Use of a Multiplex Immunoassay Platform to Investigate Multifaceted Antibody Responses in SARS-CoV-2 Vaccinees with and Without Prior Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Serum Samples
2.2. Antibody Concentrations
2.3. Antibody Avidity Assay
2.4. Multiplex Inhibition Test (MINT)
2.5. Pseudovirus Microneutralization Assay
2.6. Non-Human Primate (NHP) Study Design
2.7. Recombinant Protein Expression and Purification
2.8. Wild-Type SARS-CoV-2 Virus Assay
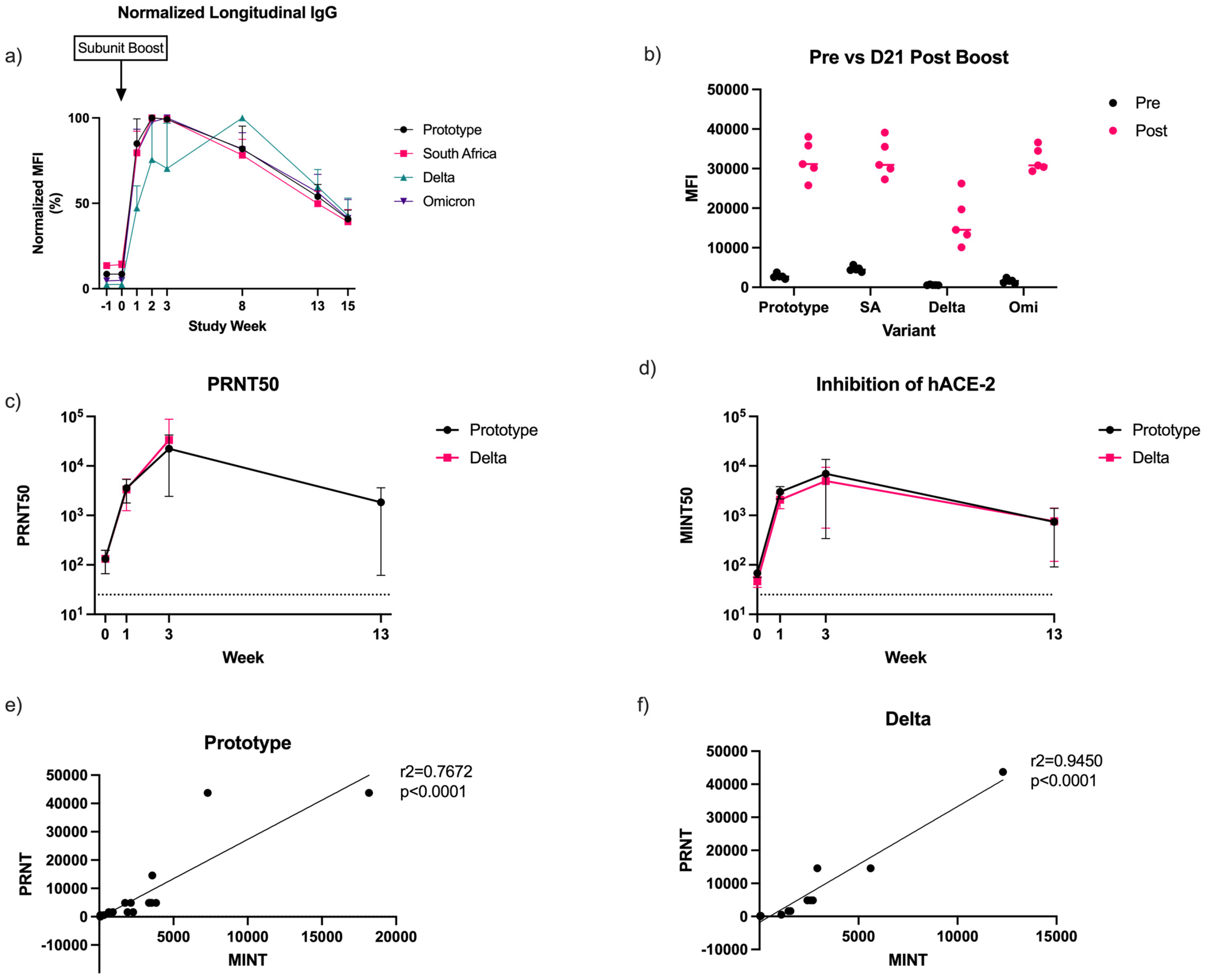
3. Results
3.1. Anti-Spike Antibody Concentrations
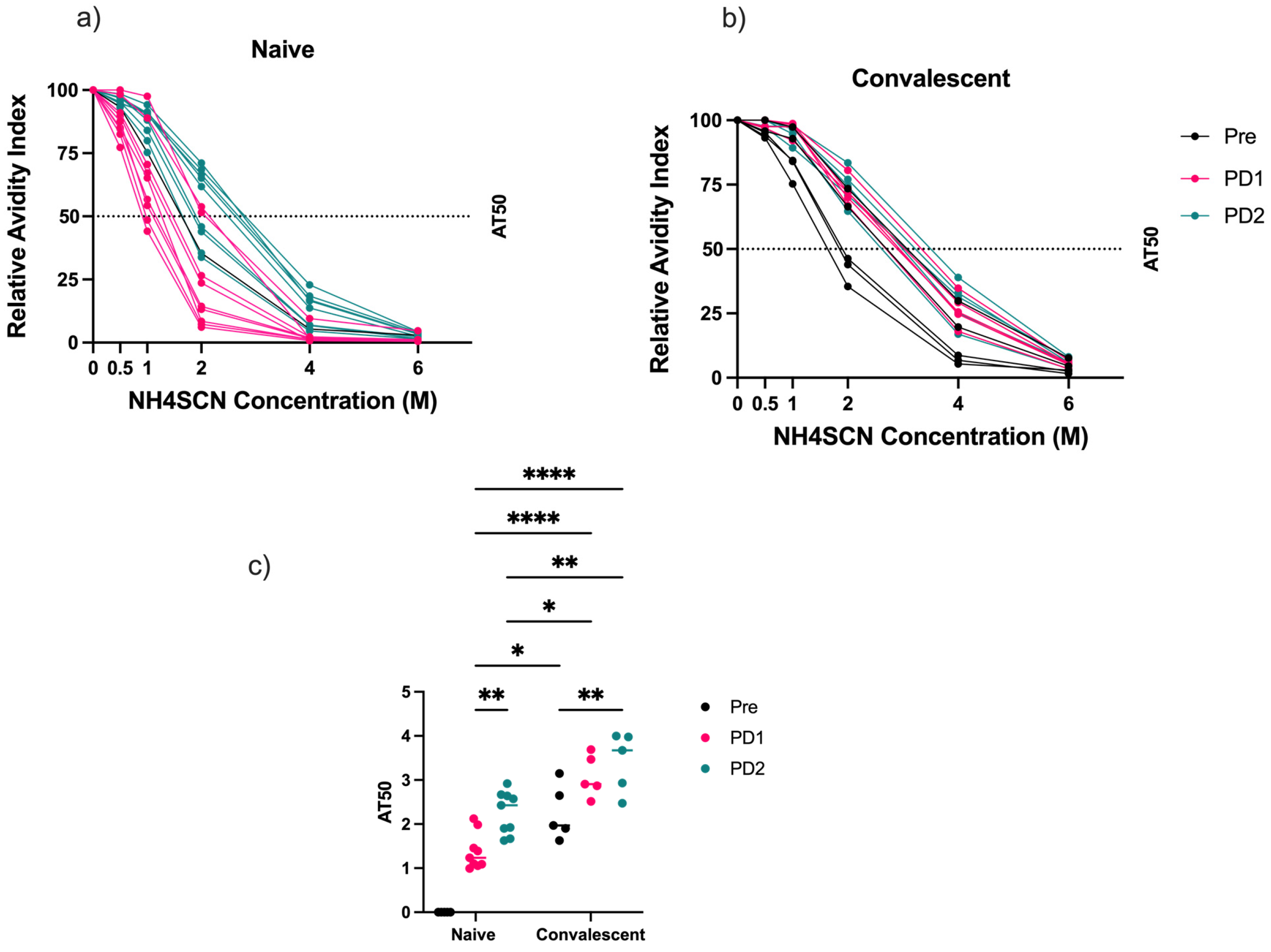
3.2. Antibody Avidity
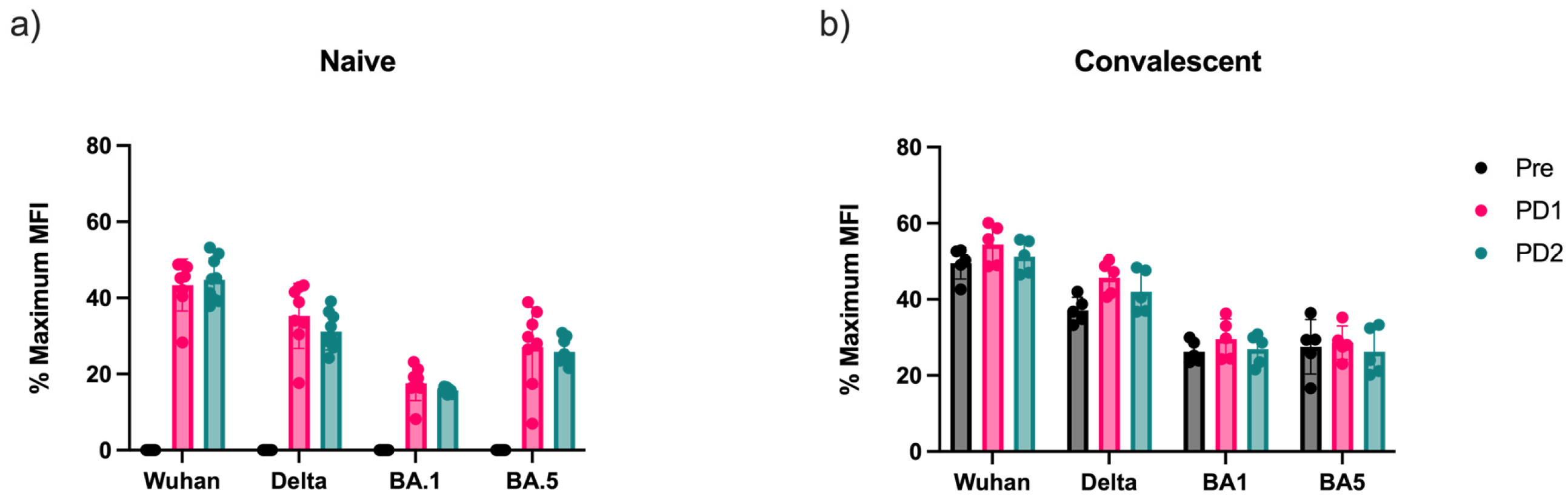
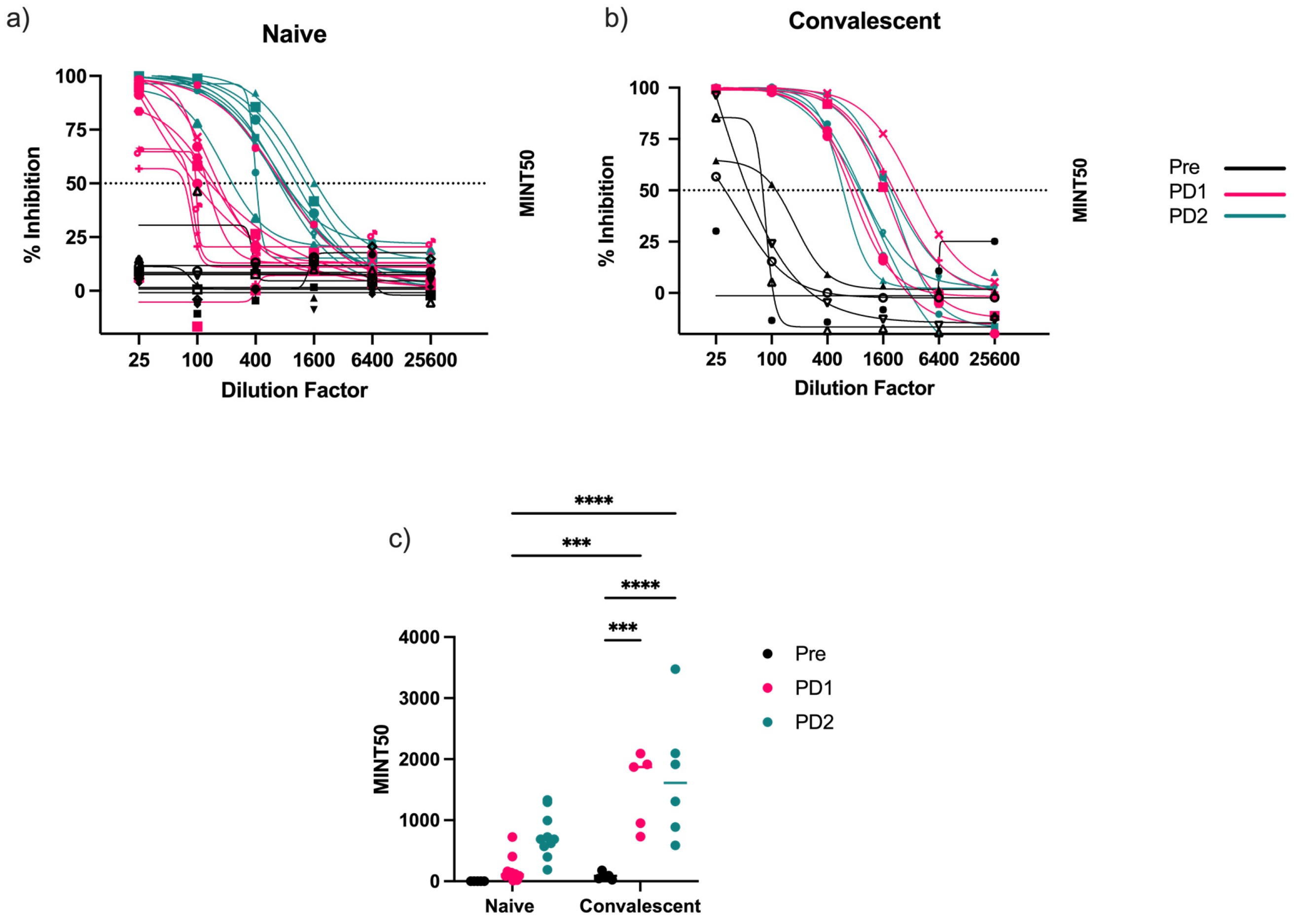
3.3. Antibody-Mediated Receptor-Binding Inhibition
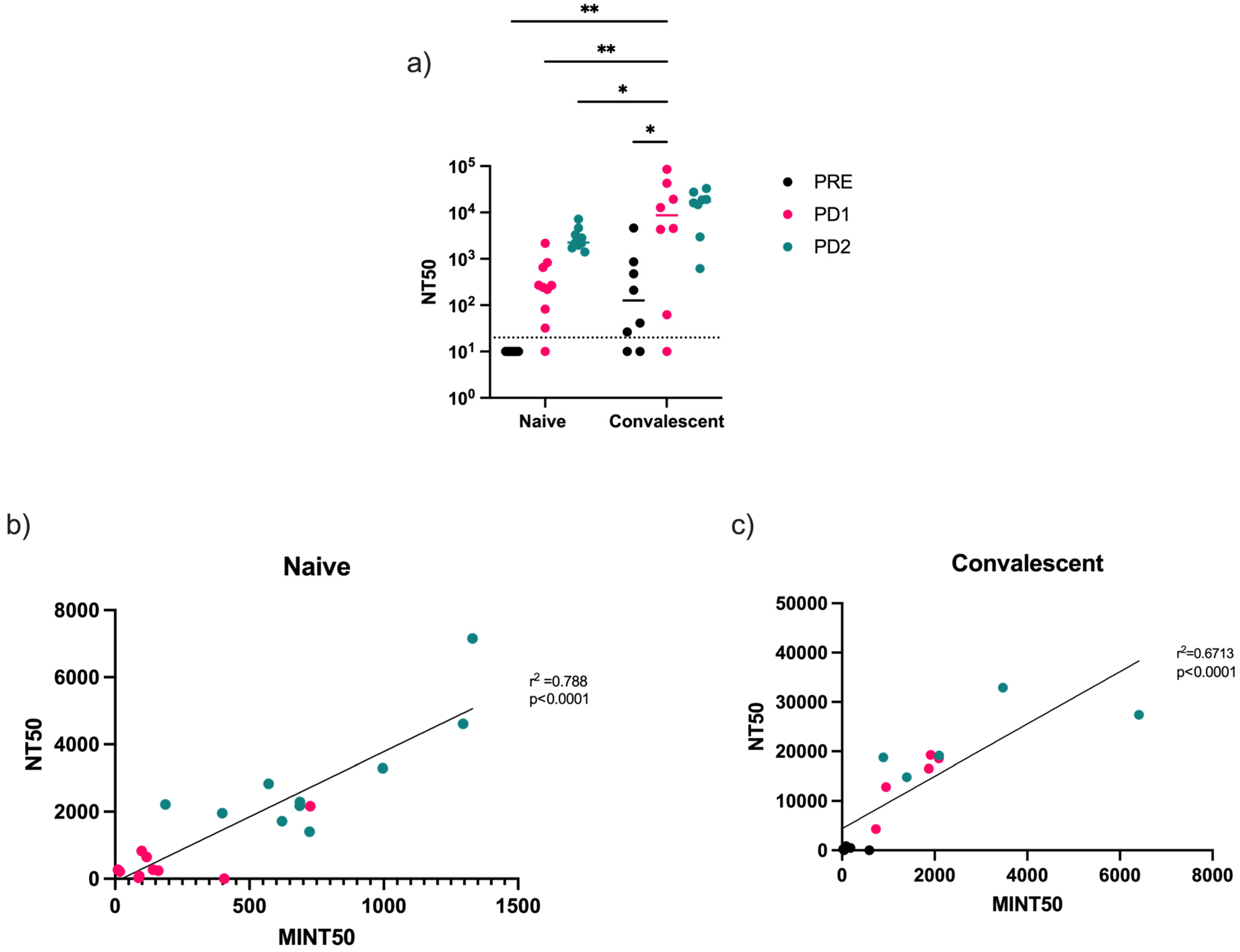
3.4. Virus-Neutralizing Antibodies Correlate with Receptor-Binding Inhibition
3.5. PRNT50 Correlates with Receptor-Binding Inhibition in Non-Human Primates
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, A.; Koch, M.; Wu, K.; Chu, L.; Ma, L.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T.; et al. Safety and Immunogenicity of SARS-CoV-2 Variant mRNA Vaccine Boosters in Healthy Adults: An Interim Analysis. Nat. Med. 2021, 27, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Gal Levin, E.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Hewins, B.; Rahman, M.; Bermejo-Martin, J.F.; Kelvin, A.A.; Richardson, C.D.; Rubino, S.; Kumar, A.; Ndishimye, P.; Toloue Ostadgavahi, A.; Mahmud-Al-Rafat, A.; et al. Alpha, Beta, Delta, Omicron, and SARS-CoV-2 Breakthrough Cases: Defining Immunological Mechanisms for Vaccine Waning and Vaccine-Variant Mismatch. Front. Virol. 2022, 2, 849936. [Google Scholar] [CrossRef]
- Jacobsen, H.; Sitaras, I.; Katzmarzyk, M.; Cobos Jiménez, V.; Naughton, R.; Higdon, M.M.; Deloria Knoll, M. Systematic Review and Meta-Analysis of the Factors Affecting Waning of Post-Vaccination Neutralizing Antibody Responses against SARS-CoV-2. Npj Vaccines 2023, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Innate Immune Recognition of Viral Infection. Nat. Immunol. 2006, 7, 131–137. [Google Scholar] [CrossRef]
