Abstract
An increase in immunoglobulin G4 (IgG4) levels is typically associated with immunological tolerance states and develops after prolonged exposure to antigens. Accordingly, IgG4 is considered an anti-inflammatory antibody with a limited ability to trigger efficient immune responses. Additionally, IgG4 reduces allergic reactions by blocking immunoglobulin E (IgE) activity. In the case of COVID-19, it has been reported that the repeated administration of some vaccines induces high IgG4 levels. The latest research data have revealed a surprising IgE anti-receptor binding domain response after both natural infection and several SARS-CoV-2 vaccines. The presence of IgG4 and IgE in COVID-19 disease suggests that the virus may induce an “allergic-like” response to evade immune surveillance, leading to a shift from T helper 1 (Th1) to T helper 2 (Th2) cells, which promotes tolerance to the virus and potentially contributes to chronic infection. The spike protein from vaccines could also induce such a response. Interestingly, “allergen-like” epitopes and IgE responses have been reported for other viruses, such as influenza, human immunodeficiency virus (HIV), and respiratory syncytial virus (RSV). The impact of this viral-induced tolerance will be discussed, concerning long COVID and the protective efficacy of vaccines.
Keywords:
SARS-CoV-2; influenza; HIV; RSV; IgG4; IgE; COVID-19 vaccines; immune evasion; immune tolerance; long COVID 1. Introduction
Attempts to rapidly contain the COVID-19 pandemic were limited by a lack of precise understanding of SARS-CoV-2–host interactions, particularly regarding viral biology and host immune response. All approved COVID-19 vaccines, including mRNA, adenoviral vector-based, inactivated, and protein-based platforms, have demonstrated efficacy in preventing severe disease, hospitalization, and death, though their immunological profiles may differ [1,2,3,4,5]. However, emerging data suggest that repeated vaccination may have unexpected consequences. One of these is the increased production of IgG4 antibodies, a subclass of immunoglobulins traditionally associated with immune regulation and tolerance. IgG4 antibodies are unique among the IgG subclasses due to their ability to undergo Fab-arm exchange, resulting in bispecific antibodies that are functionally monovalent. They have a lower avidity for antigens when compared with other IgG subclasses [6,7,8,9]. This property allows IgG4 to act as an anti-inflammatory agent by blocking immune complex formation and reducing Fc-mediated effector functions, such as antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement activation [6,10,11]. For example, IgG4 is sometimes referred to as a protective blocking antibody because it can inhibit or stop inflammation in allergies by competing with inflammatory IgE for antigen binding [12,13,14,15]. It is also implicated in certain autoimmune diseases, including IgG4-related disease, which is characterized by inflammation and fibrosis in various organs [16,17,18].
An increase in IgE production is often associated with natural exposure to a relevant allergen. IgG4 appears to inhibit IgE via various routes. First, through direct competition, IgG4 can prevent IgE from binding to allergens in serum. The second and most important mechanism is through direct suppression of IgE-mediated mast cell activation (Figure 1). IgE causes mast cells to degranulate when interacting with the high-affinity Fc epsilon I receptor (FcϵRI). On the other hand, IgG4 inhibits the aforementioned effects during both the initiation and effector phases of allergic immune responses by binding to the inhibitory Fc gamma IIb receptor (FcγRIIb) [19].
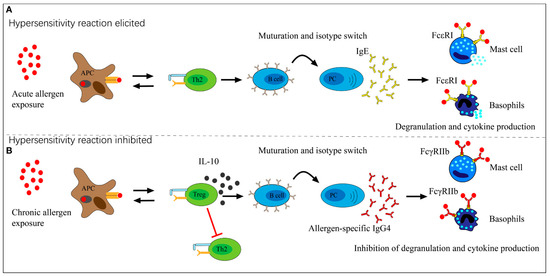
Figure 1.
The function that allergen-specific IgG4 plays in exposure to allergens. (A) Acute allergen exposure may cause IgE-mediated histamine-induced hypersensitivity reactions through the high-affinity receptor FcϵRI. (B) Long-term exposure to allergens may cause the development of IgG4, which then binds to the allergen. IgG4 then binds to the inhibitory receptor FcγRIIb located on mast cells and basophils, thus limiting hypersensitive reactions and promoting allergy tolerance. Source: [19]. This is an open-access article and distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Clinical improvement has been associated with increases in IgG4 levels, according to data from several studies [20,21]. The protection provided by treatment correlates with increased anti-venom IgG levels in venom allergy, at least at the onset of desensitization [22,23]. IgG4-blocking antibodies appear to block several allergic responses, for example, IgE-facilitated allergen presentation to T cells; allergen-induced increases in memory IgE synthesis following high allergen exposure during the pollen season; and allergen-induced release of inflammatory mediators from mast cells and basophils. Grass pollen immunotherapy leads to a significant increase in allergen-specific IgG4 antibodies. These are crucial for modulating allergic responses by blocking IgE-facilitated allergen binding to B cells, T cells, mast cells, and basophils [24,25]. Both total and allergen-specific IgE levels are strongly suppressed by interleukin 10 (IL-10), which also causes an increase in IgG4 synthesis [26]. Therefore, in addition to producing tolerance in T cells, IL-10 also controls the generation of specific isotypes and skews the specific response from an IgE- to an IgG4-dominated phenotype. During allergen-specific immunotherapy (AIT), there is an initial rise in IgE levels, which then decline, followed by a sustained increase in specific IgG4 levels [27].
Although this regulatory role is beneficial in conditions like allergies, it may be counterproductive in the case of viral infections, where robust and sustained immune responses are required for protection and viral clearance [28]. In the context of the vaccination campaign against SARS-CoV-2, scientists have demonstrated that the antibody subclasses IgG1 and IgG3 immediately dominated the IgG response following the first two mRNA injections [29]. Nonetheless, an unanticipated long-term side effect has been reported following the second mRNA vaccine injection: a change in the isotype of IgG antibodies towards IgG4 [29]. Several human investigations [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45] and at least one animal study [46] have now reported the phenomenon of increased IgG4 antibodies following mRNA vaccination. In comparison to other spike-specific IgG antibodies, IgG4 antibodies increased, on average, from 0.04% soon after the second vaccination to 19.27% late after the third [29]. A further study confirmed this, showing that the median level of IgG4 antibodies against the spike protein was 21.2% of total IgG antibodies [34]. On the other hand, this phenomenon has not been documented in unvaccinated individuals [31,32,38,40,43].
Numerous studies have shown that mRNA-based vaccines (Pfizer/BioNTech or Moderna) and inactivated vaccines such as CoronaVac and BBIBP-CorV cause this response [29,30,31,33,35,38,47], whereas recipients of adenoviral vector-based or protein-based vaccines did not produce such an increase in IgG4 concentrations [30,42]. IgG4 levels slightly increased [30,33,39] or not at all [29,35] when heterologous SARS-CoV-2 vaccines were used (i.e., administration of mRNA and adenoviral vector vaccines).
It has been proposed that an increase in IgG4 levels may protect against immunological overactivation, similar to what occurs after successful AIT by suppressing IgE-induced effects [29]. In contrast, other authors proposed that such an increase may not be a mechanism of protection but rather an immunological tolerance mechanism to the spike protein that, by inhibiting natural antiviral responses, may encourage unopposed SARS-CoV-2 infection and replication [28]. In this work, we expand on the previous hypothesis and argue that SARS-CoV-2 presents “allergen-like” epitopes, which induce IgE synthesis to evade the immune system during acute disease. To persist within the host, the virus “mimics” an allergen and promotes tolerance through IgG4 antibodies, possibly leading to chronic infection. Experimental evidence shows that other viruses, such as influenza A [48] and RSV [49], may also use IgE to suppress immune responses. Thus, in this work, we focus on IgE epitopes due to their potential role in driving immune tolerance, a mechanism that may compromise long-term vaccine efficacy and facilitate viral persistence. This has been observed with several viruses.
2. Evidence of the Involvement of IgE Antibodies in COVID-19 and in Vaccination
Only limited studies have investigated the role of IgE antibodies and their association with disease severity. One study assessed how changes in inflammatory markers and basal serum total IgE levels affected the clinical development of COVID-19 patients after hospital admission. Clinically deteriorated COVID-19 patients had considerably lower serum eosinophil levels (0.015 [0–1.200] vs. 0.040 [0–1.360], p = 0.002) and significantly higher serum total IgE concentrations (172.90 [0–2124] vs. 38.70 [0–912], p < 0.001). That study found that high IgE levels could be used as a predictor of negative clinical outcomes in severe COVID-19 [50]. Regarding the role of eosinophils in COVID-19, it has been reported that the disease was less severe in allergic individuals with high blood eosinophil numbers [51]. Conversely, low blood eosinophil counts are considered a potential indicator of disease progression. Such a reduction was often observed in individuals who passed away from COVID-19 [52]. A study analyzed the medical records of 85 fatal cases of COVID-19 and observed that 81% of the patients had blood eosinophil numbers below the normal level (absolute eosinophil counts < 0.02 × 109 cells/L) at the time of admission [53]. During respiratory viral infections, eosinophils are known to have a role in the immune response that facilitates cytokine release and the recruitment of CD8+ T cells. Additionally, the virus can be neutralized by enzymes produced by eosinophils [54].
Another study found that IgE antibodies produced against the SARS-CoV-2 N protein were detected in 100% of patients with severe disease, 92.6% with moderate disease, and 66.7% with mild disease [55]. Furthermore, a recent study discovered an unusual IgE anti-receptor binding domain response after spontaneous infection and receiving different SARS-CoV-2 vaccines [45]. Following SARS-CoV-2 infection, samples from 59 subjects were evaluated after primary vaccination with vectored (ChAdOx1), inactivated (CoronaVac) vaccines, and booster vaccination with mRNA (BNT162b2) vaccine. IgE was naturally induced by SARS-CoV-2 infection, but immunization increased its levels. All groups showed comparable IgE levels following boosting with BNT162b2, but those who received two doses of ChAdOx1 showed a more robust response than those who received two doses of CoronaVac. Particularly following the booster vaccination, IgE exhibited moderate to high avidity. Additionally, they detected IgG4 antibodies, primarily after the booster, which showed a moderate correlation with IgE [45]. Th2 cytokines facilitate class-switching to IgG4 and IgE antibodies, encoded in the Ig locus’s upstream area, and B cells require sustained antigenic stimulation for this to occur [56,57].
Although it is well known that aluminum hydroxide creates a potent Th2 milieu facilitating this response [58], ChAdOx1, which does not contain aluminum, produced significantly greater IgE and IgG4 levels than CoronaVac, an alum-adjuvanted vaccine. The researchers suggested that the active immunogen (the spike protein) triggers such IgE responses [45]. When an IgE-coated antigen binds to mast cell FcεRI receptors, the IgE-FcεRI complex is cross-linked, which activates mast cells and releases histamine along with several pro-inflammatory chemokines and cytokines, such as interleukin 6 (IL-6), interleukin 1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α) [59,60]. Mast cell activation syndrome may be connected to the development of the cytokine storm and hyper-inflammation, as several studies have shown that mast cells are activated in patients with severe COVID-19 [60,61,62]. Another study supported the use of IgE-blocking drugs, such as omalizumab for treating COVID-19 by indicating a link between mast cell activation and high antibody levels in severe COVID-19 cases [63].
An inevitable question arises: What purpose would it serve for a virus to have an allergenic epitope? This is highly unusual in a viral infection. It is supposed that, upon contact with a virus, the immune system would produce only IgG1 and IgG3, which are the main antiviral antibodies [64,65,66,67]. A plausible explanation is that the virus possesses an epitope that triggers an “allergic-like” response to evade the immune system during acute disease, thus contributing to its severity and potentially facilitating chronic infection. There is evidence that viral protein antigens can elicit IgE responses. Examples include infection by measles [68,69,70], rhinoviruses [71,72,73,74,75], influenza [48,76,77], HIV [78,79,80,81,82,83,84,85,86,87], RSV [88,89,90], varicella zoster infections [91], hepatitis B [92], measles–mumps–rubella [93] and influenza vaccinations [94]. In COVID-19, several investigations have described the presence of IgE antibodies after a spontaneous infection with SARS-CoV-2. Seric IgE was reported to correlate with the severity of COVID-19 infection, which likely contributed to hyper-inflammation [50,55,95,96].
