Association Between Methylprednisolone and the Increase of Respiratory Infections in COVID-19 Patients in the Intensive Care Unit
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Clinical Variables
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Comparison Between Patients Treated With and Without Methylprednisolone
3.2. Analysis of RTI-ICU
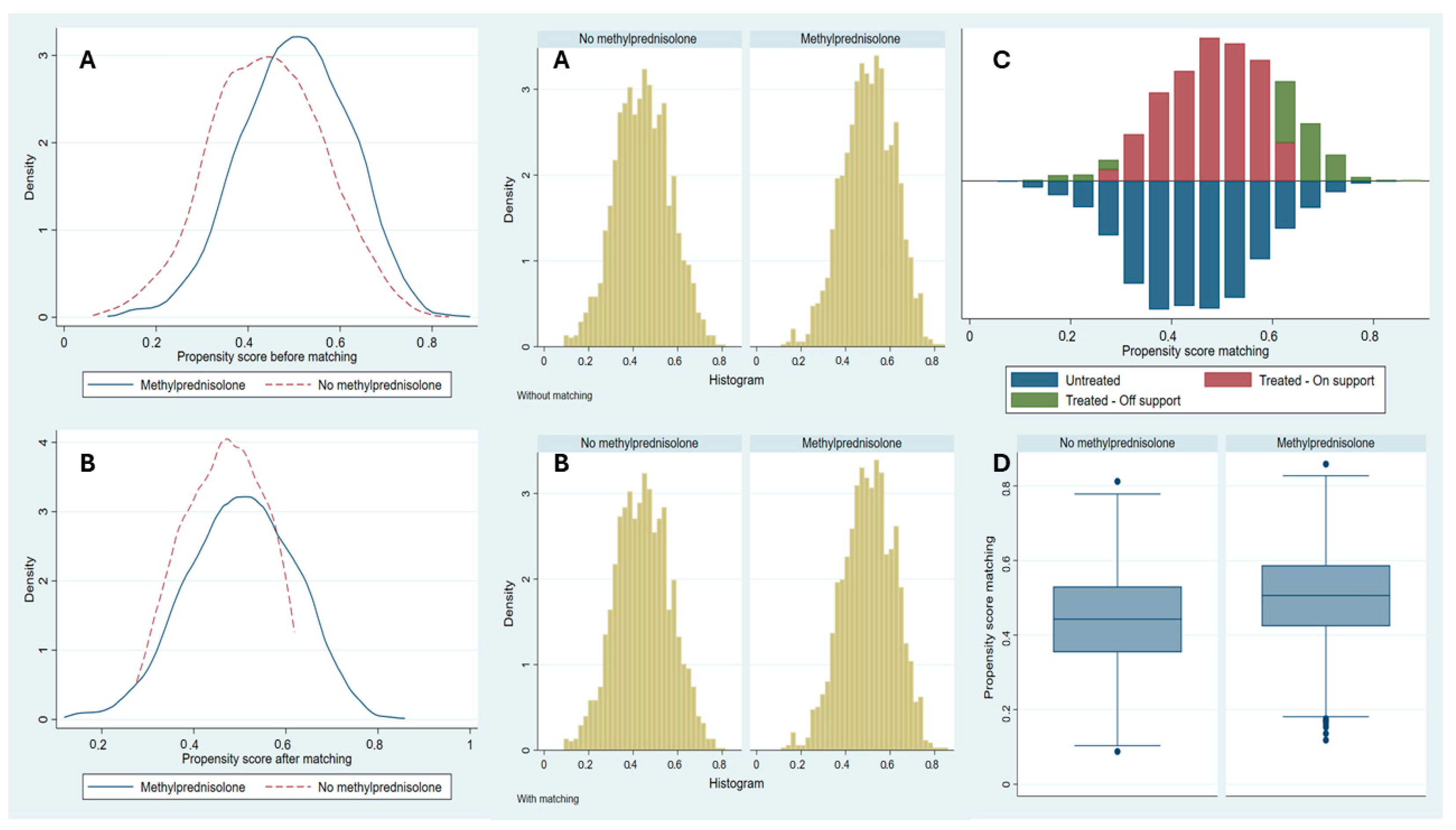
3.3. PSM
3.4. Factors Associated with Methylprednisolone Administration and RTI-ICU Risk in the Logistic Regression Model
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Abrahao-Machado, L.F.; Dahal, S.; Kumar, H.; Kv, D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758. [Google Scholar] [CrossRef]
- World Health Organization. WHO COVID-19 Dashboard. [Updated 2024]. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 19 June 2024).
