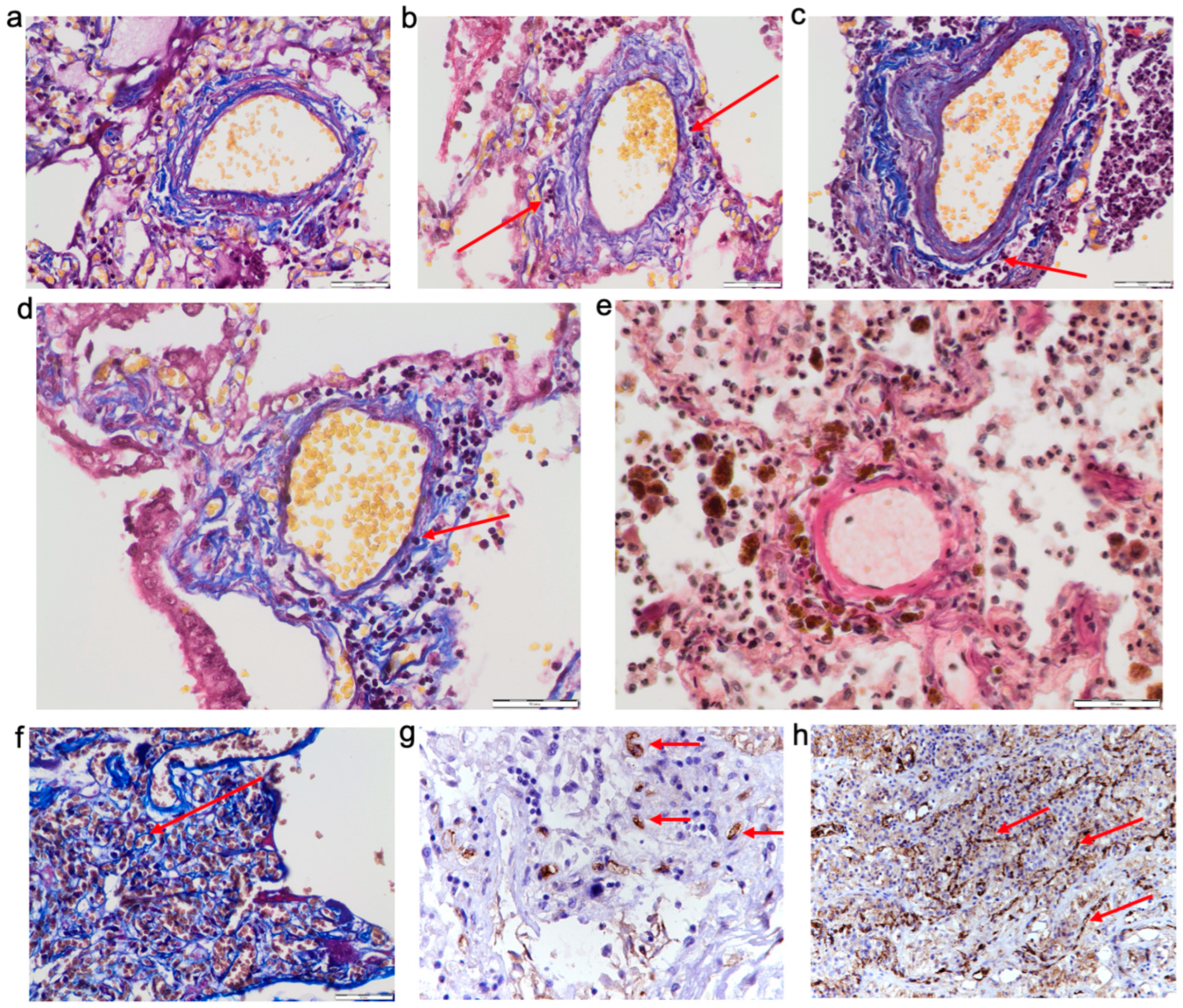
In COVID-19 cases, we observed swelling of the lung parenchyma, including the pulmonary artery walls (
Figure 5a), which causes decompensation of the lymph outflow (
Figure 5b).
We observed perivascular inflammatory infiltration and vasculitis in 15 cases (26.78% of all COVID-19 cases) (
Figure 5c,d). Statistical processing of the data showed no difference between the COVID-19 groups.