In another study, the serum levels of anti-SARS-CoV-2–spike S1 protein-specific IgE (SP-IgE) and anti-SARS-CoV-2 nucleocapsid protein-specific IgE (NP-IgE) in COVID-19 were examined. The PaO2/FiO2 ratio is a measure of the partial pressure of oxygen in arterial blood (PaO2) compared to the fraction of inspired oxygen (FiO2). Serum SP-IgE and NP-IgE levels were considerably higher in severe cases and were associated with the PaO2/FiO2 ratio and total lung severity ratings.
Furthermore, as the levels of anti-SARS-CoV-2-specific IgE antibodies were substantially higher in patients who had hypoxemic respiratory failure than in those who did not, it was concluded that the severity of COVID-19 at admission and its progression are related to IgE levels. Also, compared to specific SP-IgE, the blood level of specific NP-IgE showed a higher correlation with lung damage and hypoxemia [95].
Interestingly, other studies discovered that lethal COVID-19 was associated with high IgG4 levels [41,97,98,99]. Specifically, it was reported that high serum IgG4 concentrations (>700 mg/dL) were associated with a considerably higher 30-day death rate and were significantly correlated with IL-6 levels [97].
Another study found that patients who passed away between 8–14 and 15–21 days also had higher levels of anti-RBD IgG4 compared to those who recovered (p < 0.05). This indicates that certain patients in critical condition developed an IgG4-to-RBD antibody response during the initial weeks after the appearance of symptoms. The investigation also revealed that over 50% of blood samples from deceased patients exhibited positive IgG4 antibody tests. Conversely, the majority of recovered patients exhibited negative IgG4 antibody test results within the same period [98]. On the other hand, in mild COVID-19 cases, strong IgG1 and IgG3 antibody responses targeting the RBD predominated, while IgG4 levels remained low [66,67].
Therefore, it is hypothesized that in severe COVID-19 pneumonia, SARS-CoV-2 induces IgG4 synthesis in an IgE-independent manner, inhibiting IgG3 binding to its Fc receptor (Figure 2), thus impairing viral phagocytosis [100].
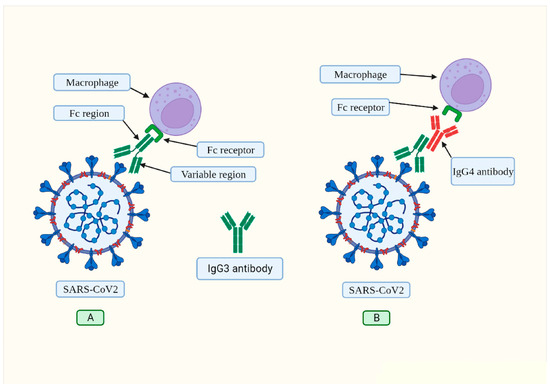
Figure 2.
(A) The IgG3 antibody typically binds to spike protein via its variable region. The corresponding receptor on macrophages and other immune cells recognizes the constant region (Fc) of this antibody. This process, known as “opsonization,” identifies foreign pathogens so that phagocytes can destroy them. (B) The presence of SARS-CoV-2 has been observed to trigger the production of IL-6, which in turn prompts a shift in the typical phenotype of B cells, resulting in the production of IgG4 antibodies (illustrated in red). The Fc region of the IgG4 antibody interacts with the Fc region of IgG3, hindering its ability to bind to its receptor on macrophages. As a result, IgG3 effector functions are inhibited. Source: [100]. This article is open access and is distributed under the Creative Commons Attribution Non-Commercial (CC BY-NC 4.0) license, which enables others to distribute, remix, adapt, and build upon it for non-commercial purposes and to license their derivative works under different conditions as long as the original work is properly cited, duly credited, any changes are noted, and the use is for non-commercial purposes.
According to a recent study, the fatal outcome of severe COVID-19 was correlated to significantly increased IgG4 levels in lung tissue. The acquisition of autopsy materials took place between 28 February 2020, and May 2022. IgG4 expression peaked at 80–90% in acute stages (Figure 3), whereas in the organization and fibrosis stages, it decreased to 5–10% [99]. It would be interesting to investigate the presence of IgE antibodies in lung samples from patients who died of severe pneumonia and who also showed high levels of IgG4. This would help determine whether death resulted from elevated IgE levels, and whether the increase in IgG4 was an attempt by the immune system to minimize IgE effects, or if IgG4 independently contributed to the inhibition of phagocytosis of virus-infected cells.
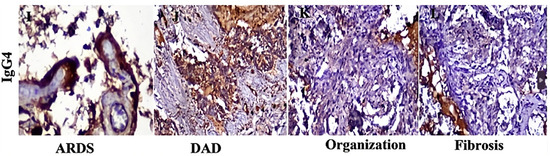
Figure 3.
Immunochemical staining of lung tissues. In the acute phase (ARDS and DAD), IgG4 was intensely expressed in lung tissue, reaching 80–90%, but was essentially absent during the stages of organization and fibrosis. ARDS (acute respiratory distress syndrome), DAD (diffuse alveolar damage). Modified from [99]. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
In addition to high IgE and IgG4 levels, several studies have reported the presence of autoantibodies, such as antiphospholipid antibodies, antinuclear antibodies (ANAs), and anti-cytoplasmic neutrophil antibodies (ANCAs). Fifteen out of thirty-three patients (45%) had at least one autoantibody test positive, including eleven with ANAs (33%), eight with anti-cardiolipin antibodies (immunoglobulin (Ig)G and/or IgM; 24%), and three with anti-β2-glycoprotein antibodies (IgG and/or IgM; 9%). ANCA reactivity was not found in any patient. The prognosis for patients who tested positive for auto-antibodies was significantly worse than that of other patients; only one out of eighteen patients (5.5%) who did not have auto-antibodies died (p = 0.03), whereas six out of fifteen patients (40%) with auto-antibodies passed away as a result of COVID-19 complications [101,102,103].
Does SARS-CoV-2 Possess “Allergen-Like” Epitopes?
To date, several works have investigated this interesting topic. Heterologous immunity mediated by virus-induced T cells has been shown to either promote immunopathology or provide protection against different antigens [104,105,106,107]. The shared characteristics between the SARS-CoV-2 proteome and some aeroallergens may offer a protective benefit to individuals sensitized to such allergens, potentially mitigating the risk of an excessive Th1 response and the subsequent cytokine storm. In contrast, these heterologous immune responses may have a negative impact by weakening the antiviral response. Due to reduced CD8+ responses, a Th2 immune skew may result in insufficient viral clearance [104].
This can be exemplified by findings from a large population-based cohort investigation, which discovered that adults with non-allergic asthma had a higher risk of severe COVID-19. In contrast, the risk of severe COVID-19 was not found to be significantly increased in patients with allergic asthma [108]. A large-scale study utilizing the Optum database identified over a million patients with allergic conditions and over two million without. The analysis showed that allergic conditions were associated with an increased risk of receiving a COVID-19 diagnosis but reduced mortality after infection [109]. From the immunological perspective, the differential risk of severe COVID-19 in individuals with non-allergic versus allergic asthma can be attributed to the distinct immune pathways underlying these conditions [108,110,111]. As mentioned above, COVID-19 was less severe in allergic people with elevated blood eosinophil counts [51]. Conversely, low eosinophil counts have been identified as a negative disease predictor. In people who died from COVID-19, such a decrease was frequently seen [52]. Eosinophils have been found to contain and produce molecules with antiviral activity, including RNases and reactive nitrogen species. Furthermore, eosinophils have the capacity to participate in adaptive immunity, functioning as antigen-presenting cells. The eosinophil antiviral response has been demonstrated against various respiratory viruses in vitro and in vivo, including RSV and influenza [112,113,114,115,116].
These viruses induce an “allergic-like” response that counteracts antiviral defenses, potentially facilitating viral persistence. This suggests that certain viral epitopes could trigger IgE production, not to cause typical allergic symptoms in the traditional sense, but rather as a part of a viral strategy to subvert the host’s defense mechanism. For example, infection with Ascaris lumbricoides (a large parasitic roundworm) has been shown to induce high total IgE levels. It should be noted that the majority of these antibodies are not specific [117], resulting from polyclonal B cell activation [118]. This nonspecific IgE binds to its receptors on mast cells and eosinophils, preventing their degranulation and reducing the effector response against larvae [119]. Although helminths and viruses are not phylogenetically related, they have likely evolved similar immune evasion mechanisms through convergent evolution. Our comparison to parasitic infections, such as Ascaris lumbricoides, is primarily immunological rather than symptomatic. We hypothesize that both SARS-CoV-2 and certain parasites may exploit “allergen-like” epitopes to induce a Th2-skewed response, characterized by elevated IgE and immune tolerance, as a means of evading host defenses. Direct symptomatic overlap is limited, and our work does not claim that SARS-CoV-2 mimics parasitic disease clinically. Rather, the similarity lies in the immunological strategy; both may leverage IgE to subvert Th1-mediated clearance.
An in silico study investigated potentially reactive T cell epitopes between allergens and SARS-CoV-2. Regardless of the bioinformatic method used, allergen sources including Aspergillus fumigatus, Phleum pratense, and Dermatophagoides species were particularly noteworthy because they correlated with several cross-reactive peptides. A major candidate was found to be the fungus Aspergillus fumigatus [104]. Notably, an amino acid region in the HIV-1 gp41 heptad repeat 2 (HR2) protein also resembles the allergen domain in the Aspergillus fumigatus Asp f1 protein [120]. This fungus employs multiple sophisticated strategies to evade the human immune system, particularly in immunocompromised individuals [121,122,123,124]. Intriguingly, Aspergillus fumigatus can indeed induce a Th2 shift after chronic exposure. Such a shift is associated with the production of cytokines such as IL-4, IL-5, and IL-13, contributing to disease progression [125,126,127,128]. IL-4 and IL-13 suppress protective Th1 responses, creating a permissive environment for fungal colonization [125,129].
Many viruses have developed sophisticated immune evasion techniques, and SARS-CoV-2 is no exception [130]. In this work, we hypothesize that during its evolution, this virus has acquired epitopes in the N and Spike proteins that, acting as allergens, induce the production of IgE antibodies to impair antiviral defenses. In respect of the Spike protein, a galectin-3 (Gal-3)-like fold has been identified within the N-terminal domain (NTD) of SARS-CoV-2 [131]. Indeed, Gal-3 was originally identified as an IgE-binding protein [132]. The “galectin-fold” of the S1-NTD region of the spike protein has a structural homology almost identical to that of human Gal-3. This supports our hypothesis that signaling pathways promoted by binding to antigenic sites on the NTD would likely correlate with signaling pathways and pathologies caused by human Gal-3 [133]. Further significant experimental support can be found through research published in 2022. The authors sought to test the hypothesis that S1-NTD is specifically responsible for a pattern of cytokines and innate immune mechanisms that match those of Gal-3. The authors used microtiter wells to immobilize S components and confirmed that S1-NTD produced a near-identical cytokine pattern to that of Gal-3 through activation of human monocytes. In contrast, ACE2-binding S1-CTD/RBD failed to trigger the same response. Researchers postulated that the spike protein’s S1-NTD would thereafter function similarly to epithelial cell-Gal-3, activating innate immune cells, including monocytes and macrophages that invade virally infected lesion sites. While it is currently uncertain whether such a mechanism has a role in the cytokine storm frequently observed in severe COVID-19, the theory is plausible and warrants further investigation [133].
Gal-3-induced IgE may also contribute to post-acute COVID-19 syndrome in children, adolescents, and adults [134,135]. This and other research support the hypothesis that multiple “allergen-like” epitopes on SARS-CoV-2 are implicated [136,137]. Since allergies are associated with a switch from Th1 to Th2 response, and Th2 induces immune tolerance [138], it is proposed that SARS-CoV-2 induces a Th2 response to promote tolerance. Such a response would be evolutionarily advantageous for the virus since the immune system would ignore it instead of attacking it. During the acute phase of COVID-19, evasion of the immune system would enable rapid viral replication. In the long term, this could lead to the establishment of a chronic infection. While chronic SARS-CoV-2 infections have been well documented in immunocompromised individuals, there is emerging evidence suggesting that viral persistence may also occur in immunocompetent individuals, though it is less frequently reported. Some studies have detected SARS-CoV-2 RNA or proteins for months after acute infection in immunocompetent hosts, including in tissues such as the gut, brain, and lungs [139,140,141,142,143].