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Boechat, J.L.; Chora, I.; Morais, A.; Delgado, L. The immune response to SARS-CoV-2 and COVID-19 immunopathology—Current perspectives. Pulmonology 2021, 27, 423–437. [Google Scholar] [CrossRef]
- Choi, H.S.; Choi, A.Y.; Kopp, J.B.; Winkler, C.A.; Cho, S.K. Review of COVID-19 Therapeutics by Mechanism: From Discovery to Approval. J. Korean Med. Sci. 2024, 39, e134. [Google Scholar] [CrossRef]
- Zhang, G.; Su, L.; Wu, W.; Qiao, Q.; Gao, S.; Zhang, Y.; Zhang, Y. Efficacy of different doses of corticosteroids in treating severe COVID-19 pneumonia. Virol J. 2024, 21, 74. [Google Scholar] [CrossRef]
- Wagner, C.; Griesel, M.; Mikolajewska, A.; Metzendorf, M.I.; Fischer, A.L.; Stegemann, M.; Spagl, M.; Nair, A.A.; Daniel, J.; Fichtner, F.; et al. Systemic corticosteroids for the treatment of COVID-19: Equity-related analyses and update on evidence. Cochrane Database Syst. Rev. 2022, 11, CD014963. [Google Scholar] [CrossRef] [PubMed]
- Salton, F.; Confalonieri, P.; Centanni, S.; Mondoni, M.; Petrosillo, N.; Bonfanti, P.; Lapadula, G.; Lacedonia, D.; Voza, A.; Carpene, N.; et al. Prolonged higher dose methylprednisolone versus conventional dexamethasone in COVID-19 pneumonia: A randomised controlled trial (MEDEAS). Eur. Respir. J. 2023, 61, 2201514. [Google Scholar] [CrossRef] [PubMed]
- Aljuhani, O.; Korayem, G.B.; Altebainawi, A.F.; AlMohammady, D.; Alfahed, A.; Altebainawi, E.F.; Aldhaeefi, M.; Badreldin, H.A.; Vishwakarma, R.; Almutairi, F.E.; et al. Dexamethasone versus methylprednisolone for multiple organ dysfunction in COVID-19 critically ill patients: A multicenter propensity score matching study. BMC Infect. Dis. 2024, 24, 189. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.J.; Wu, C.; Mehta, N.; Wald-Dickler, N.; Yang, W.; Qiao, R. A Comparison of Methylprednisolone and Dexamethasone in Intensive Care Patients with COVID-19. J. Intensive Care Med. 2021, 36, 673–680. [Google Scholar] [CrossRef]
- Al Sulaiman, K.; Aljuhani, O.; Korayem, G.B.; Altebainawi, A.; Alharbi, R.; Assadoon, M.; Aldhaeefi, M.; Badreldin, H.A.; Vishwakarma, R.; Almutairi, F.E.; et al. Evaluation of the use of methylprednisolone and dexamethasone in asthma critically ill patients with COVID-19: A multicenter cohort study. BMC Pulm. Med. 2023, 23, 315. [Google Scholar]
- Hong, S.; Wang, H.; Li, S.; Liu, J.; Qiao, L. A systematic review and meta-analysis of glucocorticoids treatment in severe COVID-19: Methylprednisolone versus dexamethasone. BMC Infect. Dis. 2023, 23, 290. [Google Scholar] [CrossRef]
- Ebrahimi Chaharom, F.; Pourafkari, L.; Ebrahimi Chaharom, A.A.; Nader, N.D. Effects of corticosteroids on COVID-19 patients: A systematic review and meta-analysis on clinical outcomes. Pulm. Pharmacol. Ther. 2022, 72, 102107. [Google Scholar] [CrossRef] [PubMed]