In 16 cases (28.57% of all COVID-19 cases), we observed pronounced hemosiderosis in the perivascular areas around the pulmonary arteries (
Figure 5e).
In COVID-19, we observed the activation of neoangiogenesis and an increased number of vessels in the perivascular areas around the pulmonary arteries (
Figure 5f). In 20 cases (35.71% of all COVID-19 cases), we found a pronounced positive reaction of endoglin (CD105), which is expressed in newly formed vessels (
Figure 5g). Also, we observed a positive reaction of CD31 in perivascular angiomatosis areas (
Figure 5h).
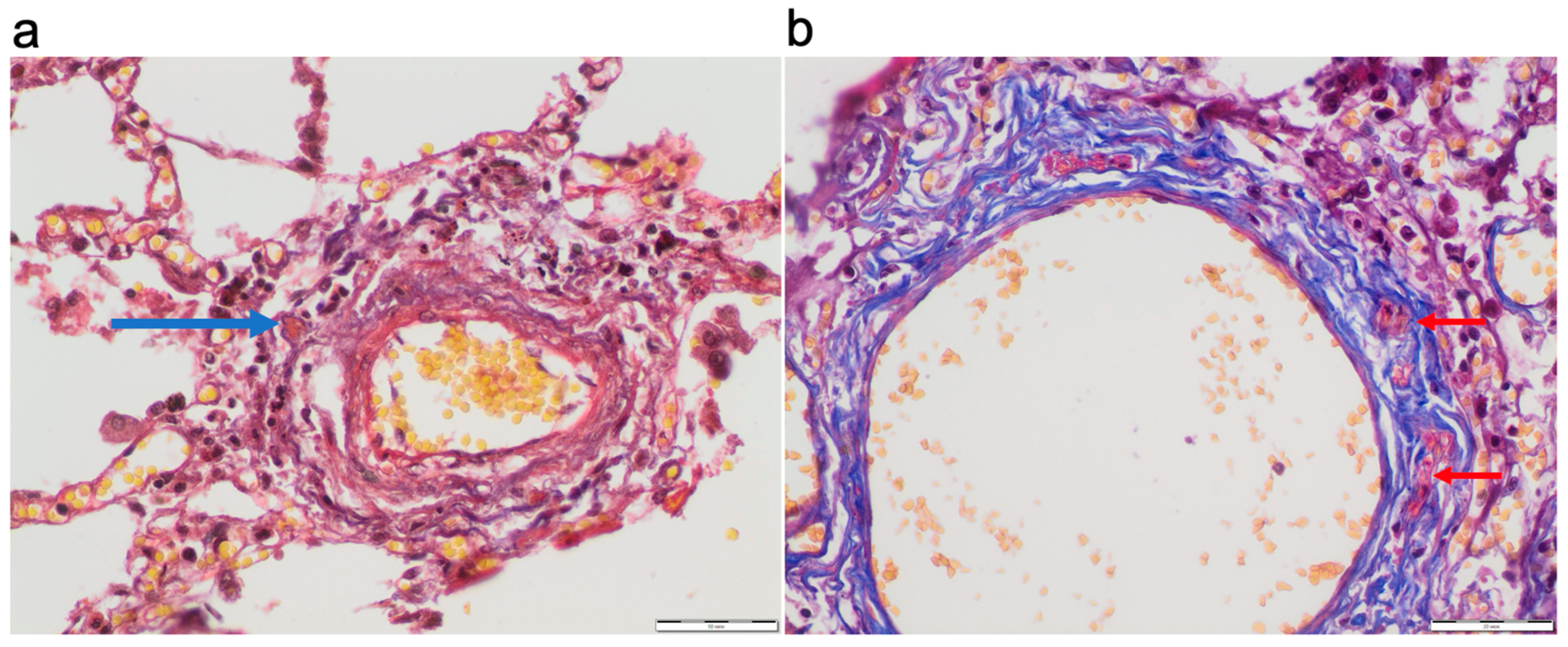
Pulmonary arterial thrombosis was observed in 42.86% of all cases without a significant difference between the study groups. Vasa vasorum thrombosis was detected in 8.9% of all cases (
Figure 6).