Considering the limited information on the mechanism mediating the induction of IgE antibodies by HIV and RSV, the following sections will describe how they have evolved an immune evasion strategy by incorporating “allergen-like” epitopes.
3. Allergen-like Epitopes in HIV Envelope Proteins
It is well known that no effective vaccine has been produced against HIV. In 2004, it was suggested that the steady rise in IL-4 and IgE levels during HIV-1 infection, as well as the stimulation of Th2 cytokine generation, could be an allergic reaction to HIV-1 proteins [144]. For example, one study found that the HIV-1 gp41 HR2 protein contains an amino acid domain similar to the allergen domain in the fungus Aspergillus fumigatus Asp f1 protein [120]. The same author expanded on this theory in a later review, citing experimental evidence that shows gp120 proteins released by HIV-1 resemble allergens, which bind to IgE molecules attached to FcεRI cell receptors (mast cells, basophils, monocytes, and dendritic cells (DCs)) and cause them to produce and release IL-4. This cytokine was shown to induce the following responses: the inactivation of Th1 cells, the inhibition of the antiviral cytotoxic T lymphocyte (CTL) response, the synthesis of IgE by B cells, and the inhibition of antiviral IgG3 synthesis [144]. It was discovered that whereas CD4+ Th1 cells did not effectively support HIV replication, CD4+ Th2 and the majority of Th0 cells did. These findings indicated that HIV replication preferentially occurs in T cells that produce Th2-type cytokines [145], given that interferon-gamma (IFN-γ) has been demonstrated to inhibit HIV infection of Th1 cells [146].
This is highly relevant, since the Th1 and Th2 pathways are mutually inhibitory; IFN-γ restricts TH2 cell growth, while IL-4 and IL-10 preferentially promote TH2 cell growth and inactivate Th1 cells [147,148]. These results imply that HIV-1 attacks the human immune system in two ways; first, it infects Th2 cells, macrophages, and DCs, where it then replicates, and second, the gp120 proteins it sheds trigger an allergic reaction. As a result, this “allergic-like” context inhibits Th1 cell differentiation, cytokine release, and antiviral CTLs, which are essential to destroy virus-infected cells [149]. The theory that HIV-induced acquired immune deficiency syndrome (AIDS) is an allergy and that gp120 is the allergen was proposed. Thus, it can be concluded that the ongoing replication of HIV-1 in infected individuals continuously exposes the immune system to an increase in allergen concentration until immune tolerance is achieved, ultimately compromising host immunity [149]. At the time of its formulation, this theory was probably considered to have no logical basis. However, considering the recent discoveries referenced in this review, there is a strong probability that the hypothesis is accurate, although the statement that AIDS is an allergy is an oversimplification. In our opinion, such a hypothesis can be reformulated as follows. The induction of a Th1 to Th2 switch during HIV infection mimics the human immune system’s response to environmental allergens. When the immune system recognizes an allergen as a harmless particle rather than a pathogen, it develops a tolerance response to ignore it. HIV has probably developed a similar mechanism through convergent evolution. HIV hijacks the immune system into inducing a tolerance response (Th2) instead of an antiviral response (Th1) via the gp120 protein (which has allergen-like epitopes).
Several works have evaluated IgE levels and their association with disease severity. A study revealed that in the initial phases of HIV-1 infection, there is an increase in plasma IgE levels before a decrease in CD4+ cell levels [82]. Furthermore, it is noteworthy that the degree of immunodeficiency (as determined by the CD4+ cell counts) was significantly correlated with the IgE concentration, i.e., the higher the concentration of IgE antibodies, the lower the CD4+ count [85]. It was suggested that IgE overproduction during HIV infection may be associated with CD4+ cell depletion and their decreased ability to produce interferon (IFN) [85].
In a particular study, the IgE levels of 315 HIV-seropositive patients, with or without AIDS, were measured, and the results were then compared to those of 100 HIV-seronegative participants. The research found that HIV-infected patients had higher IgE levels compared to the seronegative control subjects (p < 0.05). This difference was particularly pronounced between the AIDS patients and the control volunteers (p < 0.005). Furthermore, survival analysis revealed a 24-month incidence rate of AIDS of 83% in subjects with IgE > 150 KIU/L versus 44% in those with IgE < 150 (p = 0.016).
Researchers also evaluated the prognostic value of IgE elevation during the progression of the illness. Analysis revealed that individuals with IgE levels greater than 150 had a 100% risk of developing AIDS, while those with IgE levels less than 150 had a 9% risk (p = 0.003). These findings suggest that IgE levels may serve as a highly selective indicator among patients in the later stages of HIV infection [85].
In another study, the total blood IgE levels of 18 uninfected homosexual male participants, 27 uninfected heterosexual male subjects, and 67 infected male subjects were examined. Analysis revealed that participants with a helper T-lymphocyte number ≤200/mm3 exhibited a mean IgE level of 132 IU/mL, which was significantly higher than the mean IgE levels observed in the uninfected heterosexual (38 IU/mL) and homosexual (35 IU/mL) groups. Furthermore, the study identified a negative correlation between IgE levels, helper T cells, and suppressor/CTL counts. However, these correlations were not observed for IgG or IgA levels [78].
In research involving children, persistent elevation of IgE was significantly associated with a substantial decrease in CD4+ counts (≥30% over one year) and an increased susceptibility to bacterial infections. A significant reduction in CD4+ cell count appears to be associated with prolonged IgE hyperproduction, suggesting that this clinical test (as in adults) serves as a valuable indicator of the progression of the disease [150].
4. The Role of IgE in RSV Infection
It is well known that reinfection is common and immunity to RSV is not optimal. Relative protection is associated with RSV-specific IgG, IgM, and IgA antibodies. One study showed an association between wheezing and hypoxia in RSV infection and histamine and RSV-specific IgE levels in nasopharyngeal secretions [90]. There is experimental evidence that allergic inflammation can interfere with antiviral responses [151,152,153,154], and observational investigations have shown strong correlations between allergy sensitization and the incidence of acute virus-induced wheezing [155,156,157]. The mechanisms by which IgE antibodies against RSV modulate the inhibition of the immune response have been described. Uncommon blood immune cells known as plasmacytoid dendritic cells (pDCs) have been shown to release significant amounts of type 1 IFN in response to exposure to virus-derived nucleic acids that are identified by toll-like receptors 7 and 9 [158].
pDCs generate significant levels of type I IFN, primarily IFN-α and IFN-β. They are essential for producing antiviral molecules, up to 1000 times more than any other white blood cell type [159]. Purified pDCs were exposed to influenza viruses, and IFN-α production was measured to assess their function in asthma patients and controls. Flow cytometry was used to assess the expression of FcεRIα on pDCs in blood samples from both controls and patients with allergic asthma. When exposed to the influenza A virus, pDCs from asthmatic patients secreted significantly less IFN-α (572 versus 2815; p = 0.03), and secretion was inversely associated with blood IgE levels. These findings demonstrated that IgE binding reduces antiviral responses by pDCs [151]. Notably, anti-IgE therapy reduces viral exacerbations in asthma by downregulating the high-affinity IgE receptor FcεRI on pDCs, thus improving antiviral immunity [160,161,162]. In HIV infection, pDCs not only lose their ability to secrete IFN but also undergo apoptosis, which accelerates disease progression [163], and age-related decreases in pDCs counts have been linked to higher COVID-19 severity [164].
Another study found that, in contact with an allergen, IL-33 reduced the expression of the genes for viperin and interferon regulatory factor 7. It also quickly reduced the expression of IL-1 receptor-associated kinase 1 in pDCs, both in vivo and in vitro. This resulted in reduced IFN-α production and hypo-responsiveness of TLR7 [153]. All these data suggest that by shifting the balance of Th1/Th2 cytokines towards elevated levels of Th2 cytokines and IgE (both of which are indicators of allergy), RSV evades the human adaptive immune system [89].
5. Repeated Vaccination with Allergen-like Epitopes
The presence of “allergen-like” epitopes in some viruses raises an intriguing possibility; they may use such epitopes to induce immune tolerance, allowing them to persist in the host. The immunological effects of repeated immunization with viral antigens that share structural similarities to allergens require thorough investigation. In certain situations, vaccine-induced immunity may shift from a strong protective immunity to a state resembling immunological tolerance. Allergenic viral epitopes may preferentially stimulate a Th2-skewed immune response, which results in the generation of IgE and IgG4 antibodies. For strong antiviral immunity, a Th1 response—which is defined by the generation of IFN-γ and the activation of CTLs—is usually necessary.
A shift toward a Th2 response, promoting immune tolerance, could explain why HIV and RSV vaccines have failed to prevent infection and disease progression. It is important to note that the consequences of immune tolerance induction exhibit variations depending on the pathogen. In the context of SARS-CoV-2, it has been documented that emerging variants exhibit reduced pathogenicity compared to the original lineage [165,166,167,168]. However, in the case of HIV, no comparable reduction in pathogenicity has been observed, as the virus continues to evade immune control and drives disease progression [169].
According to a recent study, delayed disease progression was attributed to low IgG2 and IgG4 levels and high levels of HIV-1-specific IgG1 three years after seroconversion. In particular, there was a significant correlation between delayed disease progression and the ability to interact with all Fcγ receptors (FcγRs) and complement component C1q, specifically with FcγRIIa [170]. Since AIDS progression is also associated with high IgE levels [85,150], the combined elevated presence of IgE and IgG4 antibodies supports the hypothesis that “allergen-like” proteins in the HIV envelope induce a state of tolerance to establish chronic infection.
A study was conducted to compare the efficacy of repeated immunization with similar HIV vaccines in a trial scenario. The protection (31.2%) provided by one vaccine (RV144) was attributed to the production of IgG1 and IgG3 antibodies, whereas the protection achieved by the other vaccine (VAX003) was found to be negligible and was linked to the production of IgG4 antibodies after multiple vaccinations [171]. Because VAX003 was associated with higher levels of IgG4, which are typically associated with lower functionality, scientists sought to determine whether the induction of these antibodies was simply associated with a poorly coordinated functional response or if it contributed directly to it. To determine that, IgG4 antibodies were selectively removed from 16 samples from the two vaccine trials (VAX003 and RV144) to assess their impact on effector functions [171].
In VAX003, the removal of IgG4 antibodies resulted in a significant increase in ADCP, suggesting that IgG4 may competitively inhibit Fcγ receptor-mediated phagocytosis. Additionally, a tendency toward increased ADCC was discovered, indicating that IgG4 may dampen natural killer cell-mediated cytotoxicity. In RV144, no significant difference was observed between total and IgG4-depleted samples. This is likely due to the inherently low levels of IgG4 in RV144-induced immune responses [171].
Regarding the relevance of the elevated levels of IgG4 antibodies detected in the failed HIV trials, it has been suggested that the shift in the IgG subclass from fully efficacious antibodies (IgG3) to IgG4 would be a significant barrier to the effectiveness of HIV vaccines [172]. Indeed, in the RV144 trial, high IgG3 levels were associated with a lower risk of infection [173], while IgG4 production was directly associated with reduced efficacy in protecting people from being infected with HIV. Moreover, it favored breakthrough infections [171].
The induction of tolerance to a virus and enhancement by repeated vaccination is not recommended, as this may result in the immune system ignoring the pathogen, thereby facilitating reinfection and unopposed replication [28]. Indeed, in COVID-19, several studies demonstrated that the more vaccines a person receives, the greater the likelihood of acquiring SARS-CoV-2 [174,175,176,177,178,179,180]. Depletion assays were used in a study with convalescent participants, and results showed that IgG4 had little to no impact on SARS-CoV-2 neutralization [181], thus confirming similar results from other studies [66,182]. Other research evaluated IgG4 levels in healthy and immunocompromised patients who received three mRNA vaccines and showed that they peaked suddenly, remained elevated for a long time, and had little effect on neutralizing capacity [40]. Furthermore, a decline in the capacity of spike-specific antibodies to stimulate ADCP and complement deposition was also observed, coinciding with a class switch to IgG4 antibodies [29,33]. Although the virus is now considered less dangerous, it can still lead to chronic inflammation and severe damage or death in adults with comorbidities and immunosuppressed individuals [183].