- Seth, R.B.; Sun, L.; Chen, Z.J. Antiviral Innate Immunity Pathways. Cell Res. 2006, 16, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Tomalka, J.A.; Suthar, M.S.; Diamond, M.S.; Sekaly, R.P. Innate Antiviral Immunity: How Prior Exposures Can Guide Future Responses. Trends Immunol. 2022, 43, 696–705. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of Vaccine Immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Wang, M.; Islam, M.S.; Liao, P.; Hu, Y.; Chen, X. Humoral and Cellular Immune Responses of COVID-19 Vaccines against SARS-Cov-2 Omicron Variant: A Systemic Review. Int. J. Biol. Sci. 2022, 18, 4629–4641. [Google Scholar] [CrossRef]
- Sebina, I.; Pepper, M. Humoral Immune Responses to Infection: Common Mechanisms and Unique Strategies to Combat Pathogen Immune Evasion Tactics. Curr. Opin. Immunol. 2018, 51, 46–54. [Google Scholar] [CrossRef]
- Khoury, D.S.; Schlub, T.E.; Cromer, D.; Steain, M.; Fong, Y.; Gilbert, P.B.; Subbarao, K.; Triccas, J.A.; Kent, S.J.; Davenport, M.P. Correlates of Protection, Thresholds of Protection, and Immunobridging among Persons with SARS-CoV-2 Infection. Emerg. Infect. Dis. 2023, 29, 381–388. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of Protection against Symptomatic and Asymptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Garcia, L.; Woudenberg, T.; Rosado, J.; Dyer, A.H.; Donnadieu, F.; Planas, D.; Bruel, T.; Schwartz, O.; Prazuck, T.; Velay, A.; et al. Kinetics of the SARS-CoV-2 Antibody Avidity Response Following Infection and Vaccination. Viruses 2022, 14, 1491. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G. The Variability of the Serological Response to SARS-Corona Virus-2: Potential Resolution of Ambiguity through Determination of Avidity (Functional Affinity). J. Med. Virol. 2021, 93, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Eisen, H.N. Affinity Enhancement of Antibodies: How Low-Affinity Antibodies Produced Early in Immune Responses Are Followed by High-Affinity Antibodies Later and in Memory B-Cell Responses. Cancer Immunol. Res. 2014, 2, 381–392. [Google Scholar] [CrossRef]