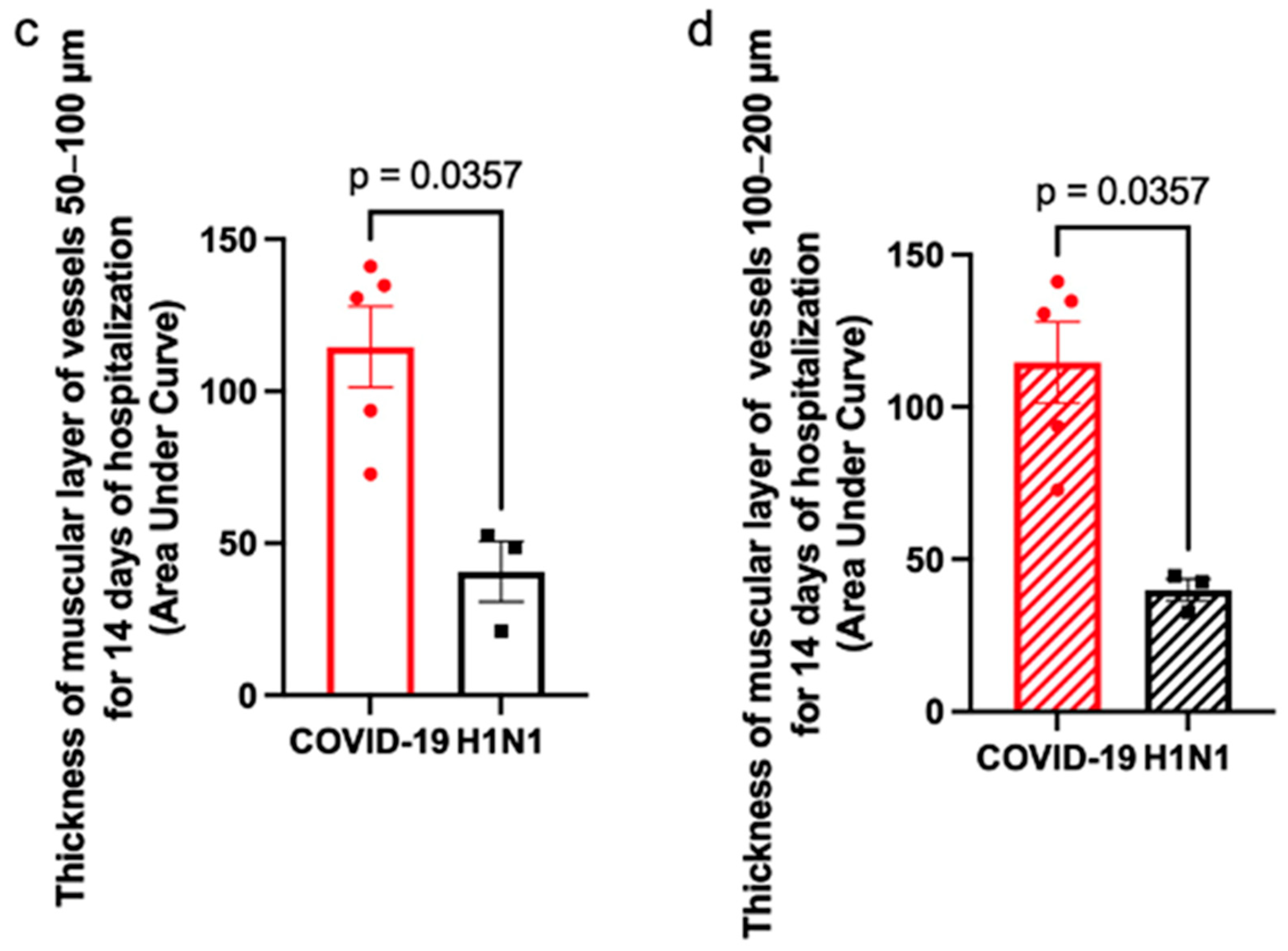
Finally, we found a correlation between the duration of hospital stay and the thickness of the muscular layer of the pulmonary artery walls (
Figure 7).
Figure 7a,b demonstrate the thickness of the smooth muscle layer of the vessels over a 14-day hospitalization period. The thickness of the smooth muscle layer significantly increased in COVID-19 patients by day 14 of the disease compared to H1N1 patients. The AUC analysis (
Figure 7c,d) indicates that COVID-19 patients experienced a greater cumulative increase in smooth muscle layer thickness over the 14-day hospitalization period compared to H1N1 patients, with this difference being statistically significant.