- Latarissa, I.R.; Rendrayani, F.; Iftinan, G.N.; Suhandi, C.; Meiliana, A.; Sormin, I.P.; Barliana, M.I.; Lestari, K. The Efficacy of Oral/Intravenous Corticosteroid Use in COVID-19 Patients: A Systematic Review. J. Exp. Pharmacol. 2024, 16, 321–337. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, C.; Hu, W.; Lu, D.; Chen, C.; Gong, S.; Yan, J.; Mao, W. Efficacy and Safety of Glucocorticoid in the Treatment of Acute Respiratory Distress Syndrome caused by COVID-19: A Systematic Review and Meta-Analysis. Clin. Investig. Med. 2023, 46, E03–E18. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Ruiz-Botella, M.; Martín-Loeches, I.; Jimenez Herrera, M.; Solé-Violan, J.; Gómez, J.; Bodí, M.; Trefler, S.; Papiol, E.; Díaz, E.; et al. Deploying unsupervised clustering analysis to derive clinical phenotypes and risk factors associated with mortality risk in 2022 critically ill patients with COVID-19 in Spain. Crit. Care 2021, 25, 63. [Google Scholar] [CrossRef]
- Reyes, L.F.; Rodriguez, A.; Bastidas, A.; Parra-Tanoux, D.; Fuentes, Y.V.; García-Gallo, E.; Moreno, G.; Ospina-Tascon, G.; Hernandez, G.; Silva, E.; et al. Dexamethasone as risk-factor for ICU-acquired respiratory tract infections in severe COVID-19. J. Crit. Care 2022, 69, 154014. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; p. 162. [Google Scholar]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Geronimi, C.B.; et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Med. 2021, 47, 188–198. [Google Scholar] [CrossRef]
- Luyt, C.E.; Sahnoun, T.; Gautier, M.; Vidal, P.; Burrel, S.; de Chambrun, M.P.; Chommeloux, J.; Desnos, C.; Arzoine, J.; Nieszkowska, A.; et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: A retrospective cohort study. Ann. Intensive Care 2020, 10, 158. [Google Scholar] [CrossRef]
- Reyes, L.F.; Rodriguez, A.; Fuentes, Y.V.; Duque, S.; García-Gallo, E.; Bastidas, A.; Serrano-Mayorga, C.C.; Ibanez-Prada, E.D.; Moreno, G.; Ramirez-Valbuena, P.C.; et al. Risk factors for developing ventilator-associated lower respiratory tract infection in patients with severe COVID-19: A multinational, multicentre study, prospective, observational study. Sci. Rep. 2023, 13, 6553. [Google Scholar] [CrossRef]
- Pinzón, M.A.; Ortiz, S.; Holguín, H.; Betancur, J.F.; Cardona Arango, D.; Laniado, H.; Arias, C.A.; Munoz, B.; Quiceno, J.; Jaramillo, D.; et al. Dexamethasone vs methylprednisolone high dose for COVID-19 pneumonia. PLoS ONE 2021, 16, e0252057. [Google Scholar] [CrossRef]