- Sun, K.; Bhiman, J.N.; Tempia, S.; Kleynhans, J.; Madzorera, V.S.; Mkhize, Q.; Kaldine, H.; McMorrow, M.L.; Wolter, N.; Moyes, J.; et al. SARS-CoV-2 Correlates of Protection from Infection against Variants of Concern. Nat. Med. 2024, 30, 2805–2812. [Google Scholar] [CrossRef]
- Sather, D.N.; Armann, J.; Ching, L.K.; Mavrantoni, A.; Sellhorn, G.; Caldwell, Z.; Yu, X.; Wood, B.; Self, S.; Kalams, S.; et al. Factors Associated with the Development of Cross-Reactive Neutralizing Antibodies during Human Immunodeficiency Virus Type 1 Infection. J. Virol. 2009, 83, 757–769. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Denis, K.S.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 Variants Escape Neutralization by Vaccine-Induced Humoral Immunity. Cell 2021, 184, 2372–2383.e9. [Google Scholar] [CrossRef]
- Syed, A.M.; Ciling, A.; Taha, T.Y.; Chen, I.P.; Khalid, M.M.; Sreekumar, B.; Chen, P.-Y.; Kumar, G.R.; Suryawanshi, R.; Silva, I.; et al. Omicron Mutations Enhance Infectivity and Reduce Antibody Neutralization of SARS-CoV-2 Virus-like Particles. Proc. Natl. Acad. Sci. USA 2022, 119, e2200592119. [Google Scholar] [CrossRef]
- Dapporto, F.; Marchi, S.; Leonardi, M.; Piu, P.; Lovreglio, P.; Decaro, N.; Buonvino, N.; Stufano, A.; Lorusso, E.; Bombardieri, E.; et al. Antibody Avidity and Neutralizing Response against SARS-CoV-2 Omicron Variant after Infection or Vaccination. J. Immunol. Res. 2022, 2022, e4813199. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Kemp, T.J.; Bullock, J.; Bouk, A.; Metz, J.; Neish, A.; Cherry, J.; Lowy, D.R.; Pinto, L.A. SARS-CoV-2 IgG Spike Antibody Levels and Avidity in Natural Infection or Following Vaccination with mRNA-1273 or BNT162b2 Vaccines. Hum. Vaccin. Immunother. 2023, 19, 2215677. [Google Scholar] [CrossRef] [PubMed]
- Nakagama, Y.; Candray, K.; Kaku, N.; Komase, Y.; Rodriguez-Funes, M.-V.; Dominguez, R.; Tsuchida, T.; Kunishima, H.; Nagai, E.; Adachi, E.; et al. Antibody Avidity Maturation Following Recovery From Infection or the Booster Vaccination Grants Breadth of SARS-CoV-2 Neutralizing Capacity. J. Infect. Dis. 2023, 227, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Struck, F.; Schreiner, P.; Staschik, E.; Wochinz-Richter, K.; Schulz, S.; Soutschek, E.; Motz, M.; Bauer, G. Vaccination versus Infection with SARS-CoV-2: Establishment of a High Avidity IgG Response versus Incomplete Avidity Maturation. J. Med. Virol. 2021, 93, 6765–6777. [Google Scholar] [CrossRef]
