Does SARS-CoV-2 Possess “Allergen-Like” Epitopes?
Abstract
:1. Introduction
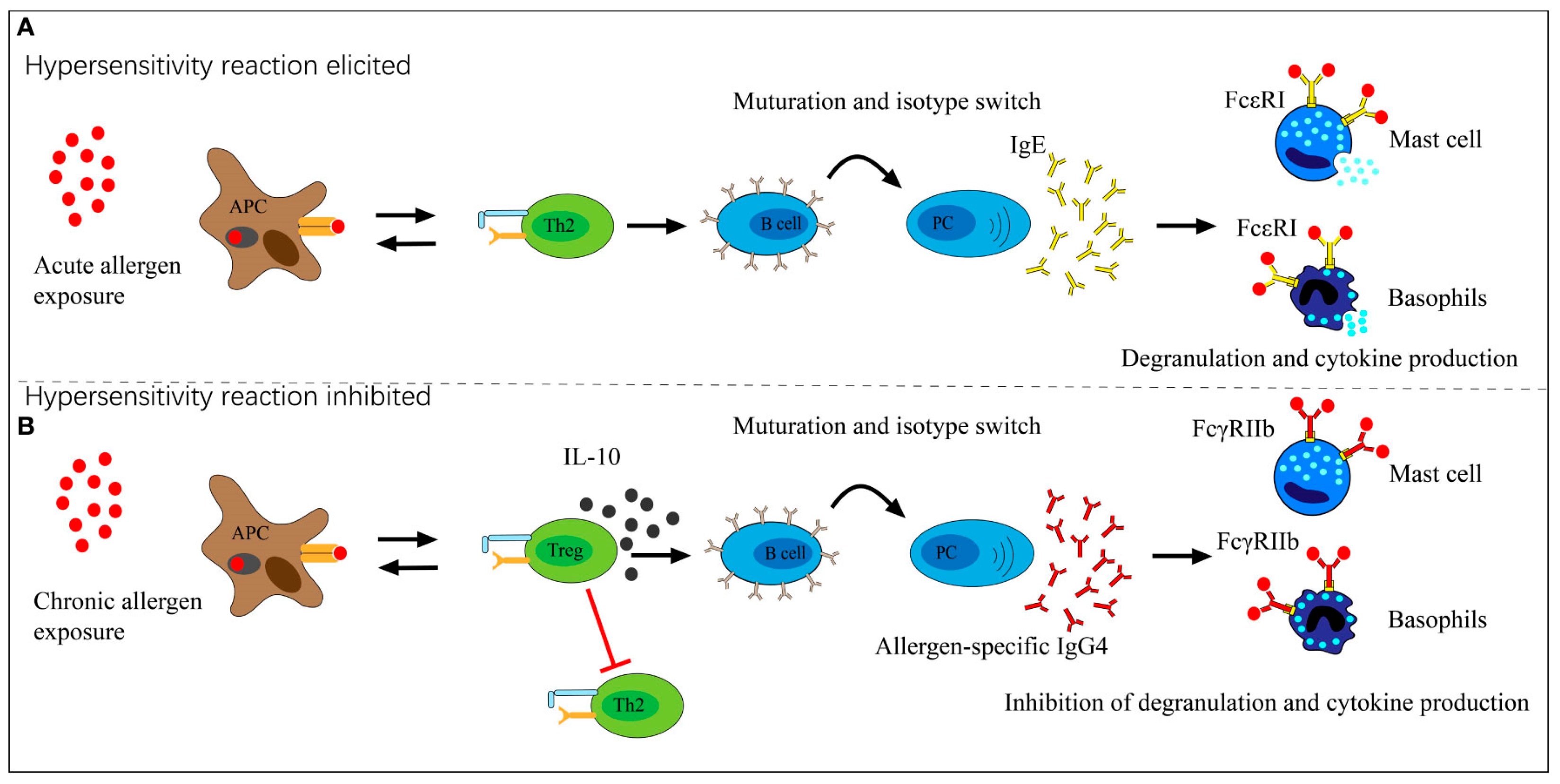
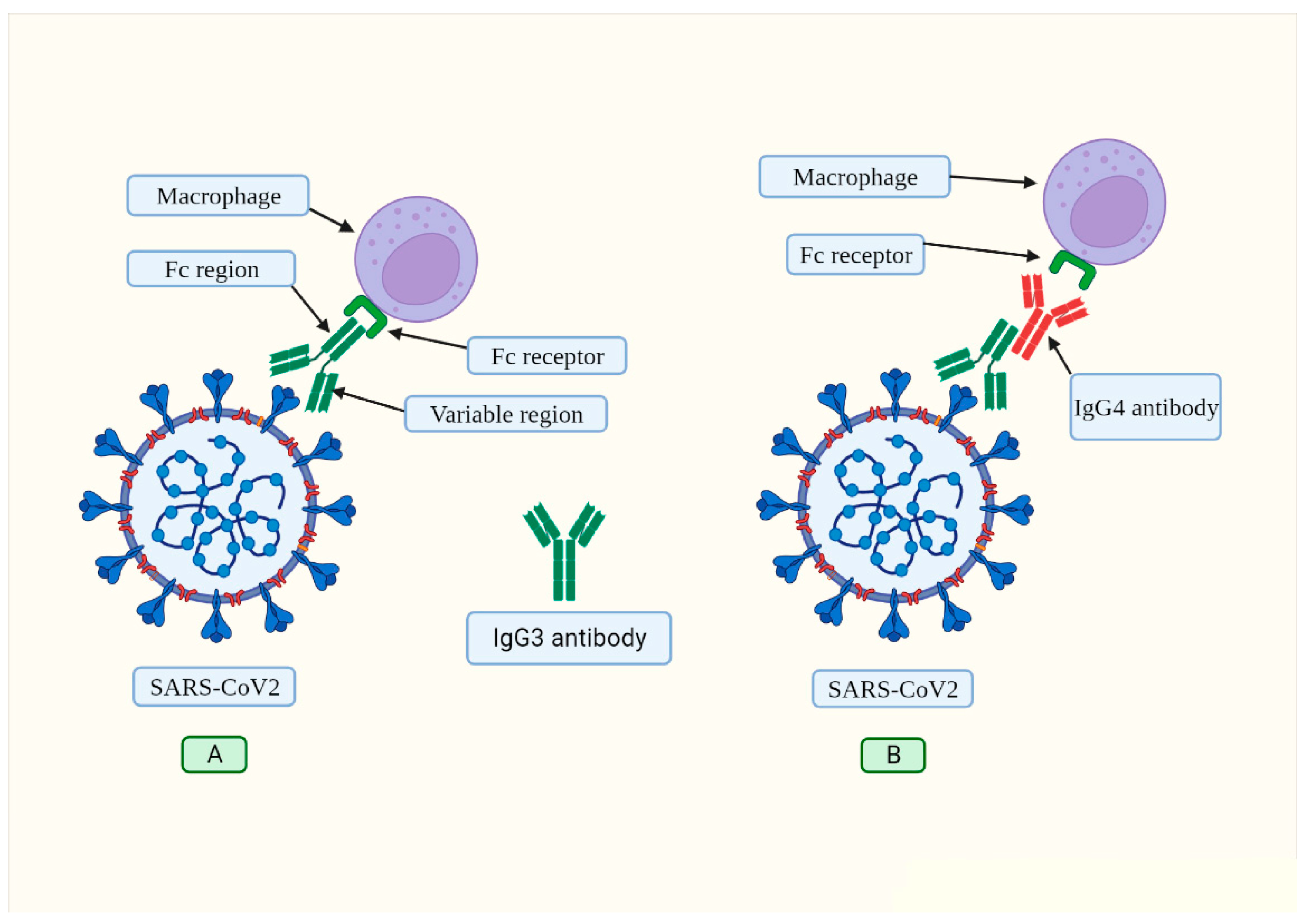
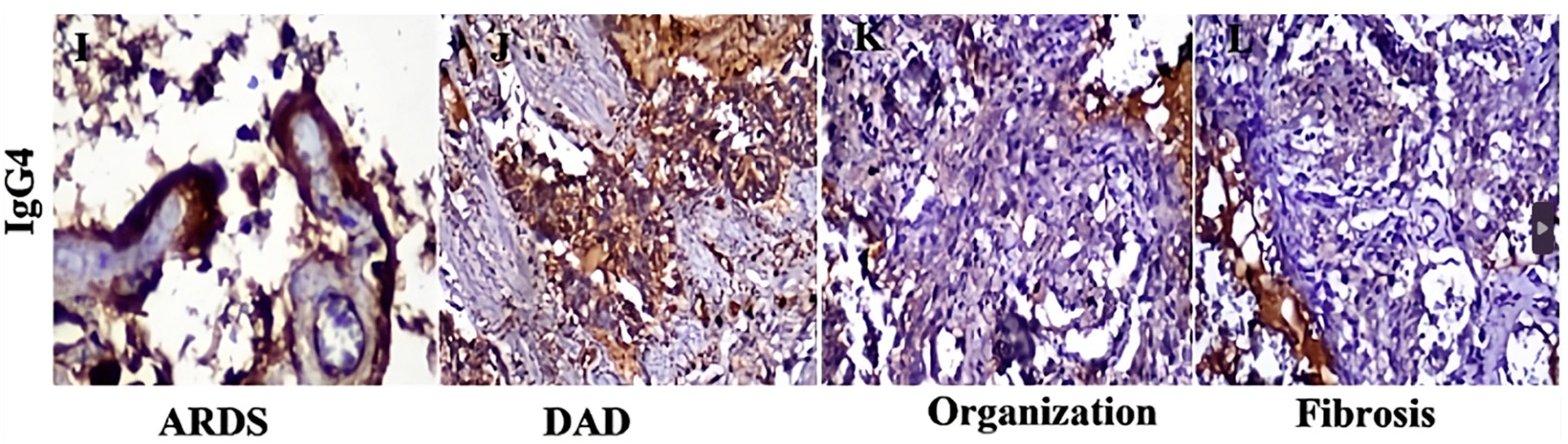
2. Evidence of the Involvement of IgE Antibodies in COVID-19 and in Vaccination
Does SARS-CoV-2 Possess “Allergen-Like” Epitopes?