- Morsali, M.; Doosti-Irani, A.; Amini, S.; Nazemipour, M.; Mansournia, M.A.; Aliannejad, R. Comparison of corticosteroids types, dexamethasone, and methylprednisolone in patients hospitalized with COVID-19: A systematic review and network meta-analysis. Glob. Epidemiol. 2023, 6, 100116. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, D.; Gao, F.; Huang, W.; Wang, J.; Li, Y.; Chen, B.; Zhong, Y.; Chen, R.; Huang, M. Efficacy of corticosteroids in patients with acute respiratory distress syndrome: A meta-analysis. Ann. Med. 2024, 56, 2381086. [Google Scholar] [CrossRef]
- Ruan, S.Y.; Lin, H.H.; Huang, C.T.; Kuo, P.H.; Wu, H.D.; Yu, C.J. Exploring the heterogeneity of effects of corticosteroids on acute respiratory distress syndrome: A systematic review and meta-analysis. Crit. Care 2014, 18, R63. [Google Scholar] [CrossRef]
- Li, Z.; Xue, Y.; Li, L.; Li, C. Methylprednisolone or dexamethasone? How should we choose to respond to COVID-19?: A systematic review and meta-analysis of randomized controlled trials. Medicine 2023, 102, e34738. [Google Scholar] [CrossRef] [PubMed]
- Haan, B.J.; Blackmon, S.N.; Cobb, A.M.; Cohen, H.E.; DeVier, M.T.; Perez, M.M.; Winslow, S.F. Corticosteroids in critically ill patients: A narrative review. Pharmacotherapy 2024, 44, 581–602. [Google Scholar] [CrossRef] [PubMed]
- Kapugi, M.; Cunningham, K. Corticosteroids. Orthop. Nurs. 2019, 38, 336–339. [Google Scholar] [CrossRef]
- Gibbison, B.; López-López, J.A.; Higgins, J.P.; Miller, T.; Angelini, G.D.; Lightman, S.L.; Annane, D. Corticosteroids in septic shock: A systematic review and network meta-analysis. Crit. Care 2017, 21, 78. [Google Scholar] [CrossRef]
- Guzzardella, A.; Motos, A.; Vallverdú, J.; Torres, A. Corticosteroids in sepsis and community-acquired pneumonia. Med. Klin. Intensivmed. Notfmed. 2023, 118, 86–92. [Google Scholar] [CrossRef]
- Akter, F.; Araf, Y.; Hosen, M.J. Corticosteroids for COVID-19: Worth it or not? Mol. Biol. Rep. 2022, 49, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Camirand-Lemyre, F.; Merson, L.; Tirupakuzhi Vijayaraghavan, B.K.; Burrell, A.J.C.; Citarella, B.W.; Domingue, M.P.; Levesque, S.; Usuf, E.; Wils, E.-J.; Ohshimo, S.; et al. Implementation of Recommendations on the Use of Corticosteroids in Severe COVID-19. JAMA Netw. Open 2023, 6, e2346502. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group. Higher dose corticosteroids in patients admitted to hospital with COVID-19 who are hypoxic but not requiring ventilatory support (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2023, 401, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Granholm, A.; Kjær, M.N.; Munch, M.W.; Myatra, S.N.; Vijayaraghavan, B.K.T.; Cronhjort, M.; Wahlin, R.R.; Jakob, S.M.; Cioccari, L.; Vesterlund, G.K.; et al. Long-term outcomes of dexamethasone 12 mg versus 6 mg in patients with COVID-19 and severe hypoxaemia. Intensive Care Med. 2022, 48, 580–589. [Google Scholar] [CrossRef]