- To, A.; Wong, T.A.S.; Lieberman, M.M.; Thompson, K.; Ball, A.H.; Pessaint, L.; Greenhouse, J.; Daham, N.; Cook, A.; Narvaez, B.; et al. A Recombinant Subunit Vaccine Induces a Potent and Broadly Neutralizing, Durable Antibody Response in Macaques against SARS-CoV-2 P.1 (Gamma) Variant. ACS Infect. Dis. 2022, 8, 825–840. [Google Scholar] [CrossRef]
- Tré-Hardy, M.; Wilmet, A.; Beukinga, I.; Favresse, J.; Dogné, J.-M.; Douxfils, J.; Blairon, L. Analytical and Clinical Validation of an ELISA for Specific SARS-CoV-2 IgG, IgA, and IgM Antibodies. J. Med. Virol. 2021, 93, 803–811. [Google Scholar] [CrossRef]
- Adams, E.; Ainsworth, M.; Anand, R.; Andersson, M.I.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beer, S.; Bell, J.I.; Berry, T.; et al. Evaluation of Antibody Testing for SARS-CoV-2 Using ELISA and Lateral Flow Immunoassays. medRxiv 2020. [Google Scholar] [CrossRef]
- Satterly, N.G.; Voorhees, M.A.; Ames, A.D.; Schoepp, R.J. Comparison of MagPix Assays and Enzyme-Linked Immunosorbent Assay for Detection of Hemorrhagic Fever Viruses. J. Clin. Microbiol. 2017, 55, 68–78. [Google Scholar] [CrossRef]
- Ayouba, A.; Touré, A.; Butel, C.; Keita, A.K.; Binetruy, F.; Sow, M.S.; Foulongne, V.; Delaporte, E.; Peeters, M. Development of a Sensitive and Specific Serological Assay Based on Luminex Technology for Detection of Antibodies to Zaire Ebola Virus. J. Clin. Microbiol. 2017, 55, 165–176. [Google Scholar] [CrossRef]
- Haun, B.K.; To, A.; Williams, C.A.; Ball, A.; Fong, K.; Wong, T.A.S.; Shobayo, B.; Teahton, J.; Ching, L.; Kamara, V.; et al. A Serological Multiplexed Immunoassay (MIA) Detects Antibody Reactivity to SARS-CoV-2 and Other Viral Pathogens in Liberia and Is Configurable as a Multiplexed Inhibition Test (MINT). Immuno 2024, 4, 108–124. [Google Scholar] [CrossRef]