3. Allergen-like Epitopes in HIV Envelope Proteins
4. The Role of IgE in RSV Infection
5. Repeated Vaccination with Allergen-like Epitopes
6. Implications for Vaccine Design
- AlgPred 2.0 (https://webs.iiitd.edu.in/raghava/algpred2/), which uses multiple machine learning approaches to predict allergen-like sequences (accessed on 25 February 2025).
- AllergenFP (http://ddg-pharmfac.net/AllergenFP/), which uses a descriptor-based fingerprint to detect potential allergens (accessed 25 February 2025).
- BLASTp (https://blast.ncbi.nlm.nih.gov/Blast.cgi), which finds regions of similarity between biological sequences. The program compares nucleotide or protein sequences to the sequence database and calculates the statistical significance (accessed on 25 February 2025).
- AlphaFold (https://swissmodel.expasy.org/) predicts protein structures for docking studies (Accessed on 25 February 2025).
- Molecular docking (Autodock, HADDOCK) (https://rascar.science.uu.nl/haddock2.4/) (accessed on 25 February 2025).
- -
- Optimizing vaccination schedules to avoid excessive boosting, which may induce tolerance and immune exhaustion.
- -
- Incorporating adjuvants that enhance Th1 responses (e.g., CpG oligodeoxynucleotides) rather than Th2-skewing agents like aluminum hydroxide.
- -
- Heterologous vaccination strategies might also mitigate IgG4 induction, as they have demonstrated only slight or no increases in IgG4 levels.
- -
- Comprehensive assessment of the route of administration for vaccines: intramuscular vs. nasal route.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeSilva, M.B.; Mitchell, P.K.; Klein, N.P.; Dixon, B.E.; Tenforde, M.W.; Thompson, M.G.; Naleway, A.L.; Grannis, S.J.; Ong, T.C.; Natarajan, K.; et al. Protection of two and three mRNA vaccine doses against severe outcomes among adults hospitalized with COVID-19—Vision Network, August 2021 to March 2022. J. Infect. Dis. 2023, 227, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA 2021, 326, 2043–2054. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Self, W.H.; Zhu, Y.; Naioti, E.A.; Gaglani, M.; Ginde, A.A.; Jensen, K.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Protection of mRNA vaccines against hospitalized COVID-19 in adults over the first year following authorization in the United States. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 76, e460–e468. [Google Scholar] [CrossRef]
- Sibanda, B.; Haryanto, B. Assessing the Impact of COVID-19 Vaccination Programs on the Reduction of COVID-19 Cases: A Systematic Literature Review. Ann. Glob. Health 2024, 90, 45. [Google Scholar] [CrossRef]
- Rispens, T.; Huijbers, M.G. The unique properties of IgG4 and its roles in health and disease. Nat. Rev. Immunol. 2023, 23, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Rispens, T.; Ooijevaar-de Heer, P.; Bende, O.; Aalberse, R.C. Mechanism of immunoglobulin G4 Fab-arm exchange. J. Am. Chem. Soc. 2011, 133, 10302–10311. [Google Scholar] [CrossRef]
- Aalberse, R.; Stapel, S.; Schuurman, J.; Rispens, T. Immunoglobulin G4: An odd antibody. Clin. Exp. Allergy 2009, 39, 469–477. [Google Scholar] [CrossRef]
- Schuurman, J.; Van Ree, R.; Perdok, G.a.; Van Doorn, H.; Tan, K.; Aalberse, R. Normal human immunoglobulin G4 is bispecific: It has two different antigen-combining sites. Immunology 1999, 97, 693–698. [Google Scholar] [CrossRef]
- Van Der Neut Kolfschoten, M.; Schuurman, J.; Losen, M.; Bleeker, W.K.; Martínez-Martínez, P.; Vermeulen, E.; Den Bleker, T.H.; Wiegman, L.; Vink, T.; Aarden, L.A.; et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007, 317, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.; Blaser, K. Mechanisms of allergen-specific immunotherapy. Allergy 2000, 55, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M. Healthy immune response to allergens: T regulatory cells and more. Curr. Opin. Immunol. 2006, 18, 738–744. [Google Scholar] [CrossRef]
- Larché, M.; Akdis, C.A.; Valenta, R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006, 6, 761–771. [Google Scholar] [CrossRef]
- Durham, S.R.; Walker, S.M.; Varga, E.-M.; Jacobson, M.R.; O’Brien, F.; Noble, W.; Till, S.J.; Hamid, Q.A.; Nouri-Aria, K.T. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 1999, 341, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahi, A.; Stone, J.H. A clinical overview of IgG4-related systemic disease. Curr. Opin. Rheumatol. 2011, 23, 57–66. [Google Scholar] [CrossRef]
- Della-Torre, E.; Lanzillotta, M.a.; Doglioni, C. Immunology of IgG4-related disease. Clin. Exp. Immunol. 2015, 181, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Shiokawa, M.; Kodama, Y.; Kuriyama, K.; Yoshimura, K.; Tomono, T.; Morita, T.; Kakiuchi, N.; Matsumori, T.; Mima, A.; Nishikawa, Y.; et al. Pathogenicity of IgG in patients with IgG4-related disease. Gut 2016, 65, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Tang, L.-F.; Cheng, L.; Wang, H.-Y. The clinical significance of allergen-specific IgG4 in allergic diseases. Front. Immunol. 2022, 13, 1032909. [Google Scholar] [CrossRef]
- Flicker, S.; Valenta, R. Renaissance of the blocking antibody concept in type I allergy. Int. Arch. Allergy Immunol. 2003, 132, 13–24. [Google Scholar] [CrossRef]
- Wachholz, P.A.; Durham, S.R. Mechanisms of immunotherapy: IgG revisited. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Golden, D.B.; Meyers, D.A.; Kagey-Sobotka, A.; Valentine, M.D.; Lichtenstein, L.M. Clinical relevance of the venom-specific immunoglobulin G antibody level during immunotherapy. J. Allergy Clin. Immunol. 1982, 69, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Helbling, A.; Bischof, M. Predictive value of venom-specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy 1989, 44, 412–418. [Google Scholar] [CrossRef]
- Nouri-Aria, K.T.; Wachholz, P.A.; Francis, J.N.; Jacobson, M.R.; Walker, S.M.; Wilcock, L.K.; Staple, S.Q.; Aalberse, R.C.; Till, S.J.; Durham, S.R. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J. Immunol. 2004, 172, 3252–3259. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.; Ljørring, C.; Francis, J.; A Calderon, M.; Larche, M.; Kimber, I.; Frew, A.; Ipsen, H.; Lund, K.; Würtzen, P.; et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy 2012, 67, 217–226. [Google Scholar] [CrossRef]
- Meiler, F.; Klunker, S.; Zimmermann, M.; Akdis, C.A.; Akdis, M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy 2008, 63, 1455–1463. [Google Scholar] [CrossRef]
- Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy: Multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014, 133, 621–631. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2022, 8, eade2798. [Google Scholar] [CrossRef]
