Abstract
Patients who survive critical COVID-19 frequently report post-acute sequelae of COVID-19 (PASC) such as psychosomatic and neurocognitive health problems. The goal of this study was to identify clinical risk factors and other predictors for such long-term consequences in severely ill COVID-19 patients. Adult COVID-19 intensive care unit (ICU) survivors from August 2020 to May 2021 were enrolled. A broad range of clinical, laboratory and chest computed tomography (CT) data was collected during their ICU stays. The association between ICU predictors and psychosomatic, respiratory, and neurocognitive assessments 12 months after ICU discharge was analyzed using univariate regression analysis. In 17 patients (mean age 58.9 ± 11.4 years), laboratory markers (CRP, lymphocytes, hemoglobin), ICU severity (SOFA, SAPS II, need for mechanical ventilation), complications (ARDS), and lung CT data (ground-glass opacity) were promising predictors of depressive and anxiety symptoms, fatigue, and sleep problems. Recovery of psychosomatic health such as fatigue, depression, and anxiety correlated with lower levels of inflammation and high hemoglobin levels. ARDS, mechanical ventilation, and worse SOFA and SAPS II scores were further risk factors for depressive and anxiety symptoms. Our study identified novel associations such as pulmonary ground-glass opacity being positively associated with depression, anxiety, fatigue, and insomnia levels.
1. Introduction
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pushed worldwide healthcare systems to their limits. Most affected individuals were asymptomatic or suffered from mild flu-like symptoms, while the global hospitalization rate was about 5%, with 12–25% of patients requiring intensive care unit (ICU) admission [1,2,3]. In Switzerland, about 15% of hospitalized patients were admitted to the ICU during the first and second waves [4,5]. Up to 98% of hospitalized coronavirus disease 2019 (COVID-19) patients had at least one underlying comorbidity like hypertension, chronic kidney disease, chronic respiratory disease, type 2 diabetes mellitus, or were immunocompromised [4,6]. These patients are also at risk of developing ICU-acquired weakness (ICUAW), a multifactorial condition in which polyneuropathy, myopathy, and/or disuse muscle atrophy result in motor weakness [7].
Reports indicate that critically ill COVID-19 patients who survived their ICU stay frequently reported persistent mental health problems at 1 and 6 months after ICU discharge. Up to one-third of patients experienced moderate symptoms of anxiety and depression, alongside medium to high stress levels [8]. COVID-19 survivors further showed somatic symptoms like loss of appetite, ageusia, anosmia, nausea, diarrhea, and weight and muscle loss in post-ICU recovery [9,10]. Post-acute sequelae of COVID-19 (PASC) encompass persistent, exacerbated, or newly occurring neuropsychological and physical symptoms following acute COVID-19 illness [11,12]. These include persistent shortness of breath, reduced mobility, fatigue, and memory impairment [13]. The development of PASC during or after a COVID-19 infection that persists for longer than four weeks and cannot be explained by any alternative diagnosis has been defined as “Long COVID”. At present, the exact definition of Long COVID is still evolving [14,15]. It poses a substantial challenge to healthcare systems, with WHO estimating a prevalence between 10 and 20% [16]. The elusive diagnosis and lack of treatment options for Long COVID often result in referrals to multiple specialists, amplifying healthcare costs [17].
In this study, we assessed the frequency and severity of long-term complaints in a cohort of COVID-19 patients needing ICU treatment. Further, we investigated associations between these complaints and clinical risk factors collected during the acute ICU phase. The clinical outcomes focused on psychosomatic aspects alongside respiratory and neurocognitive aspects 12 months after the initial ICU admission. The goal of this study was to identify ICU factors, in addition to pre-existing factors, that may predict 12-month follow-up outcomes in critically ill COVID-19 patients.
2. Materials and Methods
2.1. Screening and Informed Consent
Adult patients (≥18 years) with laboratory-confirmed (polymerase chain reaction; PCR) and severe COVID-19 disease needing ICU treatment between 24 February 2020, and 1 March 2021 were enrolled in a multicenter observational cohort study in three Swiss tertiary care hospitals [18]. Exclusion criteria were a COVID-19 diagnosis after ICU discharge or a lack of informed consent. All the ICU survivors were invited by e-mail and phone to participate in this single-center follow-up study at the Cantonal Hospital St. Gallen.
2.2. Study Procedure and Data Collection
The survivors were investigated for long-term outcomes at the Cantonal Hospital St. Gallen, a tertiary hospital in Eastern Switzerland, between March 2021 and April 2022, 12 (±1) months after ICU discharge. Informed consent was obtained from all the patients. If a patient was unable to provide informed consent, proxy consent was obtained from relatives. We extracted health-related data including demographics, medical history, chest computed tomography (CT) scans, laboratory data, ICU severity scores (within the first 24h after ICU admission), complications, and treatment forms from the electronic medical records. We quantified lung CT data at ICU admission, focusing on typical COVID-19 radiological patterns, such as crazy-paving, ground-glass opacity, as well as light and heavy consolidation, as a relative expression of healthy lung tissue [19,20]. Concerning dietary behavior, we retrospectively assessed patients’ consumption of various food products (individual foods and food groups such as fruit, vegetables, meat, and fish) and beverages over a 4-week period at the time of COVID-19 onset with the Food Frequency Questionnaire (FFQ) originated from the University Hospitals of Geneva [21]. When evaluating the FFQ, reference was made to the recommended nutritional portion of a specific food group based on Swiss recommendations [21]. Patients fulfilled these recommendations with the intake of fruit (≥2/day), vegetables (≥3/day), meat (≤5/week), and fish (≥1/week). According to the overall Swiss recommendations, healthy eating means meeting at least three of the four food group recommendations mentioned above [21].