- Müller, N.F.; Wagner, C.; Frazar, C.D.; Roychoudhury, P.; Lee, J.; Moncla, L.H.; Pelle, B.; Richardson, M.; Ryke, E.; Xie, H.; et al. Viral Genomes Reveal Patterns of the SARS-CoV-2 Outbreak in Washington State. Sci. Transl. Med. 2021, 13, eabf0202. [Google Scholar] [CrossRef] [PubMed]
- Smith, O.A.; Fujimoto, B.; Wong, T.A.S.; To, A.; Odo, T.; Ball, A.; Haun, B.K.; Muramatsu, H.; Tam, Y.K.; Pardi, N.; et al. Impact of Metabolic States on SARS-CoV-2 Vaccine Responses in Mouse Models of Obesity and Diabetes. COVID 2025, 5, 2. [Google Scholar] [CrossRef]
- Lai, C.-Y.; To, A.; Wong, T.A.S.; Lieberman, M.M.; Clements, D.E.; Senda, J.T.; Ball, A.H.; Pessaint, L.; Andersen, H.; Furuyama, W.; et al. Recombinant Protein Subunit SARS-CoV-2 Vaccines Formulated with CoVaccine HTTM Adjuvant Induce Broad, Th1 Biased, Humoral and Cellular Immune Responses in Mice. Vaccine X 2021, 9, 100126. [Google Scholar] [CrossRef]
- LeMaster, C.; Geanes, E.S.; Fraley, E.R.; Selvarangan, R.; Bradley, T. Vaccination after SARS-CoV-2 Infection Increased Antibody Avidity against the Omicron Variant Compared to Vaccination Alone. J. Infect. Dis. 2022, 226, 1712–1716. [Google Scholar] [CrossRef]
- Braunstein, G.D.; Schwartz, L.; Hymel, P.; Fielding, J. False Positive Results With SARS-CoV-2 RT-PCR Tests and How to Evaluate a RT-PCR-Positive Test for the Possibility of a False Positive Result. J. Occup. Environ. Med. 2021, 63, e159–e162. [Google Scholar] [CrossRef] [PubMed]
- Chandler, C.M.; Bourassa, L.; Mathias, P.C.; Greninger, A.L. Estimating the False-Positive Rate of Highly Automated SARS-CoV-2 Nucleic Acid Amplification Testing. J. Clin. Microbiol. 2021, 59, e01080-21. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Steel, K.J.A.; Hemmings, O.; O’Bryne, A.; Kouphou, N.; Pickering, S.; Galao, R.P.; et al. Longitudinal Evaluation and Decline of Antibody Responses in SARS-CoV-2 Infection. medRxiv 2020. [Google Scholar] [CrossRef]
- Ward, H.; Cooke, G.; Atchison, C.; Whitaker, M.; Elliott, J.; Moshe, M.; Brown, J.C.; Flower, B.; Daunt, A.; Ainslie, K.; et al. Declining Prevalence of Antibody Positivity to SARS-CoV-2: A Community Study of 365,000 Adults. medRxiv 2020. [Google Scholar] [CrossRef]