- Buhre, J.S.; Pongracz, T.; Künsting, I.; Lixenfeld, A.S.; Wang, W.; Nouta, J.; Lehrian, S.; Schmelter, F.; Lunding, H.B.; Dühring, L.; et al. mRNA vaccines against SARS-CoV-2 induce comparably low long-term IgG Fc galactosylation and sialylation levels but increasing long-term IgG4 responses compared to an adenovirus-based vaccine. Front. Immunol. 2023, 13, 1020844. [Google Scholar] [CrossRef]
- Kiszel, P.; Sík, P.; Miklós, J.; Kajdácsi, E.; Sinkovits, G.; Cervenak, L.; Prohászka, Z. Class switch towards spike protein-specific IgG4 antibodies after SARS-CoV-2 mRNA vaccination depends on prior infection history. Sci. Rep. 2023, 13, 13166. [Google Scholar] [CrossRef] [PubMed]
- Emmenegger, M.; Fiedler, S.; Brugger, S.D.; Devenish, S.R.; Morgunov, A.S.; Ilsley, A.; Ricci, F.; Malik, A.Y.; Scheier, T.; Batkitar, L.; et al. Both COVID-19 infection and vaccination induce high-affinity cross-clade responses to SARS-CoV-2 variants. Iscience 2022, 25, 104766. [Google Scholar] [CrossRef] [PubMed]
- Selva, K.J.; Ramanathan, P.; Haycroft, E.R.; Reynaldi, A.; Cromer, D.; Tan, C.W.; Wang, L.-F.; Wines, B.D.; Hogarth, P.M.; Downie, L.E.; et al. Preexisting immunity restricts mucosal antibody recognition of SARS-CoV-2 and Fc profiles during breakthrough infections. JCI Insight 2023, 8, e172470. [Google Scholar] [CrossRef] [PubMed]
- Valk, A.M.; Keijser, J.B.; van Dam, K.P.; Stalman, E.W.; Wieske, L.; Steenhuis, M.; Kummer, L.Y.; Spuls, P.I.; Bekkenk, M.W.; Musters, A.H.; et al. Suppressed IgG4 class switching in dupilumab-and TNF inhibitor-treated patients after mRNA vaccination. Allergy 2024, 79, 1952–1961. [Google Scholar] [CrossRef]
- Hartley, G.E.; Fryer, H.A.; Gill, P.A.; Boo, I.; Bornheimer, S.J.; Hogarth, P.M.; Drummer, H.E.; O’Hehir, R.E.; Edwards, E.S.; van Zelm, M.C. Third dose COVID-19 mRNA vaccine enhances IgG4 isotype switching and recognition of Omicron subvariants by memory B cells after mRNA but not adenovirus priming. bioRxiv 2023. [Google Scholar] [CrossRef]
- Farkash, I.; Feferman, T.; Cohen-Saban, N.; Avraham, Y.; Morgenstern, D.; Mayuni, G.; Barth, N.; Lustig, Y.; Miller, L.; Shouval, D.S.; et al. Anti-SARS-CoV-2 antibodies elicited by COVID-19 mRNA vaccine exhibit a unique glycosylation pattern. Cell Rep. 2021, 37, 110114. [Google Scholar] [CrossRef]
- Sheehan, J.; Ardizzone, C.M.; Khanna, M.; Trauth, A.J.; Hagensee, M.E.; Ramsay, A.J. Dynamics of Serum-Neutralizing Antibody Responses in Vaccinees through Multiple Doses of the BNT162b2 Vaccine. Vaccines 2023, 11, 1720. [Google Scholar] [CrossRef]
- Yoshimura, M.; Sakamoto, A.; Ozuru, R.; Kurihara, Y.; Itoh, R.; Ishii, K.; Shimizu, A.; Chou, B.; Nabeshima, S.; Hiromatsu, K. The appearance of anti-spike receptor binding domain immunoglobulin G4 responses after repetitive immunization with messenger RNA-based COVID-19 vaccines. Int. J. Infect. Dis. 2024, 139, 1–5. [Google Scholar] [CrossRef]
- Akhtar, M.; Islam, M.R.; Khaton, F.; Soltana, U.H.; Jafrin, S.A.; Rahman, S.I.A.; Tauheed, I.; Ahmed, T.; Khan, I.I.; Akter, A.; et al. Appearance of tolerance-induction and non-inflammatory SARS-CoV-2 spike-specific IgG4 antibodies after COVID-19 booster vaccinations. Front. Immunol. 2023, 14, 1309997. [Google Scholar] [CrossRef]
- Espino, A.M.; Armina-Rodriguez, A.; Alvarez, L.; Ocasio-Malavé, C.; Ramos-Nieves, R.; Rodriguez Martinó, E.I.; López-Marte, P.; Torres, E.A.; Sariol, C.A. The Anti-SARS-CoV-2 IgG1 and IgG3 Antibody Isotypes with Limited Neutralizing Capacity against Omicron Elicited in a Latin Population a Switch toward IgG4 after Multiple Doses with the mRNA Pfizer–BioNTech Vaccine. Viruses 2024, 16, 187. [Google Scholar] [CrossRef]
- Adhikari, B.; Oltz, E.; Bednash, J.; Horowitz, J.; Rubinstein, M.; Vlasova, A.N. Brief Research Report: Impact of vaccination on antibody responses and mortality from severe COVID-19. Front. Immunol. 2024, 15, 1325243. [Google Scholar]
- Kalkeri, R.; Zhu, M.; Cloney-Clark, S.; Plested, J.S.; Parekh, A.; Gorinson, D.; Cai, R.; Mahato, S.; Ramanathan, P.; Aurelia, L.C.; et al. Altered IgG4 Antibody Response to Repeated mRNA versus Protein COVID Vaccines. medRxiv 2024. [Google Scholar] [CrossRef]
- Nziza, N.; Deng, Y.; Wood, L.; Dhanoa, N.; Dulit-Greenberg, N.; Chen, T.; Kane, A.S.; Swank, Z.; Davis, J.P.; Demokritou, M.; et al. Humoral profiles of toddlers and young children following SARS-CoV-2 mRNA vaccination. Nat. Commun. 2024, 15, 905. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kumar, S.; Lai, L.; Linderman, S.; Malik, A.A.; Ellis, M.L.; Godbole, S.; Solis, D.; Sahoo, M.K.; Bechnak, K.; et al. XBB. 1.5 monovalent booster improves antibody binding and neutralization against emerging SARS-CoV-2 Omicron variants. bioRxiv 2024. [Google Scholar] [CrossRef]
- Portilho, A.I.; Silva, V.O.; Da Costa, H.H.M.; Yamashiro, R.; de Oliveira, I.P.; de Campos, I.B.; Prudencio, C.R.; Matsuda, E.M.; de Macedo Brígido, L.F.; De Gaspari, E. An unexpected IgE anti-receptor binding domain response following natural infection and different types of SARS-CoV-2 vaccines. Sci. Rep. 2024, 14, 20003. [Google Scholar] [CrossRef] [PubMed]
- Routhu, N.K.; Stampfer, S.D.; Lai, L.; Akhtar, A.; Tong, X.; Yuan, D.; Chicz, T.M.; McNamara, R.P.; Jakkala, K.; Davis-Gardner, M.E.; et al. Efficacy of mRNA-1273 and Novavax ancestral or BA. 1 spike booster vaccines against SARS-CoV-2 BA. 5 infection in non-human primates. Sci. Immunol. 2023, 8, eadg7015. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, L.; Xing, M.; Qiao, N.; Liu, J.; Li, X.; Zhang, C.; Tang, N.; Xu, Z.; Guo, Y.; et al. Evaluation of antibody responses in healthy individuals receiving SARS-CoV-2 inactivated vaccines. Biosaf. Health 2024, 6, 153–164. [Google Scholar] [CrossRef]
- Suzuki, S.; Suzuki, Y.; Yamamoto, N.; Matsumoto, Y.; Shirai, A.; Okubo, T. Influenza A virus infection increases IgE production and airway responsiveness in aerosolized antigen-exposed mice. J. Allergy Clin. Immunol. 1998, 102, 732–740. [Google Scholar] [CrossRef]
- Dakhama, A.; Lee, Y.-M.; Ohnishi, H.; Jing, X.; Balhorn, A.; Takeda, K.; Gelfand, E.W. Virus-specific IgE enhances airway responsiveness on reinfection with respiratory syncytial virus in newborn mice. J. Allergy Clin. Immunol. 2009, 123, 138–145.e5. [Google Scholar] [CrossRef]
- Guclu, O.A.; Goktas, S.S.; Dilektasli, A.G.; Ozturk, N.A.A.; Demirdogen, E.; Coskun, F.; Ediger, D.; Ursavas, A.; Uzaslan, E.; Erol, H.A.; et al. A pilot study for IgE as a prognostic biomarker in COVID-19. Intern. Med. J. 2022, 52, 1495–1504. [Google Scholar]
- Ferastraoaru, D.; Hudes, G.; Jerschow, E.; Jariwala, S.; Karagic, M.; de Vos, G.; Rosenstreich, D.; Ramesh, M. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J. Allergy Clin. Immunol. Pract. 2021, 9, 1152–1162.e3. [Google Scholar] [CrossRef]