6. Implications for Vaccine Design
A new pandemic virus is likely to emerge in the future. In such a scenario, it is suggested that virologists should analyze its genetic sequence to find “allergen-like” epitopes. Such identification requires a combination of bioinformatics, structural analysis, and immunological validation. Some useful tools are:
- AlgPred 2.0 (https://webs.iiitd.edu.in/raghava/algpred2/), which uses multiple machine learning approaches to predict allergen-like sequences (accessed on 25 February 2025).
- AllergenFP (http://ddg-pharmfac.net/AllergenFP/), which uses a descriptor-based fingerprint to detect potential allergens (accessed 25 February 2025).
- BLASTp (https://blast.ncbi.nlm.nih.gov/Blast.cgi), which finds regions of similarity between biological sequences. The program compares nucleotide or protein sequences to the sequence database and calculates the statistical significance (accessed on 25 February 2025).
- AlphaFold (https://swissmodel.expasy.org/) predicts protein structures for docking studies (Accessed on 25 February 2025).
- Molecular docking (Autodock, HADDOCK) (https://rascar.science.uu.nl/haddock2.4/) (accessed on 25 February 2025).
If such epitopes are identified using these tools, they should be modified or excluded from vaccines. A gene-editing technique known as CRISPR-Cas9 [184] could be utilized to selectively delete or modify an “allergen-like” epitope from a virus or a bacterium, thereby preventing a Th2-skewed response and redirecting it toward a Th1-dominant response. Beyond epitope modification, additional measures to enhance vaccine efficacy could include:
- -
- Optimizing vaccination schedules to avoid excessive boosting, which may induce tolerance and immune exhaustion.
- -
- Incorporating adjuvants that enhance Th1 responses (e.g., CpG oligodeoxynucleotides) rather than Th2-skewing agents like aluminum hydroxide.
- -
- Heterologous vaccination strategies might also mitigate IgG4 induction, as they have demonstrated only slight or no increases in IgG4 levels.
- -
- Comprehensive assessment of the route of administration for vaccines: intramuscular vs. nasal route.
7. Conclusions
A comprehensive review of the existing literature suggests that certain viruses, such as influenza, HIV, RSV, and SARS-CoV-2, possess “allergen-like” epitopes. These viruses induce an “allergic-like” response that counteracts antiviral defenses, potentially facilitating viral persistence. The proposed viral-induced immune tolerance mechanism may be a key factor contributing to the failure of vaccines targeting these viruses, as it leads to the induction of a Th2 rather than a Th1 response.
Recent work demonstrated the appearance of tolerance induction and non-inflammatory SARS-CoV-2 spike-specific IgG4 antibodies after COVID-19 booster vaccinations. The study also discovered that individuals who received mRNA for both the initial and booster doses had higher serum anti-inflammatory IL-10 levels following each vaccination treatment [39]. This aligns with research showing that prolonged immunization with SARS-CoV-2 RBD boosters (more than four doses) generated IL-10 responses that ultimately caused humoral and cellular immunological tolerance in mice [185]. Furthermore, the study discovered that extended administration of RBD booster vaccinations significantly elevated the levels of the Programmed Cell Death Protein 1 (PD-1) and the Lymphocyte Activation Gene 3 (LAG-3, also known as CD223), accompanied by a considerable decline in memory CD8+ T cells. PD-1 and LAG-3 activation is commonly associated with T-cell exhaustion [185]. This is especially significant because it has been demonstrated that memory CD8+ T cell responses are crucial for a successful defense against new SARS-CoV-2 variants. These may significantly compromise humoral immunity due to the accumulation of neutralization escape mutations [186,187,188].
Several HIV vaccine trials showed no protection against infection, and that was associated with high IgG4 levels. In addition, vaccines against pertussis and malaria have also been shown to increase IgG4 production beyond normal levels (for review, see ref. [28]). For instance, research demonstrated that a decreased risk of malaria in the second year was associated with higher concentrations of IgG1 and IgG3 antibodies. The probability of contracting malaria was reduced by almost 50% when IgG1 levels doubled and approximately 60% when IgG3 levels doubled. In stark contrast, when levels of non-neutralizing IgG4 doubled, the likelihood of getting malaria increased by about three times [189]. Interestingly, a different study also discovered a connection between elevated IgG4 levels and an increased risk of infection and malaria exacerbations [190]. These findings strongly suggest that pertussis and malaria pathogens also possess “allergen-like” epitopes.
Additionally, it has been shown that IgG4 inhibits IgG1 and IgG3 from opsonizing malaria-infected erythrocytes in vitro [191]. Opsonization is an immunological process in which invading pathogens are tagged with opsonins (antibodies) so that phagocytes can eliminate them. The negatively charged cell walls of the pathogen and the phagocyte repel each other in the absence of antibodies. Consequently, the infection can persist and spread in the human body without being eliminated. Opsonins help the macrophage ingest the pathogen by overcoming the repulsive barrier between negatively charged cell walls. Opsonization is an antimicrobial mechanism that helps eradicate pathogens and prevents disease transmission [192].
Recent studies [104,107] have demonstrated the presence of “allergen-like” epitopes in SARS-CoV-2, with evidence showing that the N and spike proteins induce IgE responses [45,50,55]. These findings strongly suggest that the virus has developed an additional immune evasion mechanism by mimicking an allergen. This IgE- and IgG4-mediated evasion mechanism contributed to a more severe infection with the early variants. In the long term, the presence of these “allergen-like” epitopes could induce tolerance to the virus, promoting its persistence and potentially contributing to long COVID. Research has shown that SARS-CoV-2 RNA or proteins can remain detectable for months after the acute phase of infection in immunocompetent individuals, including in tissues such as the gut, brain, and lungs [139,140,141,142,143]. In addition, repeated vaccination could unintentionally promote unopposed reinfection and replication, allowing chronic infection [28]. In the context of SARS-CoV-2 vaccines, repeated mRNA immunization produces elevated IgG4 antibody levels, likely generated to promote tolerance to the spike protein. Finally, while tolerance induction to an allergen benefits the host in AIT, in the context of a viral infection, it may enable pathogen persistence rather than adequate clearance.
Author Contributions
Conceptualization, A.R.-C., V.N.U. and E.M.R.; formal analysis, V.N.U., A.R.-C. and E.M.R.; investigation, E.M.R., D.C., V.N.U., M.P., C.B. and A.R.-C.; data curation, E.M.R., D.C., V.N.U., C.B. and A.R.-C.; writing—original draft preparation, V.N.U., A.R.-C. and E.M.R.; writing—review and editing, E.M.R., D.C., V.N.U., M.P., C.B. and A.R.-C.; visualization, A.R.-C. and V.N.U.; supervision, V.N.U. and E.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- DeSilva, M.B.; Mitchell, P.K.; Klein, N.P.; Dixon, B.E.; Tenforde, M.W.; Thompson, M.G.; Naleway, A.L.; Grannis, S.J.; Ong, T.C.; Natarajan, K.; et al. Protection of two and three mRNA vaccine doses against severe outcomes among adults hospitalized with COVID-19—Vision Network, August 2021 to March 2022. J. Infect. Dis. 2023, 227, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA 2021, 326, 2043–2054. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Self, W.H.; Zhu, Y.; Naioti, E.A.; Gaglani, M.; Ginde, A.A.; Jensen, K.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Protection of mRNA vaccines against hospitalized COVID-19 in adults over the first year following authorization in the United States. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 76, e460–e468. [Google Scholar] [CrossRef]
- Sibanda, B.; Haryanto, B. Assessing the Impact of COVID-19 Vaccination Programs on the Reduction of COVID-19 Cases: A Systematic Literature Review. Ann. Glob. Health 2024, 90, 45. [Google Scholar] [CrossRef]
- Rispens, T.; Huijbers, M.G. The unique properties of IgG4 and its roles in health and disease. Nat. Rev. Immunol. 2023, 23, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Rispens, T.; Ooijevaar-de Heer, P.; Bende, O.; Aalberse, R.C. Mechanism of immunoglobulin G4 Fab-arm exchange. J. Am. Chem. Soc. 2011, 133, 10302–10311. [Google Scholar] [CrossRef]
- Aalberse, R.; Stapel, S.; Schuurman, J.; Rispens, T. Immunoglobulin G4: An odd antibody. Clin. Exp. Allergy 2009, 39, 469–477. [Google Scholar] [CrossRef]
- Schuurman, J.; Van Ree, R.; Perdok, G.a.; Van Doorn, H.; Tan, K.; Aalberse, R. Normal human immunoglobulin G4 is bispecific: It has two different antigen-combining sites. Immunology 1999, 97, 693–698. [Google Scholar] [CrossRef]
- Van Der Neut Kolfschoten, M.; Schuurman, J.; Losen, M.; Bleeker, W.K.; Martínez-Martínez, P.; Vermeulen, E.; Den Bleker, T.H.; Wiegman, L.; Vink, T.; Aarden, L.A.; et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007, 317, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Akdis, C.; Blaser, K. Mechanisms of allergen-specific immunotherapy. Allergy 2000, 55, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M. Healthy immune response to allergens: T regulatory cells and more. Curr. Opin. Immunol. 2006, 18, 738–744. [Google Scholar] [CrossRef]
- Larché, M.; Akdis, C.A.; Valenta, R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006, 6, 761–771. [Google Scholar] [CrossRef]
- Durham, S.R.; Walker, S.M.; Varga, E.-M.; Jacobson, M.R.; O’Brien, F.; Noble, W.; Till, S.J.; Hamid, Q.A.; Nouri-Aria, K.T. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 1999, 341, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Khosroshahi, A.; Stone, J.H. A clinical overview of IgG4-related systemic disease. Curr. Opin. Rheumatol. 2011, 23, 57–66. [Google Scholar] [CrossRef]