- Perner, A.; Venkatesh, B. Higher-dose dexamethasone for patients with COVID-19 and hypoxaemia? Lancet 2023, 401, 1474–1476. [Google Scholar] [CrossRef]
| Original Cohort | Match Cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | All Cohort n = 3239 | Methylprednisolone n = 1527 | No Methylprednisolone n = 1712 | Valor p | All Match n = 2444 | Methylprednisolone n = 1222 | No Methylprednisolone n = 1222 | Valor p |
| Age, mean (SD) | 61.2 (13.1) | 61.8 (12.7) | 60.6 (13.2) | 0.010 * | 60.9 (13.1) | 61.4 (12.9) | 60.6 (13.2) | 0.096 |
| Male, n (%) | 2228 (68.8) | 1084 (71) | 1144 (66.8) | 0.011 * | 1663 (68) | 846 (69.2) | 817 (66.8) | 0.169 |
| Comorbid conditions, n (%) | ||||||||
| Congestive heart failure | 180 (5.6) | 79 (5.2) | 101 (5.9) | 0.368 | 140 (5.7) | 68 (5.6) | 72 (5.9) | 0.701 |
| Hypertension | 1472 (45.4) | 727 (47.6) | 745 (43.5) | 0.020 * | 1089 (44.5) | 557 (45.6) | 532 (43.5) | 0.267 |
| chronic pulmonary disease | 260 (7.5) | 118 (7.3) | 142 (7.8) | 0.553 | 204 (8.4) | 103 (8.4) | 101 (8.3) | 0.897 |
| Asthma | 179 (5.5) | 84 (5.5) | 95 (5.5) | 0.952 | 142 (5.8) | 74 (6.1) | 68 (5.5) | 0.562 |
| Renal chronic disease | 172 (5.3) | 82 (5.4) | 90 (5.3) | 0.886 | 128 (5.2) | 64 (5.2) | 64 (5.3) | 0.981 |
| Diabetes | 711 (22) | 389 (25.5) | 322 (18.8) | <0.001 * | 473 (19.3) | 243 (19.9) | 230 (18.8) | 0.466 |
| Neurologic disease | 46 (1.4) | 23 (1.5) | 23 (1.3) | 0.696 | 29 (1.2) | 13 (1.1) | 16 (1.3) | 0.498 |
| Hematological disease | 92 (2.8) | 41 (2.7) | 51 (3) | 0.615 | 74 (3) | 38 (3.1) | 36 (3) | 0.839 |
| HIV-AIDS | 8 (0.2) | 1 (0.1) | 7 (0.4) | 0.049 | 5 (0.2) | 0 (0) | 5 (0.4) | 0.148 |
| Obesity | 1138 (35.1) | 640 (41.9) | 498 (29.1) | <0.001 * | 766 (31.4) | 411 (33.6) | 355 (29.1) | 0.009 * |
| Rheumatological disease | 106 (3.3) | 58 (3.8) | 48 (2.8) | 0.112 | 75 (3.1) | 41 (3.4) | 34 (2.8) | 0.391 |
| FiO2%, mean (SD) | 64.8 (26.9) | 68.6 (25.6) | 61.4 (27.6) | <0.001 * | 63.1 (26.8) | 65.5 (25.3) | 61.4 (27.6) | <0.001 * |
| PaO2 mmHg, mean (SD) | 75.8 (26.6) | 77.2 (27.7) | 74.6 (25.5) | 0.004 * | 75 (25.2) | 75.6 (24.8) | 74.6 (25.5) | 0.268 |
| PaO2/FiO2 mmHg, n (%) | ||||||||
| >400 | 70 (2.2) | 26 (1.7) | 44 (2.6) | <0.001 * | 56 (2.3) | 25 (2) | 31 (2.6) | 0.006 * |
| 0–50 | 109 (3.4) | 57 (3.7) | 52 (3) | 81 (3.3) | 44 (3.6) | 37 (3) | ||
| 100–150 | 800 (24.7) | 392 (25.7) | 408 (23.8) | 622 (25.5) | 331 (27.1) | 291 (23.8) | ||
| 150–200 | 475 (14.7) | 211 (13.8) | 264 (15.4) | 356 (14.6) | 168 (13.7) | 188 (15.4) | ||
| 200–250 | 262 (8.1) | 111 (7.3) | 151 (8.8) | 207 (8.5) | 99 (8.1) | 108 (8.8) | ||