- Zhang, J.-j.; Dong, X.; Cao, Y.-y.; Yuan, Y.-d.; Yang, Y.-b.; Yan, Y.-q.; Akdis, C.A.; Gao, Y.-d. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Tu, L.; Zhu, P.; Mu, M.; Wang, R.; Yang, P.; Wang, X.; Hu, C.; Ping, R.; Hu, P.; et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am. J. Respir. Crit. Care Med. 2020, 201, 1372–1379. [Google Scholar] [CrossRef]
- Rodrigo-Muñoz, J.; Sastre, B.; Cañas, J.; Gil-Martínez, M.; Redondo, N.; Del Pozo, V. Eosinophil response against classical and emerging respiratory viruses: COVID-19. J. Investig. Allergol. Clin. Immunol. 2021, 31, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Plūme, J.; Galvanovskis, A.; Šmite, S.; Romanchikova, N.; Zayakin, P.; Linē, A. Early and strong antibody responses to SARS-CoV-2 predict disease severity in COVID-19 patients. J. Transl. Med. 2022, 20, 176. [Google Scholar] [CrossRef]
- Collins, A.M.; Jackson, K.J. A temporal model of human IgE and IgG antibody function. Front. Immunol. 2013, 4, 235. [Google Scholar] [CrossRef]
- Jeannin, P.; Delneste, Y.; Lecoanet-Henchoz, S.; Gretener, D.; Bonnefoy, J.-Y. Interleukin-7 (IL-7) enhances class switching to IgE and IgG4 in the presence of T cells via IL-9 and sCD23. Blood J. Am. Soc. Hematol. 1998, 91, 1355–1361. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Kinet, J.-P. The high-affinity IgE receptor (FcϵRI): From physiology to pathology. Annu. Rev. Immunol. 1999, 17, 931–972. [Google Scholar] [CrossRef]
- Kempuraj, D.; Selvakumar, G.P.; Ahmed, M.E.; Raikwar, S.P.; Thangavel, R.; Khan, A.; Zaheer, S.A.; Iyer, S.S.; Burton, C.; James, D.; et al. COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neurosci 2020, 26, 402–414. [Google Scholar] [CrossRef]
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. COVID-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020, 100, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Motta Junior, J.d.S.; Miggiolaro, A.F.R.d.S.; Nagashima, S.; De Paula, C.B.V.; Baena, C.P.; Scharfstein, J.; De Noronha, L. Mast cells in alveolar septa of COVID-19 patients: A pathogenic pathway that may link interstitial edema to immunothrombosis. Front. Immunol. 2020, 11, 574862. [Google Scholar] [CrossRef]
- Farmani, A.R.; Mahdavinezhad, F.; Moslemi, R.; Mehrabi, Z.; Noori, A.; Kouhestani, M.; Noroozi, Z.; Ai, J.; Rezaei, N. Anti-IgE monoclonal antibodies as potential treatment in COVID-19. Immunopharmacol. Immunotoxicol. 2021, 43, 259–264. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Mendez, N.V.; Landin, A.M.; Blomberg, B.B. Effects of age on H1N1-specific serum IgG1 and IgG3 levels evaluated during the 2011–2012 influenza vaccine season. Immun. Ageing 2013, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Cavacini, L.A.; Kuhrt, D.; Duval, M.; Mayer, K.; Posner, M.R. Binding and neutralization activity of human IgG1 and IgG3 from serum of HIV-infected individuals. AIDS Res. Hum. Retroviruses 2003, 19, 785–792. [Google Scholar] [CrossRef]
- Suthar, M.S.; Zimmerman, M.G.; Kauffman, R.C.; Mantus, G.; Linderman, S.L.; Hudson, W.H.; Vanderheiden, A.; Nyhoff, L.; Davis, C.W.; Adekunle, O.; et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep. Med. 2020, 1, 100040. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, L.; Martinuzzi, D.; Hyseni, I.; Benincasa, L.; Molesti, E.; Casa, E.; Lapini, G.; Piu, P.; Trombetta, C.M.; Marchi, S.; et al. Comparative analyses of SARS-CoV-2 binding (IgG, IgM, IgA) and neutralizing antibodies from human serum samples. J. Immunol. Methods 2021, 489, 112937. [Google Scholar] [CrossRef]
- Griffin, D.E.; Cooper, S.J.; Hirsch, R.L.; Johnson, R.T.; de Soriano, I.L.; Roedenbeck, S.; Vaisberg, A. Changes in plasma IgE levels during complicated and uncomplicated measles virus infections. J. Allergy Clin. Immunol. 1985, 76, 206–213. [Google Scholar] [CrossRef]
- Imani, F.; Proud, D.; Griffin, D.E. Measles virus infection synergizes with IL-4 in IgE class switching. J. Immunol. 1999, 162, 1597–1602. [Google Scholar] [CrossRef]
- Shalit, M.; Ackerman, Z.; Wollner, S.; Morag, A.; Levo, Y. Immunoglobulin E response during measles. Int. Arch. Allergy Immunol. 1984, 75, 84–86. [Google Scholar] [CrossRef]
- Tam, J.S.; Jackson, W.T.; Hunter, D.; Proud, D.; Grayson, M.H. Rhinovirus specific IgE can be detected in human sera. J. Allergy Clin. Immunol. 2013, 132, 1241–1243. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.K.; Pyle, D.M.; Farrar, J.D.; Gill, M.A. IgE-mediated regulation of IL-10 and type I IFN enhances rhinovirus-induced Th2 differentiation by primary human monocytes. Eur. J. Immunol. 2020, 50, 1550–1559. [Google Scholar] [CrossRef]
- Zambrano, J.C.; Carper, H.T.; Rakes, G.P.; Patrie, J.; Murphy, D.D.; Platts-Mills, T.A.; Hayden, F.G.; Gwaltney, J.M., Jr.; Hatley, T.K.; Owens, A.M.; et al. Experimental rhinovirus challenges in adults with mild asthma: Response to infection in relation to IgE. J. Allergy Clin. Immunol. 2003, 111, 1008–1016. [Google Scholar] [CrossRef]
- Kantor, D.B.; Stenquist, N.; McDonald, M.C.; Schultz, B.J.; Hauptman, M.; Smallwood, C.D.; Nelson, K.A.; Perzanowski, M.S.; Matsui, E.C.; Phipatanakul, W.; et al. Rhinovirus and serum IgE are associated with acute asthma exacerbation severity in children. J. Allergy Clin. Immunol. 2016, 138, 1467–1471.e9. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Wisniewski, J.; Yu, M.; Kennedy, J.L.; Platts-Mills, T.; Heymann, P.W.; Woodfolk, J.A. Infection with human rhinovirus 16 promotes enhanced IgE responsiveness in basophils of atopic asthmatics. Clin. Exp. Allergy 2014, 44, 1266–1273. [Google Scholar] [CrossRef]
- Burleson, H.L.K.S.G.R. Effect of Influenza Virus Infection on Ovalbumin Specific Ige Responses to Inhaled Antigen in The Rat. J. Toxicol. Environ. Health Part A 1996, 49, 619–630. [Google Scholar]
- Rowe, R.K.; Pyle, D.M.; Tomlinson, A.R.; Lv, T.; Hu, Z.; Gill, M.A. IgE cross-linking impairs monocyte antiviral responses and inhibits influenza-driven TH1 differentiation. J. Allergy Clin. Immunol. 2017, 140, 294–298.e8. [Google Scholar] [CrossRef]
- Wright, D.N.; Nelson, R.P., Jr.; Ledford, D.K.; Fernandez-Caldas, E.; Trudeau, W.L.; Lockey, R.F. Serum IgE and human immunodeficiency virus (HIV) infection. J. Allergy Clin. Immunol. 1990, 85, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Ammann, A.J.; Abrams, D.; Conant, M.; Chudwin, D.; Cowan, M.; Volberding, P.; Lewis, B.; Casavant, C. Acquired immune dysfunction in homosexual men: Immunologic profiles. Clin. Immunol. Immunopathol. 1983, 27, 315–325. [Google Scholar] [CrossRef]