- Della-Torre, E.; Lanzillotta, M.a.; Doglioni, C. Immunology of IgG4-related disease. Clin. Exp. Immunol. 2015, 181, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Shiokawa, M.; Kodama, Y.; Kuriyama, K.; Yoshimura, K.; Tomono, T.; Morita, T.; Kakiuchi, N.; Matsumori, T.; Mima, A.; Nishikawa, Y.; et al. Pathogenicity of IgG in patients with IgG4-related disease. Gut 2016, 65, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Tang, L.-F.; Cheng, L.; Wang, H.-Y. The clinical significance of allergen-specific IgG4 in allergic diseases. Front. Immunol. 2022, 13, 1032909. [Google Scholar] [CrossRef]
- Flicker, S.; Valenta, R. Renaissance of the blocking antibody concept in type I allergy. Int. Arch. Allergy Immunol. 2003, 132, 13–24. [Google Scholar] [CrossRef]
- Wachholz, P.A.; Durham, S.R. Mechanisms of immunotherapy: IgG revisited. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Golden, D.B.; Meyers, D.A.; Kagey-Sobotka, A.; Valentine, M.D.; Lichtenstein, L.M. Clinical relevance of the venom-specific immunoglobulin G antibody level during immunotherapy. J. Allergy Clin. Immunol. 1982, 69, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.; Helbling, A.; Bischof, M. Predictive value of venom-specific IgE, IgG and IgG subclass antibodies in patients on immunotherapy with honey bee venom. Allergy 1989, 44, 412–418. [Google Scholar] [CrossRef]
- Nouri-Aria, K.T.; Wachholz, P.A.; Francis, J.N.; Jacobson, M.R.; Walker, S.M.; Wilcock, L.K.; Staple, S.Q.; Aalberse, R.C.; Till, S.J.; Durham, S.R. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J. Immunol. 2004, 172, 3252–3259. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.; Ljørring, C.; Francis, J.; A Calderon, M.; Larche, M.; Kimber, I.; Frew, A.; Ipsen, H.; Lund, K.; Würtzen, P.; et al. Functional rather than immunoreactive levels of IgG4 correlate closely with clinical response to grass pollen immunotherapy. Allergy 2012, 67, 217–226. [Google Scholar] [CrossRef]
- Meiler, F.; Klunker, S.; Zimmermann, M.; Akdis, C.A.; Akdis, M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy 2008, 63, 1455–1463. [Google Scholar] [CrossRef]
- Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy: Multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014, 133, 621–631. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2022, 8, eade2798. [Google Scholar] [CrossRef]
- Buhre, J.S.; Pongracz, T.; Künsting, I.; Lixenfeld, A.S.; Wang, W.; Nouta, J.; Lehrian, S.; Schmelter, F.; Lunding, H.B.; Dühring, L.; et al. mRNA vaccines against SARS-CoV-2 induce comparably low long-term IgG Fc galactosylation and sialylation levels but increasing long-term IgG4 responses compared to an adenovirus-based vaccine. Front. Immunol. 2023, 13, 1020844. [Google Scholar] [CrossRef]
- Kiszel, P.; Sík, P.; Miklós, J.; Kajdácsi, E.; Sinkovits, G.; Cervenak, L.; Prohászka, Z. Class switch towards spike protein-specific IgG4 antibodies after SARS-CoV-2 mRNA vaccination depends on prior infection history. Sci. Rep. 2023, 13, 13166. [Google Scholar] [CrossRef] [PubMed]
- Emmenegger, M.; Fiedler, S.; Brugger, S.D.; Devenish, S.R.; Morgunov, A.S.; Ilsley, A.; Ricci, F.; Malik, A.Y.; Scheier, T.; Batkitar, L.; et al. Both COVID-19 infection and vaccination induce high-affinity cross-clade responses to SARS-CoV-2 variants. Iscience 2022, 25, 104766. [Google Scholar] [CrossRef] [PubMed]
- Selva, K.J.; Ramanathan, P.; Haycroft, E.R.; Reynaldi, A.; Cromer, D.; Tan, C.W.; Wang, L.-F.; Wines, B.D.; Hogarth, P.M.; Downie, L.E.; et al. Preexisting immunity restricts mucosal antibody recognition of SARS-CoV-2 and Fc profiles during breakthrough infections. JCI Insight 2023, 8, e172470. [Google Scholar] [CrossRef] [PubMed]
- Valk, A.M.; Keijser, J.B.; van Dam, K.P.; Stalman, E.W.; Wieske, L.; Steenhuis, M.; Kummer, L.Y.; Spuls, P.I.; Bekkenk, M.W.; Musters, A.H.; et al. Suppressed IgG4 class switching in dupilumab-and TNF inhibitor-treated patients after mRNA vaccination. Allergy 2024, 79, 1952–1961. [Google Scholar] [CrossRef]
- Hartley, G.E.; Fryer, H.A.; Gill, P.A.; Boo, I.; Bornheimer, S.J.; Hogarth, P.M.; Drummer, H.E.; O’Hehir, R.E.; Edwards, E.S.; van Zelm, M.C. Third dose COVID-19 mRNA vaccine enhances IgG4 isotype switching and recognition of Omicron subvariants by memory B cells after mRNA but not adenovirus priming. bioRxiv 2023. [Google Scholar] [CrossRef]
- Farkash, I.; Feferman, T.; Cohen-Saban, N.; Avraham, Y.; Morgenstern, D.; Mayuni, G.; Barth, N.; Lustig, Y.; Miller, L.; Shouval, D.S.; et al. Anti-SARS-CoV-2 antibodies elicited by COVID-19 mRNA vaccine exhibit a unique glycosylation pattern. Cell Rep. 2021, 37, 110114. [Google Scholar] [CrossRef]
- Sheehan, J.; Ardizzone, C.M.; Khanna, M.; Trauth, A.J.; Hagensee, M.E.; Ramsay, A.J. Dynamics of Serum-Neutralizing Antibody Responses in Vaccinees through Multiple Doses of the BNT162b2 Vaccine. Vaccines 2023, 11, 1720. [Google Scholar] [CrossRef]
- Yoshimura, M.; Sakamoto, A.; Ozuru, R.; Kurihara, Y.; Itoh, R.; Ishii, K.; Shimizu, A.; Chou, B.; Nabeshima, S.; Hiromatsu, K. The appearance of anti-spike receptor binding domain immunoglobulin G4 responses after repetitive immunization with messenger RNA-based COVID-19 vaccines. Int. J. Infect. Dis. 2024, 139, 1–5. [Google Scholar] [CrossRef]
- Akhtar, M.; Islam, M.R.; Khaton, F.; Soltana, U.H.; Jafrin, S.A.; Rahman, S.I.A.; Tauheed, I.; Ahmed, T.; Khan, I.I.; Akter, A.; et al. Appearance of tolerance-induction and non-inflammatory SARS-CoV-2 spike-specific IgG4 antibodies after COVID-19 booster vaccinations. Front. Immunol. 2023, 14, 1309997. [Google Scholar] [CrossRef]
- Espino, A.M.; Armina-Rodriguez, A.; Alvarez, L.; Ocasio-Malavé, C.; Ramos-Nieves, R.; Rodriguez Martinó, E.I.; López-Marte, P.; Torres, E.A.; Sariol, C.A. The Anti-SARS-CoV-2 IgG1 and IgG3 Antibody Isotypes with Limited Neutralizing Capacity against Omicron Elicited in a Latin Population a Switch toward IgG4 after Multiple Doses with the mRNA Pfizer–BioNTech Vaccine. Viruses 2024, 16, 187. [Google Scholar] [CrossRef]
- Adhikari, B.; Oltz, E.; Bednash, J.; Horowitz, J.; Rubinstein, M.; Vlasova, A.N. Brief Research Report: Impact of vaccination on antibody responses and mortality from severe COVID-19. Front. Immunol. 2024, 15, 1325243. [Google Scholar]
- Kalkeri, R.; Zhu, M.; Cloney-Clark, S.; Plested, J.S.; Parekh, A.; Gorinson, D.; Cai, R.; Mahato, S.; Ramanathan, P.; Aurelia, L.C.; et al. Altered IgG4 Antibody Response to Repeated mRNA versus Protein COVID Vaccines. medRxiv 2024. [Google Scholar] [CrossRef]
- Nziza, N.; Deng, Y.; Wood, L.; Dhanoa, N.; Dulit-Greenberg, N.; Chen, T.; Kane, A.S.; Swank, Z.; Davis, J.P.; Demokritou, M.; et al. Humoral profiles of toddlers and young children following SARS-CoV-2 mRNA vaccination. Nat. Commun. 2024, 15, 905. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kumar, S.; Lai, L.; Linderman, S.; Malik, A.A.; Ellis, M.L.; Godbole, S.; Solis, D.; Sahoo, M.K.; Bechnak, K.; et al. XBB. 1.5 monovalent booster improves antibody binding and neutralization against emerging SARS-CoV-2 Omicron variants. bioRxiv 2024. [Google Scholar] [CrossRef]
- Portilho, A.I.; Silva, V.O.; Da Costa, H.H.M.; Yamashiro, R.; de Oliveira, I.P.; de Campos, I.B.; Prudencio, C.R.; Matsuda, E.M.; de Macedo Brígido, L.F.; De Gaspari, E. An unexpected IgE anti-receptor binding domain response following natural infection and different types of SARS-CoV-2 vaccines. Sci. Rep. 2024, 14, 20003. [Google Scholar] [CrossRef] [PubMed]
- Routhu, N.K.; Stampfer, S.D.; Lai, L.; Akhtar, A.; Tong, X.; Yuan, D.; Chicz, T.M.; McNamara, R.P.; Jakkala, K.; Davis-Gardner, M.E.; et al. Efficacy of mRNA-1273 and Novavax ancestral or BA. 1 spike booster vaccines against SARS-CoV-2 BA. 5 infection in non-human primates. Sci. Immunol. 2023, 8, eadg7015. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, L.; Xing, M.; Qiao, N.; Liu, J.; Li, X.; Zhang, C.; Tang, N.; Xu, Z.; Guo, Y.; et al. Evaluation of antibody responses in healthy individuals receiving SARS-CoV-2 inactivated vaccines. Biosaf. Health 2024, 6, 153–164. [Google Scholar] [CrossRef]
- Suzuki, S.; Suzuki, Y.; Yamamoto, N.; Matsumoto, Y.; Shirai, A.; Okubo, T. Influenza A virus infection increases IgE production and airway responsiveness in aerosolized antigen-exposed mice. J. Allergy Clin. Immunol. 1998, 102, 732–740. [Google Scholar] [CrossRef]
- Dakhama, A.; Lee, Y.-M.; Ohnishi, H.; Jing, X.; Balhorn, A.; Takeda, K.; Gelfand, E.W. Virus-specific IgE enhances airway responsiveness on reinfection with respiratory syncytial virus in newborn mice. J. Allergy Clin. Immunol. 2009, 123, 138–145.e5. [Google Scholar] [CrossRef]
- Guclu, O.A.; Goktas, S.S.; Dilektasli, A.G.; Ozturk, N.A.A.; Demirdogen, E.; Coskun, F.; Ediger, D.; Ursavas, A.; Uzaslan, E.; Erol, H.A.; et al. A pilot study for IgE as a prognostic biomarker in COVID-19. Intern. Med. J. 2022, 52, 1495–1504. [Google Scholar]
- Ferastraoaru, D.; Hudes, G.; Jerschow, E.; Jariwala, S.; Karagic, M.; de Vos, G.; Rosenstreich, D.; Ramesh, M. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J. Allergy Clin. Immunol. Pract. 2021, 9, 1152–1162.e3. [Google Scholar] [CrossRef]
- Zhang, J.-j.; Dong, X.; Cao, Y.-y.; Yuan, Y.-d.; Yang, Y.-b.; Yan, Y.-q.; Akdis, C.A.; Gao, Y.-d. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Tu, L.; Zhu, P.; Mu, M.; Wang, R.; Yang, P.; Wang, X.; Hu, C.; Ping, R.; Hu, P.; et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan. A retrospective observational study. Am. J. Respir. Crit. Care Med. 2020, 201, 1372–1379. [Google Scholar] [CrossRef]
- Rodrigo-Muñoz, J.; Sastre, B.; Cañas, J.; Gil-Martínez, M.; Redondo, N.; Del Pozo, V. Eosinophil response against classical and emerging respiratory viruses: COVID-19. J. Investig. Allergol. Clin. Immunol. 2021, 31, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Plūme, J.; Galvanovskis, A.; Šmite, S.; Romanchikova, N.; Zayakin, P.; Linē, A. Early and strong antibody responses to SARS-CoV-2 predict disease severity in COVID-19 patients. J. Transl. Med. 2022, 20, 176. [Google Scholar] [CrossRef]
- Collins, A.M.; Jackson, K.J. A temporal model of human IgE and IgG antibody function. Front. Immunol. 2013, 4, 235. [Google Scholar] [CrossRef]
- Jeannin, P.; Delneste, Y.; Lecoanet-Henchoz, S.; Gretener, D.; Bonnefoy, J.-Y. Interleukin-7 (IL-7) enhances class switching to IgE and IgG4 in the presence of T cells via IL-9 and sCD23. Blood J. Am. Soc. Hematol. 1998, 91, 1355–1361. [Google Scholar] [CrossRef]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Kinet, J.-P. The high-affinity IgE receptor (FcϵRI): From physiology to pathology. Annu. Rev. Immunol. 1999, 17, 931–972. [Google Scholar] [CrossRef]