When analyzing the dynamics of remodeling of the pulmonary artery walls, a more pronounced thickening of the muscular layer was found in medium-sized vessels in COVID-19 compared with H1N1 influenza, as well as a more rapid development of remodeling of medium-sized vessels rather than small ones. A significant difference in the thickening of the muscular layer of small vessels between COVID-19 and H1N1 influenza was observed from the 8th day of hospitalization, while for medium-sized vessels, this difference occurred earlier.
4. Discussion
In the spring of 2020, we were stunned by the surge of infection by SARS-CoV-2, which resulted in a worldwide pandemic that influenced people in many ways. In the summer of 2020, we completed our initial study examining lung histology images from autopsy samples that were available in Ukraine. In that earlier study, we analyzed postmortem lung tissues collected from 10 patients who died of COVID-19 during the period of March to July 2020 and compared these with the tissues of patients who died by H1N1 influenza. While both viruses killed the patients via acute respiratory distress syndrome (ARDS), only COVID-19 patients exhibited pronounced thickened pulmonary vascular walls [
13]. Due to the limited number of autopsies of patients who died of COVID-19 in Ukraine in 2020–2021, a sufficient amount of retrospective material for the study was collected in the following years.
Severe COVID-19 is accompanied by the development of ARDS in the early period and pneumonia in the later period. There is information about pulmonary vascular remodeling in patients with ARDS. Snow et al. [
17] observed medial thickening of preacinar pulmonary arteries in patients after ARDS of a long duration (18–60 days). Tomashefski et al. [
18] reported the moderate muscularization of the pulmonary arteries in the intermediate phase and the marked muscularization in the late phase of ARDS. Borek et al. [
19] described pathogenetic mechanisms of pulmonary vascular remodeling in ARDS which can cause pulmonary hypertension. These findings prompted us to warn that COVID-19 patients may become predisposed to pulmonary arterial hypertension [
15].
In this study, we extended these observations by using a larger number of patient samples to analyze this issue on pulmonary vascular remodeling in COVID-19.
By using 56 samples from patients who died by COVID-19, we found that COVID-19 patients exhibited increased pulmonary vascular wall thickness and reduced lumen size, indicating the occurrence of pulmonary vascular remodeling and possibly pulmonary hypertension. The development of pulmonary vascular thickening took at least 14 days to develop after the onset of the disease. The expression of the SARS-CoV-2 spike protein was detected in both endothelial and smooth muscle cells of the pulmonary arteries of COVID-19 patients. Since the spike protein may interfere with the angiotensin regulation, angiotensin II-mediated growth of pulmonary vascular cells may play a mechanistic role. In addition, the spike protein may directly elicit cell growth signaling [
13].
Our histopathological evaluations further provided mechanistic insights into the development of pulmonary vascular remodeling by showing (i) the hypertrophy of the smooth muscle layer of the pulmonary artery walls; (ii) perivascular fibrosis; (iii) edema and lymphostasis in the pulmonary artery walls; (iv) inflammatory infiltration in the perivascular areas and in the pulmonary artery walls; (v) perivascular hemosiderosis; (vi) neoangiogenesis in the perivascular areas; and (vii) vasa vasorum thrombosis.
COVID-19 may promote the development of perivascular fibrosis. The duration of the disease affects the severity of perivascular fibrosis through the inflammation caused by the production of inflammatory cytokines and chemokines by immune cells and, as a result, the activation of fibroblasts that synthesize the collagen.
Lymphostasis is one of the factors of arterial wall thickening due to the accumulation of proteins, detritus, and inflammatory cells in the perivascular space. As a result, fibroblasts are activated with the subsequent formation of connective tissue and the development of perivascular fibrosis, which causes arterial wall thickening
Another factor for arterial wall thickening was perivascular inflammatory infiltration and vasculitis. The inflammatory infiltration of perivascular spaces is primarily due to the activation of cell-mediated and humoral immunity and is manifested by type 3 hypersensitivity reactions [
20]. Perivascular fibrosis and fibrosis of pulmonary artery walls develop as a result of inflammatory damage of arterial wall tissues. As statistical processing of the data showed no difference between the COVID-19 groups, the duration of the disease does not play a significant role in the development of vasculitis.