- Ring, J.; Fröschl, M.; Brunner, R.; Braun-Falco, O. LAV/HTLV-III infection and atopy: Serum IgE and specific IgE antibodies to environmental allergens. Acta Derm. Venereol. 1986, 66, 530–532. [Google Scholar] [CrossRef]
- Lin, R.Y. Chronic diffuse dermatitis and hyper-IgE in HIV infection. Acta Derm. Venereol. 1988, 68, 486–491. [Google Scholar]
- Miguez-Burbano, M.; Shor-Posner, G.; Fletcher, M.A.; Lu, Y.; Moreno, J.; Carcamo, C.; Page, B.; Quesada, J.; Sauberlich, H.; Baum, M. Immunoglobulin E levels in relationship to HIV-1 disease, route of infection, and vitamin E status. Allergy 1995, 50, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Ouaaz, F.; Ruscetti, F.W.; Dugas, B.; Mikovits, J.; Agut, H.; Debr, P.; Mossalayi, M.D. Role of IgE Immune Complexes in the Regulation of HIV-1 Replication and Increased Cell Death of Infected U1 Monocytes: Involvement of CD23/Fc ε RII-Mediated Nitric Oxide and Cyclic AMP Pathways. Mol. Med. 1996, 2, 38–49. [Google Scholar] [CrossRef]
- Ellaurie, M.; Rubinstein, A.; Rosenstreich, D.L. IgE levels in pediatric HIV-1 infection. Ann. Allergy Asthma Immunol. 1995, 75, 332–336. [Google Scholar] [PubMed]
- Israël-Biet, D.; Labrousse, F.; Tourani, J.-M.; Sors, H.; Andrieu, J.-M.; Even, P. Elevation of IgE in HIV-infected subjects: A marker of poor prognosis. J. Allergy Clin. Immunol. 1992, 89, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Lucey, D.R.; Zajac, R.A.; Melcher, G.P.; Butzin, C.A.; Boswell, R.N. Serum IgE levels in 622 persons with human immunodeficiency virus infection: IgE elevation with marked depletion of CD4+ T-cells. AIDS Res. Hum. Retroviruses 1990, 6, 427–429. [Google Scholar] [CrossRef]
- Shor-Posner, G.; Miguez-Burbano, M.J.; Lu, Y.; Feaster, D.; Fletcher, M.; Sauberlich, H.; Baum, M.K. Elevated IgE level in relationship to nutritional status and immune parameters in early human immunodeficiency virus–1 disease. J. Allergy Clin. Immunol. 1995, 95, 886–892. [Google Scholar] [CrossRef]
- Bul, R.H.D.; Molinaro, G.A.; Kettering, J.D.; Heiner, D.C.; Imagawa, D.T.; Geme, J.W.S., Jr. Virus-specific IgE and IgG4 antibodies in serum of children infected with respiratory syncytial virus. J. Pediatr. 1987, 110, 87–90. [Google Scholar]
- Becker, Y. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy—A review. Virus Genes 2006, 33, 235–252. [Google Scholar] [CrossRef]
- Welliver, R.C.; Sun, M.; Rinaldo, D.; Ogra, P.L. Respiratory syncytial virus-specific IgE responses following infection: Evidence for a predominantly mucosal response. Pediatr. Res. 1985, 19, 420–424. [Google Scholar] [CrossRef]
- Smith-Norowitz, T.A.; Josekutty, J.; Silverberg, J.I.; Lev-Tov, H.; Norowitz, Y.M.; Kohlhoff, S.; Nowakowski, M.; Durkin, H.G.; Bluth, M.H. Long term persistence of IgE anti-Varicella Zoster Virus in pediatric and adult serum post chicken pox infection and after vaccination with Varicella Virus vaccine. Int. J. Biomed. Sci. IJBS 2009, 5, 353. [Google Scholar] [CrossRef]
- Smith-Norowitz, T.A.; Tam, E.; Norowitz, K.B.; Chotikanatis, K.; Weaver, D.; Durkin, H.G.; Bluth, M.H.; Kohlhoff, S. IgE anti Hepatitis B virus surface antigen antibodies detected in serum from inner city asthmatic and non asthmatic children. Hum. Immunol. 2014, 75, 378–382. [Google Scholar] [CrossRef]
- Imani, F.; Kehoe, K.E. Infection of human B lymphocytes with MMR vaccine induces IgE class switching. Clin. Immunol. 2001, 100, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Smith-Norowitz, T.A.; Wong, D.; Kusonruksa, M.; Norowitz, K.B.; Joks, R.; Durkin, H.G.; Bluth, M.H. Long term persistence of IgE anti-influenza virus antibodies in pediatric and adult serum post vaccination with influenza virus vaccine. Int. J. Med. Sci. 2011, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zheng, X.; Sun, F.; He, J.; Shi, H.; Chen, M.; Tu, C.; Huang, Y.; Wang, Z.; Liang, Y.; et al. Hypersensitivity may be involved in severe COVID-19. Clin. Exp. Allergy 2022, 52, 324–333. [Google Scholar] [CrossRef]
- Chen, M.; Tu, C.; Tan, C.; Zheng, X.; Sun, F.; Liang, Y.; Shi, H.; Wu, J.; Huang, Y.; Wang, Z.; et al. Hypersensitivity in the lungs is responsible for acute respiratory failure in COVID-19 patients: Case series of patients who received high-dose/short-term methylprednisolone. Clin. Transl. Allergy 2021, 11, e12056. [Google Scholar] [CrossRef]
- Della-Torre, E.; Lanzillotta, M.; Strollo, M.; Ramirez, G.A.; Dagna, L.; Tresoldi, M. Serum IgG4 level predicts COVID-19 related mortality. Eur. J. Intern. Med. 2021, 93, 107–109. [Google Scholar] [CrossRef]
- Moura, A.D.; da Costa, H.H.; Correa, V.A.; de S. Lima, A.K.; Lindoso, J.A.; De Gaspari, E.; Hong, M.A.; Cunha-Junior, J.P.; Prudencio, C.R. Assessment of avidity related to IgG subclasses in SARS-CoV-2 Brazilian infected patients. Sci. Rep. 2021, 11, 17642. [Google Scholar] [CrossRef]
- Genova, S.N.; Pencheva, M.M.; Abadjieva, T.I.; Atanasov, N.G. Cellular and immune response in fatal COVID-19 pneumonia. Pan Afr. Med. J. 2024, 49, 130. [Google Scholar] [CrossRef]
- Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. Does SARS-CoV-2 induce IgG4 synthesis to evade the immune system? Biomolecules 2023, 13, 1338. [Google Scholar] [CrossRef]
- Pascolini, S.; Granito, A.; Muratori, L.; Lenzi, M.; Muratori, P. Coronavirus disease associated immune thrombocytopenia: Causation or correlation? J. Microbiol. Immunol. Infect. 2020, 54, 531. [Google Scholar] [CrossRef] [PubMed]
- Pascolini, S.; Vannini, A.; Deleonardi, G.; Ciordinik, M.; Sensoli, A.; Carletti, I.; Veronesi, L.; Ricci, C.; Pronesti, A.; Mazzanti, L.; et al. COVID-19 and immunological dysregulation: Can autoantibodies be useful? Clin. Transl. Sci. 2021, 14, 502–508. [Google Scholar] [CrossRef]
- Muratori, P.; Lenzi, M.; Muratori, L.; Granito, A. Antinuclear antibodies in COVID 19. Clin. Transl. Sci. 2021, 14, 1627. [Google Scholar] [CrossRef] [PubMed]
- Balz, K.; Kaushik, A.; Chen, M.; Cemic, F.; Heger, V.; Renz, H.; Nadeau, K.; Skevaki, C. Homologies between SARS-CoV-2 and allergen proteins may direct T cell-mediated heterologous immune responses. Sci. Rep. 2021, 11, 4792. [Google Scholar] [CrossRef]
- Balz, K.; Trassl, L.; Härtel, V.; Nelson, P.P.; Skevaki, C. Virus-induced T cell-mediated heterologous immunity and vaccine development. Front. Immunol. 2020, 11, 513. [Google Scholar] [CrossRef]
- Pusch, E.; Renz, H.; Skevaki, C. Respiratory virus-induced heterologous immunity: Part of the problem or part of the solution? Allergo J. 2018, 27, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Karagöz, I.K.; Kaya, M.; Rückert, R.; Bozman, N.; Kaya, V.; Bayram, H.; Yıldırım, M. A bioinformatic analysis: Previous allergen exposure may support anti-SARS-CoV-2 immune response. Comput. Biol. Chem. 2023, 107, 107961. [Google Scholar] [CrossRef]