- Kempuraj, D.; Selvakumar, G.P.; Ahmed, M.E.; Raikwar, S.P.; Thangavel, R.; Khan, A.; Zaheer, S.A.; Iyer, S.S.; Burton, C.; James, D.; et al. COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neurosci 2020, 26, 402–414. [Google Scholar] [CrossRef]
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. COVID-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020, 100, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Motta Junior, J.d.S.; Miggiolaro, A.F.R.d.S.; Nagashima, S.; De Paula, C.B.V.; Baena, C.P.; Scharfstein, J.; De Noronha, L. Mast cells in alveolar septa of COVID-19 patients: A pathogenic pathway that may link interstitial edema to immunothrombosis. Front. Immunol. 2020, 11, 574862. [Google Scholar] [CrossRef]
- Farmani, A.R.; Mahdavinezhad, F.; Moslemi, R.; Mehrabi, Z.; Noori, A.; Kouhestani, M.; Noroozi, Z.; Ai, J.; Rezaei, N. Anti-IgE monoclonal antibodies as potential treatment in COVID-19. Immunopharmacol. Immunotoxicol. 2021, 43, 259–264. [Google Scholar] [CrossRef]
- Frasca, D.; Diaz, A.; Romero, M.; Mendez, N.V.; Landin, A.M.; Blomberg, B.B. Effects of age on H1N1-specific serum IgG1 and IgG3 levels evaluated during the 2011–2012 influenza vaccine season. Immun. Ageing 2013, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Cavacini, L.A.; Kuhrt, D.; Duval, M.; Mayer, K.; Posner, M.R. Binding and neutralization activity of human IgG1 and IgG3 from serum of HIV-infected individuals. AIDS Res. Hum. Retroviruses 2003, 19, 785–792. [Google Scholar] [CrossRef]
- Suthar, M.S.; Zimmerman, M.G.; Kauffman, R.C.; Mantus, G.; Linderman, S.L.; Hudson, W.H.; Vanderheiden, A.; Nyhoff, L.; Davis, C.W.; Adekunle, O.; et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep. Med. 2020, 1, 100040. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, L.; Martinuzzi, D.; Hyseni, I.; Benincasa, L.; Molesti, E.; Casa, E.; Lapini, G.; Piu, P.; Trombetta, C.M.; Marchi, S.; et al. Comparative analyses of SARS-CoV-2 binding (IgG, IgM, IgA) and neutralizing antibodies from human serum samples. J. Immunol. Methods 2021, 489, 112937. [Google Scholar] [CrossRef]
- Griffin, D.E.; Cooper, S.J.; Hirsch, R.L.; Johnson, R.T.; de Soriano, I.L.; Roedenbeck, S.; Vaisberg, A. Changes in plasma IgE levels during complicated and uncomplicated measles virus infections. J. Allergy Clin. Immunol. 1985, 76, 206–213. [Google Scholar] [CrossRef]
- Imani, F.; Proud, D.; Griffin, D.E. Measles virus infection synergizes with IL-4 in IgE class switching. J. Immunol. 1999, 162, 1597–1602. [Google Scholar] [CrossRef]
- Shalit, M.; Ackerman, Z.; Wollner, S.; Morag, A.; Levo, Y. Immunoglobulin E response during measles. Int. Arch. Allergy Immunol. 1984, 75, 84–86. [Google Scholar] [CrossRef]
- Tam, J.S.; Jackson, W.T.; Hunter, D.; Proud, D.; Grayson, M.H. Rhinovirus specific IgE can be detected in human sera. J. Allergy Clin. Immunol. 2013, 132, 1241–1243. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.K.; Pyle, D.M.; Farrar, J.D.; Gill, M.A. IgE-mediated regulation of IL-10 and type I IFN enhances rhinovirus-induced Th2 differentiation by primary human monocytes. Eur. J. Immunol. 2020, 50, 1550–1559. [Google Scholar] [CrossRef]
- Zambrano, J.C.; Carper, H.T.; Rakes, G.P.; Patrie, J.; Murphy, D.D.; Platts-Mills, T.A.; Hayden, F.G.; Gwaltney, J.M., Jr.; Hatley, T.K.; Owens, A.M.; et al. Experimental rhinovirus challenges in adults with mild asthma: Response to infection in relation to IgE. J. Allergy Clin. Immunol. 2003, 111, 1008–1016. [Google Scholar] [CrossRef]
- Kantor, D.B.; Stenquist, N.; McDonald, M.C.; Schultz, B.J.; Hauptman, M.; Smallwood, C.D.; Nelson, K.A.; Perzanowski, M.S.; Matsui, E.C.; Phipatanakul, W.; et al. Rhinovirus and serum IgE are associated with acute asthma exacerbation severity in children. J. Allergy Clin. Immunol. 2016, 138, 1467–1471.e9. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Wisniewski, J.; Yu, M.; Kennedy, J.L.; Platts-Mills, T.; Heymann, P.W.; Woodfolk, J.A. Infection with human rhinovirus 16 promotes enhanced IgE responsiveness in basophils of atopic asthmatics. Clin. Exp. Allergy 2014, 44, 1266–1273. [Google Scholar] [CrossRef]
- Burleson, H.L.K.S.G.R. Effect of Influenza Virus Infection on Ovalbumin Specific Ige Responses to Inhaled Antigen in The Rat. J. Toxicol. Environ. Health Part A 1996, 49, 619–630. [Google Scholar]
- Rowe, R.K.; Pyle, D.M.; Tomlinson, A.R.; Lv, T.; Hu, Z.; Gill, M.A. IgE cross-linking impairs monocyte antiviral responses and inhibits influenza-driven TH1 differentiation. J. Allergy Clin. Immunol. 2017, 140, 294–298.e8. [Google Scholar] [CrossRef]
- Wright, D.N.; Nelson, R.P., Jr.; Ledford, D.K.; Fernandez-Caldas, E.; Trudeau, W.L.; Lockey, R.F. Serum IgE and human immunodeficiency virus (HIV) infection. J. Allergy Clin. Immunol. 1990, 85, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Ammann, A.J.; Abrams, D.; Conant, M.; Chudwin, D.; Cowan, M.; Volberding, P.; Lewis, B.; Casavant, C. Acquired immune dysfunction in homosexual men: Immunologic profiles. Clin. Immunol. Immunopathol. 1983, 27, 315–325. [Google Scholar] [CrossRef]
- Ring, J.; Fröschl, M.; Brunner, R.; Braun-Falco, O. LAV/HTLV-III infection and atopy: Serum IgE and specific IgE antibodies to environmental allergens. Acta Derm. Venereol. 1986, 66, 530–532. [Google Scholar] [CrossRef]
- Lin, R.Y. Chronic diffuse dermatitis and hyper-IgE in HIV infection. Acta Derm. Venereol. 1988, 68, 486–491. [Google Scholar]
- Miguez-Burbano, M.; Shor-Posner, G.; Fletcher, M.A.; Lu, Y.; Moreno, J.; Carcamo, C.; Page, B.; Quesada, J.; Sauberlich, H.; Baum, M. Immunoglobulin E levels in relationship to HIV-1 disease, route of infection, and vitamin E status. Allergy 1995, 50, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Ouaaz, F.; Ruscetti, F.W.; Dugas, B.; Mikovits, J.; Agut, H.; Debr, P.; Mossalayi, M.D. Role of IgE Immune Complexes in the Regulation of HIV-1 Replication and Increased Cell Death of Infected U1 Monocytes: Involvement of CD23/Fc ε RII-Mediated Nitric Oxide and Cyclic AMP Pathways. Mol. Med. 1996, 2, 38–49. [Google Scholar] [CrossRef]
- Ellaurie, M.; Rubinstein, A.; Rosenstreich, D.L. IgE levels in pediatric HIV-1 infection. Ann. Allergy Asthma Immunol. 1995, 75, 332–336. [Google Scholar] [PubMed]
- Israël-Biet, D.; Labrousse, F.; Tourani, J.-M.; Sors, H.; Andrieu, J.-M.; Even, P. Elevation of IgE in HIV-infected subjects: A marker of poor prognosis. J. Allergy Clin. Immunol. 1992, 89, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Lucey, D.R.; Zajac, R.A.; Melcher, G.P.; Butzin, C.A.; Boswell, R.N. Serum IgE levels in 622 persons with human immunodeficiency virus infection: IgE elevation with marked depletion of CD4+ T-cells. AIDS Res. Hum. Retroviruses 1990, 6, 427–429. [Google Scholar] [CrossRef]
- Shor-Posner, G.; Miguez-Burbano, M.J.; Lu, Y.; Feaster, D.; Fletcher, M.; Sauberlich, H.; Baum, M.K. Elevated IgE level in relationship to nutritional status and immune parameters in early human immunodeficiency virus–1 disease. J. Allergy Clin. Immunol. 1995, 95, 886–892. [Google Scholar] [CrossRef]
- Bul, R.H.D.; Molinaro, G.A.; Kettering, J.D.; Heiner, D.C.; Imagawa, D.T.; Geme, J.W.S., Jr. Virus-specific IgE and IgG4 antibodies in serum of children infected with respiratory syncytial virus. J. Pediatr. 1987, 110, 87–90. [Google Scholar]
- Becker, Y. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy—A review. Virus Genes 2006, 33, 235–252. [Google Scholar] [CrossRef]
- Welliver, R.C.; Sun, M.; Rinaldo, D.; Ogra, P.L. Respiratory syncytial virus-specific IgE responses following infection: Evidence for a predominantly mucosal response. Pediatr. Res. 1985, 19, 420–424. [Google Scholar] [CrossRef]
- Smith-Norowitz, T.A.; Josekutty, J.; Silverberg, J.I.; Lev-Tov, H.; Norowitz, Y.M.; Kohlhoff, S.; Nowakowski, M.; Durkin, H.G.; Bluth, M.H. Long term persistence of IgE anti-Varicella Zoster Virus in pediatric and adult serum post chicken pox infection and after vaccination with Varicella Virus vaccine. Int. J. Biomed. Sci. IJBS 2009, 5, 353. [Google Scholar] [CrossRef]
- Smith-Norowitz, T.A.; Tam, E.; Norowitz, K.B.; Chotikanatis, K.; Weaver, D.; Durkin, H.G.; Bluth, M.H.; Kohlhoff, S. IgE anti Hepatitis B virus surface antigen antibodies detected in serum from inner city asthmatic and non asthmatic children. Hum. Immunol. 2014, 75, 378–382. [Google Scholar] [CrossRef]
- Imani, F.; Kehoe, K.E. Infection of human B lymphocytes with MMR vaccine induces IgE class switching. Clin. Immunol. 2001, 100, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Smith-Norowitz, T.A.; Wong, D.; Kusonruksa, M.; Norowitz, K.B.; Joks, R.; Durkin, H.G.; Bluth, M.H. Long term persistence of IgE anti-influenza virus antibodies in pediatric and adult serum post vaccination with influenza virus vaccine. Int. J. Med. Sci. 2011, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zheng, X.; Sun, F.; He, J.; Shi, H.; Chen, M.; Tu, C.; Huang, Y.; Wang, Z.; Liang, Y.; et al. Hypersensitivity may be involved in severe COVID-19. Clin. Exp. Allergy 2022, 52, 324–333. [Google Scholar] [CrossRef]
- Chen, M.; Tu, C.; Tan, C.; Zheng, X.; Sun, F.; Liang, Y.; Shi, H.; Wu, J.; Huang, Y.; Wang, Z.; et al. Hypersensitivity in the lungs is responsible for acute respiratory failure in COVID-19 patients: Case series of patients who received high-dose/short-term methylprednisolone. Clin. Transl. Allergy 2021, 11, e12056. [Google Scholar] [CrossRef]
- Della-Torre, E.; Lanzillotta, M.; Strollo, M.; Ramirez, G.A.; Dagna, L.; Tresoldi, M. Serum IgG4 level predicts COVID-19 related mortality. Eur. J. Intern. Med. 2021, 93, 107–109. [Google Scholar] [CrossRef]
- Moura, A.D.; da Costa, H.H.; Correa, V.A.; de S. Lima, A.K.; Lindoso, J.A.; De Gaspari, E.; Hong, M.A.; Cunha-Junior, J.P.; Prudencio, C.R. Assessment of avidity related to IgG subclasses in SARS-CoV-2 Brazilian infected patients. Sci. Rep. 2021, 11, 17642. [Google Scholar] [CrossRef]
- Genova, S.N.; Pencheva, M.M.; Abadjieva, T.I.; Atanasov, N.G. Cellular and immune response in fatal COVID-19 pneumonia. Pan Afr. Med. J. 2024, 49, 130. [Google Scholar] [CrossRef]
- Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. Does SARS-CoV-2 induce IgG4 synthesis to evade the immune system? Biomolecules 2023, 13, 1338. [Google Scholar] [CrossRef]
- Pascolini, S.; Granito, A.; Muratori, L.; Lenzi, M.; Muratori, P. Coronavirus disease associated immune thrombocytopenia: Causation or correlation? J. Microbiol. Immunol. Infect. 2020, 54, 531. [Google Scholar] [CrossRef] [PubMed]