| 250–300 | 177 (5.5) | 64 (4.2) | 113 (6.6) | 135 (5.5) | 54 (4.4) | 81 (6.6) | ||
| 300–350 | 103 (3.2) | 35 (2.3) | 68 (4) | 80 (3.3) | 31 (2.5) | 49 (4) | ||
| 350–400 | 49 (1.5) | 15 (1) | 34 (2) | 39 (1.6) | 15 (1.2) | 24 (2) | ||
| 50–100 | 1194 (36.9) | 616 (40.3) | 578 (33.8) | 868 (35.5) | 455 (37.2) | 413 (33.8) | ||
| Leucocytes cell × 103 mL, mean (SD) | 10 (5.45) | 10.3 (5.54) | 9.8 (5.3) | 0.008 * | 9.8 (5.2) | 9.9 (5) | 9.8 (5.3) | 0.428 |
| Creatinine mg/dL, mean (SD) | 1.1 (1.1) | 1.1 (0.8) | 1.1 (1.21) | 0.536 | 1.1 (1.1) | 1.1 (0.9) | 1.1 (1.2) | 0.481 |
| RCP mg/dL, mean (SD) | 26.4 (39.1) | 22.2 (30.5) | 30.2 (44.9) | <0.001 * | 26 (37.9) | 20.1 (23.9) | 30.2 (44.9) | <0.001 * |
| Chest X Ray, n (%) | ||||||||
| Lung infiltrates, n (%) | 2750 (84.9) | 1186 (77.7) | 1564 (91.4) | <0.001 * | 2080 (85.1) | 964 (78.9) | 1116 (91.4) | <0.001 * |
| Tocilizumab, n (%) | 633 (19.5) | 338 (22.1) | 295 (17.2) | <0.001 * | 452 (18.5) | 241 (19.7) | 211 (17.2) | 0.085 |
| VAP, n (%) | 462 (14.3) | 268 (17.6) | 194 (11.3) | <0.001 * | 338 (13.8) | 200 (16.4) | 138 (11.3) | <0.001 * |
| VAT, n (%) | 192 (5.9) | 102 (6.7) | 90 (5.3) | 0.087 | 142 (5.8) | 78 (6.4) | 64 (5.3) | 0.196 |
| HAP (without NAV and without VAT), n (%) | 8 (0.2) | 3 (0.2) | 5 (0.3) | 0.584 | 7 (0.3) | 3 (0.2) | 4 (0.3) | 0.812 |
| IMV, n (%) | 2364 (73) | 1248 (81.7) | 1116 (65.2) | <0.001 * | 1778 (72.7) | 981 (80.3) | 797 (65.2) | <0.001 * |
| IVM days, mean (SD) | 12.6 (14.1) | 15 (14.67) | 10.4 (13.1) | <0.001 * | 12.2 (14.1) | 14.8 (14.9) | 10.4 (13.1) | <0.001 * |
| Pronation, n (%) | 1803 (55.7) | 1029 (67.4) | 774 (45.2) | <0.001 * | 1358 (55.6) | 806 (66) | 552 (45.2) | <0.001 * |
| NIMV, n (%) | 300 (9.3) | 149 (9.8) | 151 (8.8) | 0.358 | 231 (9.4) | 123 (10.1) | 108 (8.8) | 0.253 |
| NIVM days, mean (SD) | 0.3 (1.3) | 0.3 (1.1) | 0.3 (1.4) | 0.921 | 0.3 (1.3) | 0.3 (1.1) | 0.3 (1.4) | 0.759 |
| HFNC, n (%) | 802 (24.8) | 478 (31.3) | 324 (18.9) | <0.001 * | 627 (25.7) | 396 (32.4) | 231 (18.9) | <0.001 * |
| ICU days, mean (SD) | 17.3 (15.3) | 19.3 (16.2) | 15.5 (14.3) | <0.001 * | 17.1 (15.4) | 19.3 (16.6) | 15.5 (14.3) | <0.001 * |
| Stay hospital days, mean (SD) | 27.6 (21.2) | 30.1 (21.8) | 25.3 (20.3) | <0.001 * | 27.4 (21.3) | 30.2 (22.2) | 25.3 (20.3) | <0.001 * |
| Mortality, n (%) | 1013 (31.3) | 519 (34) | 494 (28.9) | 0.002 * | 754 (30.8) | 802 (32.8) | 705 (28.9) | 0.022 * |
| Original Cohort | ||||
|---|---|---|---|---|
| Characteristic | All Cohort n = 3239 | RTI-ICU n = 660 | No RTI-ICU n = 2579 | Valor p |
| Age, mean (SD) | 61.2 (13.01) | 63 (11.97) | 60.7 (13.22) | <0.001 * |
| Male, n (%) | 2228 (68.8) | 484 (73.3) | 1744 (67.6) | 0.005 * |
| Comorbid conditions, n (%) | ||||