- Zhu, Z.; Hasegawa, K.; Ma, B.; Fujiogi, M.; Camargo, C.A.; Liang, L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J. Allergy Clin. Immunol. 2020, 146, 327–329.e4. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Murdock, D.J.; Marcus, A.; Hussein, M.; Jalbert, J.J.; Geba, G.P. Association between allergic conditions and COVID-19 susceptibility and outcomes. Ann. Allergy Asthma Immunol. 2024, 132, 637–645.e7. [Google Scholar] [CrossRef]
- Skevaki, C.; Karsonova, A.; Karaulov, A.; Fomina, D.; Xie, M.; Chinthrajah, S.; Nadeau, K.C.; Renz, H. SARS-CoV-2 infection and COVID-19 in asthmatics: A complex relationship. Nat. Rev. Immunol. 2021, 21, 202–203. [Google Scholar] [CrossRef]
- Skevaki, C.; Karsonova, A.; Karaulov, A.; Xie, M.; Renz, H. Asthma-associated risk for COVID-19 development. J. Allergy Clin. Immunol. 2020, 146, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, A.E.; Melo, R.C.; Duan, S.; LeMessurier, K.S.; Liedmann, S.; Surman, S.L.; Lee, J.J.; Hurwitz, J.L.; Thomas, P.G.; McCullers, J.A. Eosinophils promote antiviral immunity in mice infected with influenza A virus. J. Immunol. 2017, 198, 3214–3226. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.G.; Bivins-Smith, E.R.; Proskocil, B.J.; Nie, Z.; Scott, G.D.; Lee, J.J.; Lee, N.A.; Fryer, A.D.; Jacoby, D.B. Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am. J. Respir. Cell Mol. Biol. 2016, 55, 387–394. [Google Scholar] [CrossRef]
- Phipps, S.; Lam, C.E.; Mahalingam, S.; Newhouse, M.; Ramirez, R.; Rosenberg, H.F.; Foster, P.S.; Matthaei, K.I. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood J. Am. Soc. Hematol. 2007, 110, 1578–1586. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Domachowske, J.B. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J. Leukoc. Biol. 2001, 70, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Domachowske, J.B.; Dyer, K.D.; Bonville, C.A.; Rosenberg, H.F. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J. Infect. Dis. 1998, 177, 1458–1464. [Google Scholar] [CrossRef]
- Turner, K.J.; Feddema, L.; Quinn, E.H. Non-specific potentiation of IgE by parasitic infections in man. Int. Arch. Allergy Immunol. 1979, 58, 232–236. [Google Scholar] [CrossRef]
- Lee, T.D.; Xie, C.Y. IgE regulation by nematodes: The body fluid of Ascaris contains a B-cell mitogen. J. Allergy Clin. Immunol. 1995, 95, 1246–1254. [Google Scholar] [CrossRef]
- Hagel, I.; Lynch, N.; Di Prisco, M.; Rojas, E.; Perez, M.; Alvarez, N. Ascaris reinfection of slum children: Relation with the IgE response. Clin. Exp. Immunol. 1993, 94, 80–83. [Google Scholar] [CrossRef]
- Becker, Y. HIV-1 gp41 heptad repeat 2 (HR2) possesses an amino acid domain that resembles the allergen domain in Aspergillus fumigatus Asp f1 protein: Review, hypothesis and implications. Virus Genes 2007, 34, 233–240. [Google Scholar] [CrossRef]
- Dasari, P.; Shopova, I.A.; Stroe, M.; Wartenberg, D.; Martin-Dahse, H.; Beyersdorf, N.; Hortschansky, P.; Dietrich, S.; Cseresnyés, Z.; Figge, M.T.; et al. Aspf2 from Aspergillus fumigatus recruits human immune regulators for immune evasion and cell damage. Front. Immunol. 2018, 9, 1635. [Google Scholar] [CrossRef] [PubMed]
- Heinekamp, T.; Schmidt, H.; Lapp, K.; Pähtz, V.; Shopova, I.; Köster-Eiserfunke, N.; Krüger, T.; Kniemeyer, O.; Brakhage, A.A. Interference of Aspergillus fumigatus with the immune response. Semin. Immunopathol. 2015, 37, 141–152. [Google Scholar] [CrossRef]
- Abad, A.; Fernández-Molina, J.V.; Bikandi, J.; Ramírez, A.; Margareto, J.; Sendino, J.; Hernando, F.L.; Pontón, J.; Garaizar, J.; Rementeria, A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. De Micol. 2010, 27, 155–182. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Tonani, L.; Cardoso, C.R.B.; Kress, M.R.V.Z. The immune interplay between the host and the pathogen in Aspergillus fumigatus lung infection. BioMed Res. Int. 2013, 2013, 693023. [Google Scholar] [CrossRef] [PubMed]
- Dewi, I.M.; Van de Veerdonk, F.L.; Gresnigt, M.S. The multifaceted role of T-helper responses in host defense against Aspergillus fumigatus. J. Fungi 2017, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Murdock, B.J.; Shreiner, A.B.; McDonald, R.A.; Osterholzer, J.J.; White, E.S.; Toews, G.B.; Huffnagle, G.B. Coevolution of TH1, TH2, and TH17 responses during repeated pulmonary exposure to Aspergillus fumigatus conidia. Infect. Immun. 2011, 79, 125–135. [Google Scholar] [CrossRef]
- Cramer, R.A.; Rivera, A.; Hohl, T.M. Immune responses against Aspergillus fumigatus: What have we learned? Curr. Opin. Infect. Dis. 2011, 24, 315–322. [Google Scholar] [CrossRef]
- Stevens, D.A. Th1/Th2 in aspergillosis. Med. Mycol. 2006, 44, S229–S235. [Google Scholar] [CrossRef]
- Arias, M.; Santiago, L.; Vidal-García, M.; Redrado, S.; Lanuza, P.; Comas, L.; Domingo, M.P.; Rezusta, A.; Gálvez, E.M. Preparations for invasion: Modulation of host lung immunity during pulmonary aspergillosis by gliotoxin and other fungal secondary metabolites. Front. Immunol. 2018, 9, 2549. [Google Scholar] [CrossRef]
- Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. SARS-CoV-2: A master of immune evasion. Biomedicines 2022, 10, 1339. [Google Scholar] [CrossRef]
- Caetano-Anollés, K.; Hernandez, N.; Mughal, F.; Tomaszewski, T.; Caetano-Anollés, G. The seasonal behaviour of COVID-19 and its galectin-like culprit of the viral spike. Methods Microbiol. 2022, 50, 27–81. [Google Scholar] [PubMed]
- Cherayil, B.J.; Weiner, S.J.; Pillai, S. The Mac-2 antigen is a galactose-specific lectin that binds IgE. J. Exp. Med. 1989, 170, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.T.; Bieneman, A.P. The S1 subunit of the SARS-CoV-2 spike protein activates human monocytes to produce cytokines linked to COVID-19: Relevance to galectin-3. Front. Immunol. 2022, 13, 831763. [Google Scholar] [CrossRef]
- Körner, R.W.; Bansemir, O.Y.; Franke, R.; Sturm, J.; Dafsari, H.S. Atopy and elevation of IgE, IgG3, and IgG4 may be risk factors for post COVID-19 condition in children and adolescents. Children 2023, 10, 1598. [Google Scholar] [CrossRef]
- Giménez-Orenga, K.; Pierquin, J.; Brunel, J.; Charvet, B.; Martín-Martínez, E.; Perron, H.; Oltra, E. HERV-W ENV antigenemia and correlation of increased anti-SARS-CoV-2 immunoglobulin levels with post-COVID-19 symptoms. Front. Immunol. 2022, 13, 1020064. [Google Scholar] [CrossRef]
- Motta, R.V.; Culver, E.L. IgG4 autoantibodies and autoantigens in the context of IgG4-autoimmune disease and IgG4-related disease. Front. Immunol. 2024, 15, 1272084. [Google Scholar] [CrossRef] [PubMed]