- Pascolini, S.; Vannini, A.; Deleonardi, G.; Ciordinik, M.; Sensoli, A.; Carletti, I.; Veronesi, L.; Ricci, C.; Pronesti, A.; Mazzanti, L.; et al. COVID-19 and immunological dysregulation: Can autoantibodies be useful? Clin. Transl. Sci. 2021, 14, 502–508. [Google Scholar] [CrossRef]
- Muratori, P.; Lenzi, M.; Muratori, L.; Granito, A. Antinuclear antibodies in COVID 19. Clin. Transl. Sci. 2021, 14, 1627. [Google Scholar] [CrossRef] [PubMed]
- Balz, K.; Kaushik, A.; Chen, M.; Cemic, F.; Heger, V.; Renz, H.; Nadeau, K.; Skevaki, C. Homologies between SARS-CoV-2 and allergen proteins may direct T cell-mediated heterologous immune responses. Sci. Rep. 2021, 11, 4792. [Google Scholar] [CrossRef]
- Balz, K.; Trassl, L.; Härtel, V.; Nelson, P.P.; Skevaki, C. Virus-induced T cell-mediated heterologous immunity and vaccine development. Front. Immunol. 2020, 11, 513. [Google Scholar] [CrossRef]
- Pusch, E.; Renz, H.; Skevaki, C. Respiratory virus-induced heterologous immunity: Part of the problem or part of the solution? Allergo J. 2018, 27, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Karagöz, I.K.; Kaya, M.; Rückert, R.; Bozman, N.; Kaya, V.; Bayram, H.; Yıldırım, M. A bioinformatic analysis: Previous allergen exposure may support anti-SARS-CoV-2 immune response. Comput. Biol. Chem. 2023, 107, 107961. [Google Scholar] [CrossRef]
- Zhu, Z.; Hasegawa, K.; Ma, B.; Fujiogi, M.; Camargo, C.A.; Liang, L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J. Allergy Clin. Immunol. 2020, 146, 327–329.e4. [Google Scholar] [CrossRef]
- Chen, C.; Song, X.; Murdock, D.J.; Marcus, A.; Hussein, M.; Jalbert, J.J.; Geba, G.P. Association between allergic conditions and COVID-19 susceptibility and outcomes. Ann. Allergy Asthma Immunol. 2024, 132, 637–645.e7. [Google Scholar] [CrossRef]
- Skevaki, C.; Karsonova, A.; Karaulov, A.; Fomina, D.; Xie, M.; Chinthrajah, S.; Nadeau, K.C.; Renz, H. SARS-CoV-2 infection and COVID-19 in asthmatics: A complex relationship. Nat. Rev. Immunol. 2021, 21, 202–203. [Google Scholar] [CrossRef]
- Skevaki, C.; Karsonova, A.; Karaulov, A.; Xie, M.; Renz, H. Asthma-associated risk for COVID-19 development. J. Allergy Clin. Immunol. 2020, 146, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, A.E.; Melo, R.C.; Duan, S.; LeMessurier, K.S.; Liedmann, S.; Surman, S.L.; Lee, J.J.; Hurwitz, J.L.; Thomas, P.G.; McCullers, J.A. Eosinophils promote antiviral immunity in mice infected with influenza A virus. J. Immunol. 2017, 198, 3214–3226. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.G.; Bivins-Smith, E.R.; Proskocil, B.J.; Nie, Z.; Scott, G.D.; Lee, J.J.; Lee, N.A.; Fryer, A.D.; Jacoby, D.B. Human and mouse eosinophils have antiviral activity against parainfluenza virus. Am. J. Respir. Cell Mol. Biol. 2016, 55, 387–394. [Google Scholar] [CrossRef]
- Phipps, S.; Lam, C.E.; Mahalingam, S.; Newhouse, M.; Ramirez, R.; Rosenberg, H.F.; Foster, P.S.; Matthaei, K.I. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood J. Am. Soc. Hematol. 2007, 110, 1578–1586. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Domachowske, J.B. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J. Leukoc. Biol. 2001, 70, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Domachowske, J.B.; Dyer, K.D.; Bonville, C.A.; Rosenberg, H.F. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J. Infect. Dis. 1998, 177, 1458–1464. [Google Scholar] [CrossRef]
- Turner, K.J.; Feddema, L.; Quinn, E.H. Non-specific potentiation of IgE by parasitic infections in man. Int. Arch. Allergy Immunol. 1979, 58, 232–236. [Google Scholar] [CrossRef]
- Lee, T.D.; Xie, C.Y. IgE regulation by nematodes: The body fluid of Ascaris contains a B-cell mitogen. J. Allergy Clin. Immunol. 1995, 95, 1246–1254. [Google Scholar] [CrossRef]
- Hagel, I.; Lynch, N.; Di Prisco, M.; Rojas, E.; Perez, M.; Alvarez, N. Ascaris reinfection of slum children: Relation with the IgE response. Clin. Exp. Immunol. 1993, 94, 80–83. [Google Scholar] [CrossRef]
- Becker, Y. HIV-1 gp41 heptad repeat 2 (HR2) possesses an amino acid domain that resembles the allergen domain in Aspergillus fumigatus Asp f1 protein: Review, hypothesis and implications. Virus Genes 2007, 34, 233–240. [Google Scholar] [CrossRef]
- Dasari, P.; Shopova, I.A.; Stroe, M.; Wartenberg, D.; Martin-Dahse, H.; Beyersdorf, N.; Hortschansky, P.; Dietrich, S.; Cseresnyés, Z.; Figge, M.T.; et al. Aspf2 from Aspergillus fumigatus recruits human immune regulators for immune evasion and cell damage. Front. Immunol. 2018, 9, 1635. [Google Scholar] [CrossRef] [PubMed]
- Heinekamp, T.; Schmidt, H.; Lapp, K.; Pähtz, V.; Shopova, I.; Köster-Eiserfunke, N.; Krüger, T.; Kniemeyer, O.; Brakhage, A.A. Interference of Aspergillus fumigatus with the immune response. Semin. Immunopathol. 2015, 37, 141–152. [Google Scholar] [CrossRef]
- Abad, A.; Fernández-Molina, J.V.; Bikandi, J.; Ramírez, A.; Margareto, J.; Sendino, J.; Hernando, F.L.; Pontón, J.; Garaizar, J.; Rementeria, A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. De Micol. 2010, 27, 155–182. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Tonani, L.; Cardoso, C.R.B.; Kress, M.R.V.Z. The immune interplay between the host and the pathogen in Aspergillus fumigatus lung infection. BioMed Res. Int. 2013, 2013, 693023. [Google Scholar] [CrossRef] [PubMed]
- Dewi, I.M.; Van de Veerdonk, F.L.; Gresnigt, M.S. The multifaceted role of T-helper responses in host defense against Aspergillus fumigatus. J. Fungi 2017, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Murdock, B.J.; Shreiner, A.B.; McDonald, R.A.; Osterholzer, J.J.; White, E.S.; Toews, G.B.; Huffnagle, G.B. Coevolution of TH1, TH2, and TH17 responses during repeated pulmonary exposure to Aspergillus fumigatus conidia. Infect. Immun. 2011, 79, 125–135. [Google Scholar] [CrossRef]
- Cramer, R.A.; Rivera, A.; Hohl, T.M. Immune responses against Aspergillus fumigatus: What have we learned? Curr. Opin. Infect. Dis. 2011, 24, 315–322. [Google Scholar] [CrossRef]
- Stevens, D.A. Th1/Th2 in aspergillosis. Med. Mycol. 2006, 44, S229–S235. [Google Scholar] [CrossRef]
- Arias, M.; Santiago, L.; Vidal-García, M.; Redrado, S.; Lanuza, P.; Comas, L.; Domingo, M.P.; Rezusta, A.; Gálvez, E.M. Preparations for invasion: Modulation of host lung immunity during pulmonary aspergillosis by gliotoxin and other fungal secondary metabolites. Front. Immunol. 2018, 9, 2549. [Google Scholar] [CrossRef]
- Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. SARS-CoV-2: A master of immune evasion. Biomedicines 2022, 10, 1339. [Google Scholar] [CrossRef]
- Caetano-Anollés, K.; Hernandez, N.; Mughal, F.; Tomaszewski, T.; Caetano-Anollés, G. The seasonal behaviour of COVID-19 and its galectin-like culprit of the viral spike. Methods Microbiol. 2022, 50, 27–81. [Google Scholar] [PubMed]
- Cherayil, B.J.; Weiner, S.J.; Pillai, S. The Mac-2 antigen is a galactose-specific lectin that binds IgE. J. Exp. Med. 1989, 170, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.T.; Bieneman, A.P. The S1 subunit of the SARS-CoV-2 spike protein activates human monocytes to produce cytokines linked to COVID-19: Relevance to galectin-3. Front. Immunol. 2022, 13, 831763. [Google Scholar] [CrossRef]
- Körner, R.W.; Bansemir, O.Y.; Franke, R.; Sturm, J.; Dafsari, H.S. Atopy and elevation of IgE, IgG3, and IgG4 may be risk factors for post COVID-19 condition in children and adolescents. Children 2023, 10, 1598. [Google Scholar] [CrossRef]
- Giménez-Orenga, K.; Pierquin, J.; Brunel, J.; Charvet, B.; Martín-Martínez, E.; Perron, H.; Oltra, E. HERV-W ENV antigenemia and correlation of increased anti-SARS-CoV-2 immunoglobulin levels with post-COVID-19 symptoms. Front. Immunol. 2022, 13, 1020064. [Google Scholar] [CrossRef]
- Motta, R.V.; Culver, E.L. IgG4 autoantibodies and autoantigens in the context of IgG4-autoimmune disease and IgG4-related disease. Front. Immunol. 2024, 15, 1272084. [Google Scholar] [CrossRef] [PubMed]
- Raszek, M.; Cowley, D.; Redwan, E.M.; Uversky, V.N.; Rubio-Casillas, A. Exploring the possible link between the spike protein immunoglobulin G4 antibodies and cancer progression. Explor. Immunol. 2024, 4, 267–284. [Google Scholar] [CrossRef]
- Umetsu, D.T.; DeKruyff, R.H. TH1 and TH2 CD4+ cells in human allergic diseases. J. Allergy Clin. Immunol. 1997, 100, 1–6. [Google Scholar] [CrossRef]
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387.e9. [Google Scholar] [CrossRef]
- Leitao, I.d.C.; Calil, P.T.; Galliez, R.M.; Moreira, F.R.R.; Mariani, D.; Castiñeiras, A.C.P.; da Silva, G.P.D.; Maia, R.A.; Correa, I.A.; Monteiro, F.L.L.; et al. Prolonged SARS-CoV-2 positivity in immunocompetent patients: Virus isolation, genomic integrity, and transmission risk. Microbiol. Spectr. 2021, 9, e00855-21. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin. Infect. Dis. 2023, 76, e487–e490. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Tejerina, F.; Catalan, P.; Rodriguez-Grande, C.; Adan, J.; Rodriguez-Gonzalez, C.; Muñoz, P.; Aldamiz, T.; Diez, C.; Perez, L.; Fanciulli, C.; et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect. Dis. 2022, 22, 211. [Google Scholar] [CrossRef]
- Becker, Y. The changes in the T helper 1 (Th1) and T helper 2 (Th2) cytokine balance during HIV-1 infection are indicative of an allergic response to viral proteins that may be reversed by Th2 cytokine inhibitors and immune response modifiers–a review and hypothesis. Virus Genes 2004, 28, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S.; Del Prete, G.; Manetti, R.; Ravina, A.; Annunziato, F.; De Carli, M.; Mazzetti, M.; Piccinni, M.-P.; D’Elios, M.M.; Parronchi, P.; et al. Role of TH1/TH2 cytokines in HIV infection. Immunol. Rev. 1994, 140, 73–92. [Google Scholar] [CrossRef]
- Wong, G.H.; Goeddel, D.V. Tumour necrosis factors α and β inhibit virus replication and synergize with interferons. Nature 1986, 323, 819–822. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Fitch, F.W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J. Immunol. 1988, 140, 4245–4252. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Moore, K.W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol. Today 1991, 12, A49–A53. [Google Scholar] [CrossRef]
- Becker, Y. HIV-1 induced AIDS is an allergy and the allergen is the Shed gp120–a review, hypothesis, and implications. Virus Genes 2004, 28, 319–331. [Google Scholar] [CrossRef]