| Congestive heart failure | 180 (5.6) | 30 (4.5) | 150 (5.8) | 0.204 |
| Hypertension | 1472 (45.4) | 315 (47.7) | 1157 (44.9) | 0.187 |
| chronic pulmonary disease | 260 (7.5) | 51 (7.3) | 209 (7.6) | 0.751 |
| Asthma | 179 (5.5) | 37 (5.6) | 142 (5.5) | 0.920 |
| Renal chronic disease | 172 (5.3) | 41 (6.2) | 131 (5.1) | 0.247 |
| Diabetes | 711 (22) | 148 (22.4) | 563 (21.8) | 0.742 |
| Neurologic disease | 46 (1.4) | 10 (1.5) | 36 (1.4) | 0.817 |
| Hematological disease | 92 (2.8) | 23 (3.5) | 69 (2.7) | 0.264 |
| HIV-AIDS | 8 (0.2) | 2 (0.3) | 6 (0.2) | 0.745 |
| Obesity | 1138 (35.1) | 230 (34.8) | 908 (35.2) | 0.863 |
| Rheumatological disease | 106 (3.3) | 19 (2.9) | 87 (3.4) | 0.524 |
| FiO2%, mean (SD) | 64.8 (26.98) | 72.5 (25.4) | 62.8 (27.03) | <0.001 * |
| PaO2 mmHg, mean (SD) | 75.8 (26.6) | 74 (26.24) | 76.3 (26.67) | 0.110 |
| PaO2/FiO2 mmHg, n (%) | ||||
| 0–50 | 109 (3.4) | 38 (5.8) | 71 (2.8) | <0.001 * |
| 50–100 | 1194 (36.9) | 302 (45.8) | 892 (34.6) | |
| 100–150 | 800 (24.7) | 172 (26.1) | 628 (24.4) | |
| 150–200 | 475 (14.7) | 64 (9.7) | 411 (15.9) | |
| 200–250 | 262 (8.1) | 34 (5.2) | 228 (8.8) | |
| 250–300 | 177 (5.5) | 30 (4.5) | 147 (5.7) | |
| 300–350 | 103 (3.2) | 11 (1.7) | 92 (3.6) | |
| 350–400 | 49 (1.5) | 2 (0.3) | 47 (1.8) | |
| >400 | 70 (2.2) | 7 (1.1) | 63 (2.4) | |
| Leucocytes cell × 103 mL, mean (SD) | 10 (5.45) | 10.8 (5.64) | 9.8 (5.38) | <0.001 * |
| Creatinine mg/dL, mean (SD) | 1.1 (1.06) | 1.2 (1.34) | 1.1 (0.97) | 0.100 |
| RCP mg/dL, mean (SD) | 26.4 (39.05) | 37.9 (54.17) | 23.5 (33.51) | <0.001 * |
| Chest X Ray, n (%) | ||||
| Lung infiltrates, n (%) | 2750 (84.9) | 577 (87.4) | 2173 (84.3) | <0.001 * |
| Tocilizumab, n (%) | 633 (19.5) | 133 (20.2) | 500 (19.4) | <0.001 * |
| VAP, n (%) | 462 (14.3) | 462 (70) | (0) | <0.001 * |
| VAT, n (%) | 192 (5.9) | 192 (29.1) | (0) | <0.001 * |
| HAP (without NAV and without VAT), n (%) | 8 (0.2) | 8 (1.2) | (0) | <0.001 * |
| IMV, n (%) | 2364 (73) | 656 (99) | 1708 (66) | <0.001 * |
| IVM days mean (SD) | 12.6 (14.08) | 24.6 (15.97) | 9.5 (11.73) | <0.001 * |
| Prono, n (%) | 1803 (55.7) | 521 (78.9) | 1282 (49.7) | <0.001 * |
| NIMV, n (%) | 300 (9) | 46 (7) | 254 (10) | 0.023 * |
| NIVM days mean (SD) | 0.3 (1.33) | 0.2 (0.8) | 0.3 (1.43) | 0.001 * |
| HFNC, n (%) | 802 (25) | 130 (20) | 672 (26) | <0.001 * |
| ICU days mean (SD) | 17.3 (15.35) | 29.1 (17.62) | 14.3 (13.11) | <0.001 * |
| Stay hospital days mean (SD) | 27.6 (21.21) | 40 (24.94) | 24.4 (18.87) | <0.001 * |
| Mortality, n (%) | 1013 (31) | 259 (39.2) | 754 (29.2) | <0.001 * |
| Method | ATE | 95% CI | p-Value | ATET | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Logistic regression | 0.076 | 0.043–0.110 | <0.001 | 0.088 | 0.052–0.124 | <0.001 |