- Raszek, M.; Cowley, D.; Redwan, E.M.; Uversky, V.N.; Rubio-Casillas, A. Exploring the possible link between the spike protein immunoglobulin G4 antibodies and cancer progression. Explor. Immunol. 2024, 4, 267–284. [Google Scholar] [CrossRef]
- Umetsu, D.T.; DeKruyff, R.H. TH1 and TH2 CD4+ cells in human allergic diseases. J. Allergy Clin. Immunol. 1997, 100, 1–6. [Google Scholar] [CrossRef]
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387.e9. [Google Scholar] [CrossRef]
- Leitao, I.d.C.; Calil, P.T.; Galliez, R.M.; Moreira, F.R.R.; Mariani, D.; Castiñeiras, A.C.P.; da Silva, G.P.D.; Maia, R.A.; Correa, I.A.; Monteiro, F.L.L.; et al. Prolonged SARS-CoV-2 positivity in immunocompetent patients: Virus isolation, genomic integrity, and transmission risk. Microbiol. Spectr. 2021, 9, e00855-21. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin. Infect. Dis. 2023, 76, e487–e490. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Tejerina, F.; Catalan, P.; Rodriguez-Grande, C.; Adan, J.; Rodriguez-Gonzalez, C.; Muñoz, P.; Aldamiz, T.; Diez, C.; Perez, L.; Fanciulli, C.; et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect. Dis. 2022, 22, 211. [Google Scholar] [CrossRef]
- Becker, Y. The changes in the T helper 1 (Th1) and T helper 2 (Th2) cytokine balance during HIV-1 infection are indicative of an allergic response to viral proteins that may be reversed by Th2 cytokine inhibitors and immune response modifiers–a review and hypothesis. Virus Genes 2004, 28, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S.; Del Prete, G.; Manetti, R.; Ravina, A.; Annunziato, F.; De Carli, M.; Mazzetti, M.; Piccinni, M.-P.; D’Elios, M.M.; Parronchi, P.; et al. Role of TH1/TH2 cytokines in HIV infection. Immunol. Rev. 1994, 140, 73–92. [Google Scholar] [CrossRef]
- Wong, G.H.; Goeddel, D.V. Tumour necrosis factors α and β inhibit virus replication and synergize with interferons. Nature 1986, 323, 819–822. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Fitch, F.W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J. Immunol. 1988, 140, 4245–4252. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Moore, K.W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol. Today 1991, 12, A49–A53. [Google Scholar] [CrossRef]
- Becker, Y. HIV-1 induced AIDS is an allergy and the allergen is the Shed gp120–a review, hypothesis, and implications. Virus Genes 2004, 28, 319–331. [Google Scholar] [CrossRef]
- Da Silva, L.; Da Silva, J. Relationship between atopy, allergic diseases and total serum IgE levels among HIV-infected children. Eur. Ann. Allergy Clin. Immunol. 2013, 45, 155–159. [Google Scholar]
- Gill, M.A.; Bajwa, G.; George, T.A.; Dong, C.C.; Dougherty, I.I.; Jiang, N.; Gan, V.N.; Gruchalla, R.S. Counterregulation between the FcεRI pathway and antiviral responses in human plasmacytoid dendritic cells. J. Immunol. 2010, 184, 5999–6006. [Google Scholar] [CrossRef] [PubMed]
- Gielen, V.; Sykes, A.; Zhu, J.; Chan, B.; Macintyre, J.; Regamey, N.; Kieninger, E.; Gupta, A.; Shoemark, A.; Bossley, C.; et al. Increased nuclear suppressor of cytokine signaling 1 in asthmatic bronchial epithelium suppresses rhinovirus induction of innate interferons. J. Allergy Clin. Immunol. 2015, 136, 177–188.e11. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Werder, R.B.; Simpson, J.; Loh, Z.; Zhang, V.; Haque, A.; Spann, K.; Sly, P.D.; Mazzone, S.B.; Upham, J.W.; et al. Aeroallergen-induced IL-33 predisposes to respiratory virus–induced asthma by dampening antiviral immunity. J. Allergy Clin. Immunol. 2016, 138, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Durrani, S.R.; Montville, D.J.; Pratt, A.S.; Sahu, S.; DeVries, M.K.; Rajamanickam, V.; Gangnon, R.E.; Gill, M.A.; Gern, J.E.; Lemanske, R.F., Jr.; et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J. Allergy Clin. Immunol. 2012, 130, 489–495. [Google Scholar] [CrossRef]
- Duff, A.L.; Pomeranz, E.S.; Gelber, L.E.; Price, G.W.; Farris, H.; Hayden, F.G.; Platts-Mills, T.A.; Heymann, P.W. Risk factors for acute wheezing in infants and children: Viruses, passive smoke, and IgE antibodies to inhalant allergens. Pediatrics 1993, 92, 535–540. [Google Scholar] [CrossRef]
- Teach, S.J.; Gergen, P.J.; Szefler, S.J.; Mitchell, H.E.; Calatroni, A.; Wildfire, J.; Bloomberg, G.R.; Kercsmar, C.M.; Liu, A.H.; Makhija, M.M.; et al. Seasonal risk factors for asthma exacerbations among inner-city children. J. Allergy Clin. Immunol. 2015, 135, 1465–1473.e5. [Google Scholar] [CrossRef]
- Soto-Quiros, M.; Avila, L.; Platts-Mills, T.A.; Hunt, J.F.; Erdman, D.D.; Carper, H.; Murphy, D.D.; Odio, S.; James, H.R.; Patrie, J.T.; et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J. Allergy Clin. Immunol. 2012, 129, 1499–1505.e5. [Google Scholar] [CrossRef]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef]
- Bencze, D.; Fekete, T.; Pázmándi, K. Type I interferon production of plasmacytoid dendritic cells under control. Int. J. Mol. Sci. 2021, 22, 4190. [Google Scholar] [CrossRef]
- Gill, M.A.; Liu, A.H.; Calatroni, A.; Krouse, R.Z.; Shao, B.; Schiltz, A.; Gern, J.E.; Togias, A.; Busse, W.W. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J. Allergy Clin. Immunol. 2018, 141, 1735–1743.e9. [Google Scholar] [CrossRef]
- Teach, S.J.; Gill, M.A.; Togias, A.; Sorkness, C.A.; Arbes, S.J., Jr.; Calatroni, A.; Wildfire, J.J.; Gergen, P.J.; Cohen, R.T.; Pongracic, J.A.; et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J. Allergy Clin. Immunol. 2015, 136, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, A.; Busse, W.W.; Calatroni, A.; Togias, A.G.; Grindle, K.G.; Bochkov, Y.A.; Gruchalla, R.S.; Kattan, M.; Kercsmar, C.M.; Khurana Hershey, G.; et al. Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald-Bocarsly, P.; Jacobs, E.S. Plasmacytoid dendritic cells in HIV infection: Striking a delicate balance. J. Leukoc. Biol. 2010, 87, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Bartleson, J.M.; Radenkovic, D.; Covarrubias, A.J.; Furman, D.; Winer, D.A.; Verdin, E. SARS-CoV-2, COVID-19 and the aging immune system. Nat. Aging 2021, 1, 769–782. [Google Scholar] [CrossRef]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef]
- Peacock, T.P.; Brown, J.C.; Zhou, J.; Thakur, N.; Sukhova, K.; Newman, J.; Kugathasan, R.; Yan, A.W.; Furnon, W.; De Lorenzo, G.; et al. The altered entry pathway and antigenic distance of the SARS-CoV-2 Omicron variant map to separate domains of spike protein. Biorxiv 2022. [Google Scholar] [CrossRef]
- McMahan, K.; Giffin, V.; Tostanoski, L.H.; Chung, B.; Siamatu, M.; Suthar, M.S.; Halfmann, P.; Kawaoka, Y.; Piedra-Mora, C.; Jain, N.; et al. Reduced pathogenicity of the SARS-CoV-2 omicron variant in hamsters. Med 2022, 3, 262–268.e4. [Google Scholar] [CrossRef]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar] [CrossRef]