- Ibarrondo, F.J.; Fulcher, J.A.; Goodman-Meza, D.; Elliott, J.; Hofmann, C.; Hausner, M.A.; Ferbas, K.G.; Tobin, N.H.; Aldrovandi, G.M.; Yang, O.O. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N. Engl. J. Med. 2020, 383, 1085–1087. [Google Scholar] [CrossRef]
- de Gier, B.; Huiberts, A.J.; Hoeve, C.E.; den Hartog, G.; van Werkhoven, H.; van Binnendijk, R.; Hahné, S.J.M.; de Melker, H.E.; van den Hof, S.; Knol, M.J. Effects of COVID-19 Vaccination and Previous Infection on Omicron SARS-CoV-2 Infection and Relation with Serology. Nat. Commun. 2023, 14, 4793. [Google Scholar] [CrossRef]
- Henry, C.; Palm, A.-K.E.; Krammer, F.; Wilson, P.C. From Original Antigenic Sin to the Universal Influenza Virus Vaccine. Trends Immunol. 2018, 39, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Acute Bacterial Meningitis in Infants and Children. Lancet Infect. Dis. 2010, 10, 32–42. [Google Scholar] [CrossRef]
- Gagne, M.; Moliva, J.I.; Foulds, K.E.; Andrew, S.F.; Flynn, B.J.; Werner, A.P.; Wagner, D.A.; Teng, I.-T.; Lin, B.C.; Moore, C.; et al. mRNA-1273 or mRNA-Omicron Boost in Vaccinated Macaques Elicits Comparable B Cell Expansion, Neutralizing Antibodies and Protection against Omicron. medRxiv 2022. [Google Scholar] [CrossRef]
- Pérez-Alós, L.; Hansen, C.B.; Almagro Armenteros, J.J.; Madsen, J.R.; Heftdal, L.D.; Hasselbalch, R.B.; Pries-Heje, M.M.; Bayarri-Olmos, R.; Jarlhelt, I.; Hamm, S.R.; et al. Previous Immunity Shapes Immune Responses to SARS-CoV-2 Booster Vaccination and Omicron Breakthrough Infection Risk. Nat. Commun. 2023, 14, 5624. [Google Scholar] [CrossRef]
- Servellita, V.; Syed, A.M.; Morris, M.K.; Brazer, N.; Saldhi, P.; Garcia-Knight, M.; Sreekumar, B.; Khalid, M.M.; Ciling, A.; Chen, P.-Y.; et al. Neutralizing Immunity in Vaccine Breakthrough Infections from the SARS-CoV-2 Omicron and Delta Variants. Cell 2022, 185, 1539–1548.e5. [Google Scholar] [CrossRef] [PubMed]
- Pušnik, J.; Monzon-Posadas, W.O.; Zorn, J.; Peters, K.; Baum, M.; Proksch, H.; Schlüter, C.B.; Alter, G.; Menting, T.; Streeck, H. SARS-CoV-2 Humoral and Cellular Immunity Following Different Combinations of Vaccination and Breakthrough Infection. Nat. Commun. 2023, 14, 572. [Google Scholar] [CrossRef]