| Nearest Neighbor matching | 0.078 | 0.045–0.111 | <0.001 | 0.076 | 0.039–0.114 | <0.001 |
| Adjusted regression | 0.073 | 0.045–0.101 | <0.001 | 0.075 | 0.046–0.104 | <0.001 |
| Inverse probability weighting | 0.073 | 0.044–0.102 | <0.001 | 0.074 | 0.045–0.102 | <0.001 |
| Variables | OR | 95% CI | p-Value |
|---|---|---|---|
| Methylprednisolone treatment | |||
| Sex | 1.22 | 1.05–1.43 | 0.009 |
| Age | 1.01 | 1.00–1.02 | 0.009 |
| Obesity | 1.75 | 1.51–2.04 | <0.001 |
| Diabetes | 1.32 | 1.11–1.57 | 0.002 |
| Leucocytes, 109 cells/L | 1.01 | 1.00–1.03 | 0.013 |
| PaO2 | 1.01 | 1.00–1.02 | 0.004 |
| Higher FiO2 support, % | 1.01 | 1.00–1.02 | <0.001 |
| Tocilizumab | 1.38 | 1.15–1.65 | <0.001 |
| Risk of RTI-UCI | |||
| Sex | 1.26 | 1.03–1.53 | 0.021 |
| Age | 1.01 | 1.00–1.02 | <0.001 |
| Leucocytes, 109 cells/L | 1.02 | 1.00–1.03 | <0.001 |
| Proteína C reactiva, mg/dl | 1.01 | 1.00–1.02 | <0.001 |
| Higher FiO2 Support, % | 1.01 | 1.00–1.02 | <0.001 |
| Methylprednisolone | 1.59 | 1.33–1.91 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuta-Quintero, E.; Bastidas, A.; García-Gallo, E.; Díaz, E.; Bodí, M.; Solé-Violán, J.; Ferrer, R.; Albaya-Moreno, A.; Socias, L.; Estella, Á.; et al. Association Between Methylprednisolone and the Increase of Respiratory Infections in COVID-19 Patients in the Intensive Care Unit. COVID 2025, 5, 204. https://doi.org/10.3390/covid5120204
Tuta-Quintero E, Bastidas A, García-Gallo E, Díaz E, Bodí M, Solé-Violán J, Ferrer R, Albaya-Moreno A, Socias L, Estella Á, et al. Association Between Methylprednisolone and the Increase of Respiratory Infections in COVID-19 Patients in the Intensive Care Unit. COVID. 2025; 5(12):204. https://doi.org/10.3390/covid5120204
Chicago/Turabian StyleTuta-Quintero, Eduardo, Alirio Bastidas, Esteban García-Gallo, Emilio Díaz, María Bodí, Jordi Solé-Violán, Ricard Ferrer, Antonio Albaya-Moreno, Lorenzo Socias, Ángel Estella, and et al. 2025. "Association Between Methylprednisolone and the Increase of Respiratory Infections in COVID-19 Patients in the Intensive Care Unit" COVID 5, no. 12: 204. https://doi.org/10.3390/covid5120204
APA StyleTuta-Quintero, E., Bastidas, A., García-Gallo, E., Díaz, E., Bodí, M., Solé-Violán, J., Ferrer, R., Albaya-Moreno, A., Socias, L., Estella, Á., Loza-Vazquez, A., Jorge-García, R., Sancho, I., Martin-Loeches, I., Rodriguez, A., & Reyes, L. F., on behalf of the LIVEN-COVID-19 Investigators and COVID-19 SEMICYUC Study Group2. (2025). Association Between Methylprednisolone and the Increase of Respiratory Infections in COVID-19 Patients in the Intensive Care Unit. COVID, 5(12), 204. https://doi.org/10.3390/covid5120204