- Da Silva, L.; Da Silva, J. Relationship between atopy, allergic diseases and total serum IgE levels among HIV-infected children. Eur. Ann. Allergy Clin. Immunol. 2013, 45, 155–159. [Google Scholar]
- Gill, M.A.; Bajwa, G.; George, T.A.; Dong, C.C.; Dougherty, I.I.; Jiang, N.; Gan, V.N.; Gruchalla, R.S. Counterregulation between the FcεRI pathway and antiviral responses in human plasmacytoid dendritic cells. J. Immunol. 2010, 184, 5999–6006. [Google Scholar] [CrossRef] [PubMed]
- Gielen, V.; Sykes, A.; Zhu, J.; Chan, B.; Macintyre, J.; Regamey, N.; Kieninger, E.; Gupta, A.; Shoemark, A.; Bossley, C.; et al. Increased nuclear suppressor of cytokine signaling 1 in asthmatic bronchial epithelium suppresses rhinovirus induction of innate interferons. J. Allergy Clin. Immunol. 2015, 136, 177–188.e11. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Werder, R.B.; Simpson, J.; Loh, Z.; Zhang, V.; Haque, A.; Spann, K.; Sly, P.D.; Mazzone, S.B.; Upham, J.W.; et al. Aeroallergen-induced IL-33 predisposes to respiratory virus–induced asthma by dampening antiviral immunity. J. Allergy Clin. Immunol. 2016, 138, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Durrani, S.R.; Montville, D.J.; Pratt, A.S.; Sahu, S.; DeVries, M.K.; Rajamanickam, V.; Gangnon, R.E.; Gill, M.A.; Gern, J.E.; Lemanske, R.F., Jr.; et al. Innate immune responses to rhinovirus are reduced by the high-affinity IgE receptor in allergic asthmatic children. J. Allergy Clin. Immunol. 2012, 130, 489–495. [Google Scholar] [CrossRef]
- Duff, A.L.; Pomeranz, E.S.; Gelber, L.E.; Price, G.W.; Farris, H.; Hayden, F.G.; Platts-Mills, T.A.; Heymann, P.W. Risk factors for acute wheezing in infants and children: Viruses, passive smoke, and IgE antibodies to inhalant allergens. Pediatrics 1993, 92, 535–540. [Google Scholar] [CrossRef]
- Teach, S.J.; Gergen, P.J.; Szefler, S.J.; Mitchell, H.E.; Calatroni, A.; Wildfire, J.; Bloomberg, G.R.; Kercsmar, C.M.; Liu, A.H.; Makhija, M.M.; et al. Seasonal risk factors for asthma exacerbations among inner-city children. J. Allergy Clin. Immunol. 2015, 135, 1465–1473.e5. [Google Scholar] [CrossRef]
- Soto-Quiros, M.; Avila, L.; Platts-Mills, T.A.; Hunt, J.F.; Erdman, D.D.; Carper, H.; Murphy, D.D.; Odio, S.; James, H.R.; Patrie, J.T.; et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J. Allergy Clin. Immunol. 2012, 129, 1499–1505.e5. [Google Scholar] [CrossRef]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef]
- Bencze, D.; Fekete, T.; Pázmándi, K. Type I interferon production of plasmacytoid dendritic cells under control. Int. J. Mol. Sci. 2021, 22, 4190. [Google Scholar] [CrossRef]
- Gill, M.A.; Liu, A.H.; Calatroni, A.; Krouse, R.Z.; Shao, B.; Schiltz, A.; Gern, J.E.; Togias, A.; Busse, W.W. Enhanced plasmacytoid dendritic cell antiviral responses after omalizumab. J. Allergy Clin. Immunol. 2018, 141, 1735–1743.e9. [Google Scholar] [CrossRef]
- Teach, S.J.; Gill, M.A.; Togias, A.; Sorkness, C.A.; Arbes, S.J., Jr.; Calatroni, A.; Wildfire, J.J.; Gergen, P.J.; Cohen, R.T.; Pongracic, J.A.; et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J. Allergy Clin. Immunol. 2015, 136, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, A.; Busse, W.W.; Calatroni, A.; Togias, A.G.; Grindle, K.G.; Bochkov, Y.A.; Gruchalla, R.S.; Kattan, M.; Kercsmar, C.M.; Khurana Hershey, G.; et al. Effects of omalizumab on rhinovirus infections, illnesses, and exacerbations of asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald-Bocarsly, P.; Jacobs, E.S. Plasmacytoid dendritic cells in HIV infection: Striking a delicate balance. J. Leukoc. Biol. 2010, 87, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Bartleson, J.M.; Radenkovic, D.; Covarrubias, A.J.; Furman, D.; Winer, D.A.; Verdin, E. SARS-CoV-2, COVID-19 and the aging immune system. Nat. Aging 2021, 1, 769–782. [Google Scholar] [CrossRef]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef]
- Peacock, T.P.; Brown, J.C.; Zhou, J.; Thakur, N.; Sukhova, K.; Newman, J.; Kugathasan, R.; Yan, A.W.; Furnon, W.; De Lorenzo, G.; et al. The altered entry pathway and antigenic distance of the SARS-CoV-2 Omicron variant map to separate domains of spike protein. Biorxiv 2022. [Google Scholar] [CrossRef]
- McMahan, K.; Giffin, V.; Tostanoski, L.H.; Chung, B.; Siamatu, M.; Suthar, M.S.; Halfmann, P.; Kawaoka, Y.; Piedra-Mora, C.; Jain, N.; et al. Reduced pathogenicity of the SARS-CoV-2 omicron variant in hamsters. Med 2022, 3, 262–268.e4. [Google Scholar] [CrossRef]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar] [CrossRef]
- Ariën, K.K.; Vanham, G.; Arts, E.J. Is HIV-1 evolving to a less virulent form in humans? Nat. Rev. Microbiol. 2007, 5, 141–151. [Google Scholar] [CrossRef]
- Grobben, M.; Bakker, M.; Schriek, A.I.; Levels, L.J.; Umotoy, J.C.; Tejjani, K.; van Breemen, M.J.; Lin, R.N.; de Taeye, S.W.; Ozorowski, G.; et al. Polyfunctionality and breadth of HIV-1 antibodies are associated with delayed disease progression. PLoS Pathog. 2024, 20, e1012739. [Google Scholar] [CrossRef]
- Chung, A.W.; Ghebremichael, M.; Robinson, H.; Brown, E.; Choi, I.; Lane, S.; Dugast, A.-S.; Schoen, M.K.; Rolland, M.; Suscovich, T.J.; et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci. Transl. Med. 2014, 6, 228ra38. [Google Scholar] [CrossRef] [PubMed]
- Karnasuta, C.; Akapirat, S.; Madnote, S.; Savadsuk, H.; Puangkaew, J.; Rittiroongrad, S.; Rerks-Ngarm, S.; Nitayaphan, S.; Pitisuttithum, P.; Kaewkungwal, J.; et al. Comparison of antibody responses induced by RV144, VAX003, and VAX004 vaccination regimens. AIDS Res. Hum. Retroviruses 2017, 33, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Yates, N.L.; Liao, H.-X.; Fong, Y.; DeCamp, A.; Vandergrift, N.A.; Williams, W.T.; Alam, S.M.; Ferrari, G.; Yang, Z.-Y.; Seaton, K.E.; et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci. Transl. Med. 2014, 6, 228ra39. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Locke, E.R.; O’Hare, A.M.; Bohnert, A.S.; Boyko, E.J.; Hynes, D.M.; Berry, K. COVID-19 vaccination effectiveness against infection or death in a national US health care system: A target trial emulation study. Ann. Intern. Med. 2022, 175, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, E.; Morioka, H.; Kikuchi, T.; Fukushima, M. Behavioral and Health Outcomes of mRNA COVID-19 Vaccination: A Case-Control Study in Japanese Small and Medium-Sized Enterprises. Cureus 2024, 16, e75652. [Google Scholar] [CrossRef]
- Eythorsson, E.; Runolfsdottir, H.L.; Ingvarsson, R.F.; Sigurdsson, M.I.; Palsson, R. Rate of SARS-CoV-2 reinfection during an omicron wave in Iceland. JAMA Netw. Open 2022, 5, e2225320. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.K.; Burke, P.C.; Nowacki, A.S.; Gordon, S.M. Risk of coronavirus disease 2019 (COVID-19) among those up-to-date and not up-to-date on COVID-19 vaccination by US CDC criteria. PLoS ONE 2023, 18, e0293449. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Ruffin, J.; Wiegand, R.; Grant, L.; Babu, T.M.; Briggs-Hagen, M.; Burgess, J.L.; Caban-Martinez, A.J.; Chu, H.Y.; Ellingson, K.D.; et al. Protection from COVID-19 vaccination and prior SARS-CoV-2 infection among children aged 6 months–4 years, united states, september 2022–april 2023. J. Pediatr. Infect. Dis. Soc. 2025, 14, piae121. [Google Scholar] [CrossRef]
- Pérez, C.M.; Ruiz-Rius, S.; Ramírez-Morros, A.; Vidal, M.; Opi, D.H.; Santamaria, P.; Blanco, J.; Vidal-Alaball, J.; Beeson, J.G.; Molinos-Albert, L.M.; et al. Post-vaccination IgG4 and IgG2 class switch associates with increased risk of SARS-CoV-2 infections. J. Infect. 2025, 90, 106473. [Google Scholar] [CrossRef]
- Kober, C.; Manni, S.; Wolff, S.; Barnes, T.; Mukherjee, S.; Vogel, T.; Hoenig, L.; Vogel, P.; Hahn, A.; Gerlach, M.; et al. IgG3 and IgM identified as key to SARS-CoV-2 neutralization in convalescent plasma pools. PLoS ONE 2022, 17, e0262162. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, A.L.; McGregor, R.; Craigie, A.; James, A.; Charlewood, R.; Lorenz, N.; Dickson, J.M.; Sheen, C.R.; Koch, B.; Fox-Lewis, S.; et al. Comprehensive analysis of SARS-CoV-2 antibody dynamics in New Zealand. Clin. Transl. Immunol. 2021, 10, e1261. [Google Scholar] [CrossRef] [PubMed]
- Gazit, S.; Shlezinger, R.; Perez, G.; Lotan, R.; Peretz, A.; Ben-Tov, A.; Herzel, E.; Alapi, H.; Cohen, D.; Muhsen, K.; et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) naturally acquired immunity versus vaccine-induced immunity, reinfections versus breakthrough infections: A retrospective cohort study. Clin. Infect. Dis. 2022, 75, e545–e551. [Google Scholar] [CrossRef] [PubMed]
- Redman, M.; King, A.; Watson, C.; King, D. What is CRISPR/Cas9? Arch. Dis. Child. -Educ. Pract. 2016, 101, 213–215. [Google Scholar] [CrossRef]
- Gao, F.-X.; Wu, R.-X.; Shen, M.-Y.; Huang, J.-J.; Li, T.-T.; Hu, C.; Luo, F.-Y.; Song, S.-Y.; Mu, S.; Hao, Y.-N.; et al. Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice. Iscience 2022, 25, 105479. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Nathan, A.; Kaseke, C.; Berrios, C.; Khatri, A.; Choi, S.; Getz, M.A.; Tano-Menka, R.; Ofoman, O.; Gayton, A.; et al. T cell reactivity to the SARS-CoV-2 Omicron variant is preserved in most but not all individuals. Cell 2022, 185, 1041–1051.E6. [Google Scholar] [CrossRef]
- Swadling, L.; Diniz, M.O.; Schmidt, N.M.; Amin, O.E.; Chandran, A.; Shaw, E.; Pade, C.; Gibbons, J.M.; Le Bert, N.; Tan, A.T.; et al. Pre-existing polymerase-specific T cells expand in abortive seronegative SARS-CoV-2. Nature 2022, 601, 110–117. [Google Scholar] [CrossRef]
- Dobaño, C.; Quelhas, D.; Quintó, L.; Puyol, L.; Serra-Casas, E.; Mayor, A.; Nhampossa, T.; Macete, E.; Aide, P.; Mandomando, I.; et al. Age-dependent IgG subclass responses to Plasmodium falciparum EBA-175 are differentially associated with incidence of malaria in Mozambican children. Clin. Vaccine Immunol. 2012, 19, 157–166. [Google Scholar] [CrossRef]
- Aucan, C.; Traoré, Y.; Tall, F.O.; Nacro, B.; Traoré-Leroux, T.R.S.; Fumoux, F.; Rihet, P. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect. Immun. 2000, 68, 1252–1258. [Google Scholar] [CrossRef]
- Groux, H.; Gysin, J. Opsonization as an effector mechanism in human protection against asexual blood stages of Plasmodium falciparum: Functional role of IgG subclasses. Res. Immunol. 1990, 141, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Thau, L.; Asuka, E.; Mahajan, K. Physiology, opsonization. In StatPearls; StatPearls Publishing: Treausure Island, FL, USA, 2023. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).