Hemosiderosis occurs as a result of prolonged and widespread hemorrhages in the alveoli. Alveolar macrophages phagocytose red blood cells and produce a significant amount of hemosiderin, becoming so-called siderophages. These siderophages are transported by lymph flow to the perivascular spaces of the pulmonary arteries. Alveolar macrophages with intracytoplasmic ferritin are known to cause oxidative stress, which also can lead to pulmonary fibrosis and perivascular fibrosis [
21].
Neoangiogenesis may also be a factor that contributes to the thickening of pulmonary arteries. In COVID-19, we observed the activation of neoangiogenesis in the perivascular areas around the pulmonary arteries. We found high expression of endoglin (CD105), which is a part of the TGFβ receptor complex of the membrane protein located on the surface of new-formed endothelial cells. Neoangiogenesis is associated with perivascular fibrosis, also leading to thickening of the arterial walls.
Furthermore, SARS-CoV-2-induced vasa vasorum thrombosis led to the development of hypoxic stress and damage of the pulmonary artery walls, which cause their remodeling, fibrosis, and thickening.
A number of observational and case studies identified transthoracic echocardiogram-detectable pulmonary hypertension. As early as the summer of 2020, Pagnesi et al. [
22] published the results of an observational study in Italy, concluding that the prevalence of pulmonary hypertension and right ventricular dysfunction in 200 COVID-19 patients was 12.0% and 14.5%, respectively. A Romanian study of 91 COVID-19 patients concluded the prevalence of pulmonary hypertension to be 7.69% and that of right ventricular dysfunction to be 10.28% [
23]. Norderfeldt et al. [
24] reported that 26 of 67 (39%) of COVID-19 patients exhibited acute pulmonary hypertension in Sweden. A 24-month follow-up study by the same group determined that the overall mortality was 61.5% in patients with acute pulmonary hypertension [
25]. Numerous case studies also reported the occurrence of pulmonary hypertension after contracting COVID-19 in various countries, including China [
26], Netherlands [
27], the United States [
28], Pakistan [
29], and Mexico [
30]. The onset of pulmonary hypertension post-COVID-19 is unusually rapid, which is similar to two cases of COVID-19-vaccine-associated pulmonary hypertension [
31], strengthening the possibility of the role of the spike protein in the pathogenic mechanism.
In summary, the present study presents a histopathological evaluation of the pulmonary vessels in patients who died of COVID-19, demonstrating that pulmonary vascular thickening and lumen narrowing occur in response to a SARS-CoV-2 infection. Since H1N1 patients who also died of ARDS do not exhibit pulmonary vascular remodeling, some unique features of SARS-CoV-2 specifically affect the pulmonary vessels as well as possibly vessels in general. Given that the spike protein is known to affect ACE2, which regulates angiotensin II levels, it is likely that this viral membrane fusion protein plays a critical role in the pathogenesis of the complications associated with COVID-19.
6. Limitations
This study did not include cases of patients with a history of such comorbidities as chronic obstructive pulmonary disease, diffuse pulmonary emphysema, diffuse pneumosclerosis, systemic vasculitis, systemic connective tissue diseases, tuberculosis, or lung tumors.
In 33 out of 56 COVID-19 patients, a history of comorbidities was indicated: arterial hypertension in 22 patients, chronic ischemic heart disease in 5 patients, diabetes mellitus in 4 patients, polycystic kidneys in 1 patient, and liver cirrhosis in 1 patient. In the influenza H1N1 group, 13 patients out of 17 had a comorbidity: obesity in 8 patients, arterial hypertension in 2 patients, compensated diabetes mellitus in 1 patient, chronic pyelonephritis in 1 patient, and thyroiditis in 1 patient. These comorbidities are not usually associated with the development of pulmonary vascular remodeling.
The absence of preexisting demographic and comorbidities stratification is one major limitation of the current study.
In order to prevent the development of autolytic changes in postmortem samples, the time of autopsy did not exceed 12 h after death.
Possible bias may be associated with a subjective assessment of the degree of expression of markers, the severity of fibrosis, and the manifestations of vasa vasorum thrombosis (due to the fact that vasa vasorum are only clearly detected in serial sections).