- Collier, A.Y.; Brown, C.M.; McMahan, K.A.; Yu, J.; Liu, J.; Jacob-Dolan, C.; Chandrashekar, A.; Tierney, D.; Ansel, J.L.; Rowe, M.; et al. Characterization of Immune Responses in Fully Vaccinated Individuals after Breakthrough Infection with the SARS-CoV-2 Delta Variant. Sci. Transl. Med. 2022, 14, eabn6150. [Google Scholar] [CrossRef]
- Kim, S.J.; Yao, Z.; Marsh, M.C.; Eckert, D.M.; Kay, M.S.; Lyakisheva, A.; Pasic, M.; Bansal, A.; Birnboim, C.; Jha, P.; et al. Homogeneous Surrogate Virus Neutralization Assay to Rapidly Assess Neutralization Activity of Anti-SARS-CoV-2 Antibodies. Nat. Commun. 2022, 13, 3716. [Google Scholar] [CrossRef]
- Byrnes, J.R.; Zhou, X.X.; Lui, I.; Elledge, S.K.; Glasgow, J.E.; Lim, S.A.; Loudermilk, R.P.; Chiu, C.Y.; Wang, T.T.; Wilson, M.R.; et al. Competitive SARS-CoV-2 Serology Reveals Most Antibodies Targeting the Spike Receptor-Binding Domain Compete for ACE2 Binding. mSphere 2020, 5, e00802-20. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.-C.; Tiu, C.; Hu, Z.; Chen, V.C.-W.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 Surrogate Virus Neutralization Test Based on Antibody-Mediated Blockage of ACE2–Spike Protein–Protein Interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Abe, K.T.; Li, Z.; Samson, R.; Samavarchi-Tehrani, P.; Valcourt, E.J.; Wood, H.; Budylowski, P.; Dupuis, A.P.; Girardin, R.C.; Rathod, B.; et al. A Simple Protein-Based Surrogate Neutralization Assay for SARS-CoV-2. JCI Insight 2020, 5, e142362. [Google Scholar] [CrossRef]
- Lynch, K.L.; Zhou, S.; Kaul, R.; Walker, R.; Wu, A.H. Evaluation of Neutralizing Antibodies against SARS-CoV-2 Variants after Infection and Vaccination Using a Multiplexed Surrogate Virus Neutralization Test. Clin. Chem. 2022, 68, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Pitiriga, V.C.; Papamentzelopoulou, M.; Konstantinakou, K.E.; Theodoridou, K.; Vasileiou, I.V.; Tsakris, A. SARS-CoV-2 T Cell Immunity Responses Following Natural Infection and Vaccination. Vaccines 2023, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T Cell Immune Response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Vardhana, S.; Baldo, L.; Morice, W.G.; Wherry, E.J. Understanding T Cell Responses to COVID-19 Is Essential for Informing Public Health Strategies. Sci. Immunol. 2022, 7, eabo1303. [Google Scholar] [CrossRef] [PubMed]