- Ariën, K.K.; Vanham, G.; Arts, E.J. Is HIV-1 evolving to a less virulent form in humans? Nat. Rev. Microbiol. 2007, 5, 141–151. [Google Scholar] [CrossRef]
- Grobben, M.; Bakker, M.; Schriek, A.I.; Levels, L.J.; Umotoy, J.C.; Tejjani, K.; van Breemen, M.J.; Lin, R.N.; de Taeye, S.W.; Ozorowski, G.; et al. Polyfunctionality and breadth of HIV-1 antibodies are associated with delayed disease progression. PLoS Pathog. 2024, 20, e1012739. [Google Scholar] [CrossRef]
- Chung, A.W.; Ghebremichael, M.; Robinson, H.; Brown, E.; Choi, I.; Lane, S.; Dugast, A.-S.; Schoen, M.K.; Rolland, M.; Suscovich, T.J.; et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci. Transl. Med. 2014, 6, 228ra38. [Google Scholar] [CrossRef] [PubMed]
- Karnasuta, C.; Akapirat, S.; Madnote, S.; Savadsuk, H.; Puangkaew, J.; Rittiroongrad, S.; Rerks-Ngarm, S.; Nitayaphan, S.; Pitisuttithum, P.; Kaewkungwal, J.; et al. Comparison of antibody responses induced by RV144, VAX003, and VAX004 vaccination regimens. AIDS Res. Hum. Retroviruses 2017, 33, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Yates, N.L.; Liao, H.-X.; Fong, Y.; DeCamp, A.; Vandergrift, N.A.; Williams, W.T.; Alam, S.M.; Ferrari, G.; Yang, Z.-Y.; Seaton, K.E.; et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci. Transl. Med. 2014, 6, 228ra39. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Locke, E.R.; O’Hare, A.M.; Bohnert, A.S.; Boyko, E.J.; Hynes, D.M.; Berry, K. COVID-19 vaccination effectiveness against infection or death in a national US health care system: A target trial emulation study. Ann. Intern. Med. 2022, 175, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, E.; Morioka, H.; Kikuchi, T.; Fukushima, M. Behavioral and Health Outcomes of mRNA COVID-19 Vaccination: A Case-Control Study in Japanese Small and Medium-Sized Enterprises. Cureus 2024, 16, e75652. [Google Scholar] [CrossRef]
- Eythorsson, E.; Runolfsdottir, H.L.; Ingvarsson, R.F.; Sigurdsson, M.I.; Palsson, R. Rate of SARS-CoV-2 reinfection during an omicron wave in Iceland. JAMA Netw. Open 2022, 5, e2225320. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.K.; Burke, P.C.; Nowacki, A.S.; Gordon, S.M. Risk of coronavirus disease 2019 (COVID-19) among those up-to-date and not up-to-date on COVID-19 vaccination by US CDC criteria. PLoS ONE 2023, 18, e0293449. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Ruffin, J.; Wiegand, R.; Grant, L.; Babu, T.M.; Briggs-Hagen, M.; Burgess, J.L.; Caban-Martinez, A.J.; Chu, H.Y.; Ellingson, K.D.; et al. Protection from COVID-19 vaccination and prior SARS-CoV-2 infection among children aged 6 months–4 years, united states, september 2022–april 2023. J. Pediatr. Infect. Dis. Soc. 2025, 14, piae121. [Google Scholar] [CrossRef]
- Pérez, C.M.; Ruiz-Rius, S.; Ramírez-Morros, A.; Vidal, M.; Opi, D.H.; Santamaria, P.; Blanco, J.; Vidal-Alaball, J.; Beeson, J.G.; Molinos-Albert, L.M.; et al. Post-vaccination IgG4 and IgG2 class switch associates with increased risk of SARS-CoV-2 infections. J. Infect. 2025, 90, 106473. [Google Scholar] [CrossRef]
- Kober, C.; Manni, S.; Wolff, S.; Barnes, T.; Mukherjee, S.; Vogel, T.; Hoenig, L.; Vogel, P.; Hahn, A.; Gerlach, M.; et al. IgG3 and IgM identified as key to SARS-CoV-2 neutralization in convalescent plasma pools. PLoS ONE 2022, 17, e0262162. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, A.L.; McGregor, R.; Craigie, A.; James, A.; Charlewood, R.; Lorenz, N.; Dickson, J.M.; Sheen, C.R.; Koch, B.; Fox-Lewis, S.; et al. Comprehensive analysis of SARS-CoV-2 antibody dynamics in New Zealand. Clin. Transl. Immunol. 2021, 10, e1261. [Google Scholar] [CrossRef] [PubMed]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) naturally acquired immunity versus vaccine-induced immunity, reinfections versus breakthrough infections: A retrospective cohort study. Clin. Infect. Dis. 2022, 75, e545–e551. [Google Scholar] [CrossRef] [PubMed]
- Redman, M.; King, A.; Watson, C.; King, D. What is CRISPR/Cas9? Arch. Dis. Child. -Educ. Pract. 2016, 101, 213–215. [Google Scholar] [CrossRef]
- Gao, F.-X.; Wu, R.-X.; Shen, M.-Y.; Huang, J.-J.; Li, T.-T.; Hu, C.; Luo, F.-Y.; Song, S.-Y.; Mu, S.; Hao, Y.-N.; et al. Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice. Iscience 2022, 25, 105479. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Nathan, A.; Kaseke, C.; Berrios, C.; Khatri, A.; Choi, S.; Getz, M.A.; Tano-Menka, R.; Ofoman, O.; Gayton, A.; et al. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals. Cell 2022, 185, 1041–1051.E6. [Google Scholar] [CrossRef]
- Swadling, L.; Diniz, M.O.; Schmidt, N.M.; Amin, O.E.; Chandran, A.; Shaw, E.; Pade, C.; Gibbons, J.M.; Le Bert, N.; Tan, A.T.; et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 2022, 601, 110–117. [Google Scholar] [CrossRef]
- Dobaño, C.; Quelhas, D.; Quintó, L.; Puyol, L.; Serra-Casas, E.; Mayor, A.; Nhampossa, T.; Macete, E.; Aide, P.; Mandomando, I.; et al. Age-dependent IgG subclass responses to Plasmodium falciparum EBA-175 are differentially associated with incidence of malaria in Mozambican children. Clin. Vaccine Immunol. 2012, 19, 157–166. [Google Scholar] [CrossRef]
- Aucan, C.; Traoré, Y.; Tall, F.O.; Nacro, B.; Traoré-Leroux, T.R.S.; Fumoux, F.; Rihet, P. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect. Immun. 2000, 68, 1252–1258. [Google Scholar] [CrossRef]
- Groux, H.; Gysin, J. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: Functional role of IgG subclasses. Res. Immunol. 1990, 141, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Thau, L.; Asuka, E.; Mahajan, K. Physiology, opsonization. In StatPearls; StatPearls Publishing: Treausure Island, FL, USA, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Casillas, A.; Cowley, D.; Uversky, V.N.; Redwan, E.M.; Brogna, C.; Piscopo, M. Does SARS-CoV-2 Possess “Allergen-Like” Epitopes? COVID 2025, 5, 55. https://doi.org/10.3390/covid5040055
Rubio-Casillas A, Cowley D, Uversky VN, Redwan EM, Brogna C, Piscopo M. Does SARS-CoV-2 Possess “Allergen-Like” Epitopes? COVID. 2025; 5(4):55. https://doi.org/10.3390/covid5040055
Chicago/Turabian StyleRubio-Casillas, Alberto, David Cowley, Vladimir N. Uversky, Elrashdy M. Redwan, Carlo Brogna, and Marina Piscopo. 2025. "Does SARS-CoV-2 Possess “Allergen-Like” Epitopes?" COVID 5, no. 4: 55. https://doi.org/10.3390/covid5040055
APA StyleRubio-Casillas, A., Cowley, D., Uversky, V. N., Redwan, E. M., Brogna, C., & Piscopo, M. (2025). Does SARS-CoV-2 Possess “Allergen-Like” Epitopes? COVID, 5(4), 55. https://doi.org/10.3390/covid5040055