- Bertoletti, A.; Le Bert, N.; Tan, A.T. SARS-CoV-2-Specific T Cells in the Changing Landscape of the COVID-19 Pandemic. Immunity 2022, 55, 1764–1778. [Google Scholar] [CrossRef]
- Haun, B.K.; Kamara, V.; Dweh, A.S.; Garalde-Machida, K.; Forkay, S.S.E.; Takaaze, M.; Namekar, M.; Wong, T.A.S.; Bell-Gam Woto, A.E.R.; Humphreys, P.; et al. Serological Evidence of Ebola Virus Exposure in Dogs from Affected Communities in Liberia: A Preliminary Report. PLoS Negl. Trop. Dis. 2019, 13, e0007614. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odo, T.; Haun, B.K.; Williams, C.A.; Ball, A.; To, A.; Wong, T.A.S.; Ching, L.; Nakano, E.; Van Ry, A.; Pessaint, L.; et al. Use of a Multiplex Immunoassay Platform to Investigate Multifaceted Antibody Responses in SARS-CoV-2 Vaccinees with and Without Prior Infection. COVID 2025, 5, 44. https://doi.org/10.3390/covid5040044
Odo T, Haun BK, Williams CA, Ball A, To A, Wong TAS, Ching L, Nakano E, Van Ry A, Pessaint L, et al. Use of a Multiplex Immunoassay Platform to Investigate Multifaceted Antibody Responses in SARS-CoV-2 Vaccinees with and Without Prior Infection. COVID. 2025; 5(4):44. https://doi.org/10.3390/covid5040044
Chicago/Turabian StyleOdo, Troy, Brien K. Haun, Caitlin A. Williams, Aquena Ball, Albert To, Teri Ann S. Wong, Lauren Ching, Eileen Nakano, Alex Van Ry, Laurent Pessaint, and et al. 2025. "Use of a Multiplex Immunoassay Platform to Investigate Multifaceted Antibody Responses in SARS-CoV-2 Vaccinees with and Without Prior Infection" COVID 5, no. 4: 44. https://doi.org/10.3390/covid5040044
APA StyleOdo, T., Haun, B. K., Williams, C. A., Ball, A., To, A., Wong, T. A. S., Ching, L., Nakano, E., Van Ry, A., Pessaint, L., Andersen, H., Donini, O., Nerurkar, V. R., & Lehrer, A. T. (2025). Use of a Multiplex Immunoassay Platform to Investigate Multifaceted Antibody Responses in SARS-CoV-2 Vaccinees with and Without Prior Infection. COVID, 5(4), 44. https://doi.org/10.3390/covid5040044