The patients completed several validated questionnaires to evaluate persistent COVID-19 symptoms, current psychosomatic health, respiratory, and neurocognitive function at the Cantonal Hospital St. Gallen 12 (±1) months after ICU discharge (Table S1). Psychosomatic questionnaires were completed at the study site either in tablet or paper and pencil format with the assistance of the study team, including an interpreter when necessary due to the questionnaires being in the German language [22,23,24,25,26,27]. Neurologists assessed neurocognitive function using validated tools and performed clinical examinations to exclude neurodegenerative or other neurological conditions that could impair cognition [28,29,30]. We further measured cognitive performance using both subjective (by patients) and external (by relatives) evaluations. Patients then underwent pulmonary function and exercise tolerance testing at the outpatient clinic of the Department of Pulmonary and Sleep Medicine at the Cantonal Hospital St. Gallen. We further used WatchPAT (Itamar Medical Ltd. Caesarea, Israel), a portable device used for sleep monitoring and diagnosing sleep disorders, to analyze sleep architecture during a typical night’s sleep at the patients’ homes. WatchPAT measures various parameters during sleep, including breathing rate, blood oxygen saturation, snoring, and movements, enabling a comprehensive assessment of sleep patterns and identification of potential sleep-related issues [31]. See Supplemental Table S1 for all the collected data 12 (±1) months after ICU discharge.
2.3. Statistical Analyses
Our study design required a total of 55 patients for an expected 80% statistical power based on a priori power analysis with Gpower software (v.3.1.9.7) for a simple linear regression and an expected medium effect size (Cohen’s F2) of 0.15 [32]. We imputed missing predictor data with the MICE (multiple imputation by chained equation) R package (v3.9.0) [33]. Using a strategy called multiple imputation, then deletion (MID), we used all the cases for imputation of the predictor variables (X) and then excluded all the cases with imputed endpoint variables (Y) from the analysis [34].
As predictor variables, we used clinical characteristics and risk factors including demographics, personal medical history, nutritional habits at COVID-19 onset, lung CT results, laboratory data, ICU severity scores, complications, and treatment forms during the ICU stay. We first analyzed clinical outcomes at the 12-month follow-up visit with a descriptive approach. Therefore, we analyzed all interval-scaled and continuous endpoints by creating nested violin boxplots with the use of the ggplot2 R package (v3.5.1) [35]. The distributions of psychosomatic and neurocognitive score results were compared visually with established cut-off values from the literature [22,23,24,25,26,27,28,29,30]. In the second step, we performed univariate linear, logistic, and ordinal regression models to investigate the association between demographic and ICU variables and 12-month clinical outcomes. Cluster analysis was performed to identify relevant groups of associations between possible predictors and endpoints using the gplots R package (v3.1.3.1) [36]. We used R statistical software (v4.1.1) and a significance level of 5% for all the statistical analyses with Bonferroni correction for multiple testing [37,38].
3. Results
Of 58 severely ill COVID-19 patients treated in the ICU at the Cantonal Hospital St. Gallen, 22 (~38%) died, and 19 (~33%) decided not to participate in this follow-up study. A total of 17 patients (mean age 58.9 ± SD 11.4, range 35–77) were included in the cohort. Death (~38%) and lack of willingness to participate in the follow-up study (~33%) were the main reasons for the small sample size. Missing predictor variables at ICU admission consisted of lung CT data (11.8%) and laboratory data, specifically, lymphocytes (23.5%), interleukin-6 (29.4%), troponin (11.8%), NT-proBNP (17.6%), LDH (5.9%), and D-dimers (29.4%). There were no missing values concerning psychosomatic endpoints. Patients showed 17.6% and 4.8% missing values in the respiratory and neurocognitive endpoints, respectively. In detail, the missing data consisted of the WatchPAT parameters and items of the MoCA test. Table 1 shows patients’ baseline characteristic symptoms at COVID-19 onset and the collected data during ICU stay, while Table S2 shows patients’ nutritional habits at COVID-19 onset.

Table 1.
Baseline and clinical variables during ICU stay.
At 12 (±1) months after ICU discharge, the most frequently reported symptoms were persisting cough (76.5%), headaches (64.7%), anosmia, dysgeusia, and loss of appetite in 58.8% of patients. When considering established cut-off points, very few patients showed score values for psychosomatic and neurocognitive diseases at the 12-month follow-up visit. Figure 1 provides a summary of the descriptive statistical analysis of all endpoints.
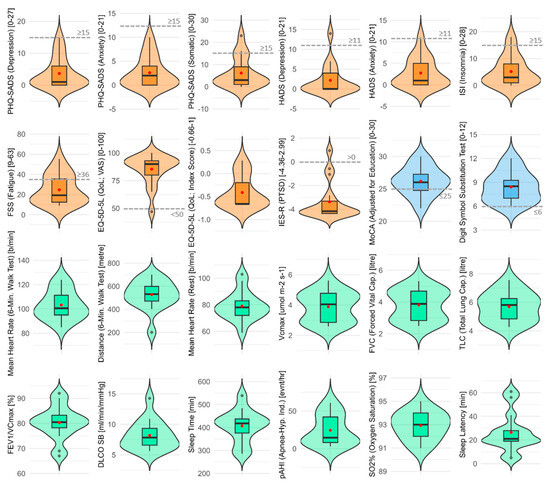
Figure 1.
Psychosomatic, respiratory, and neurocognitive outcomes 12 months after ICU discharge. Nested violin boxplots of all interval-scaled and continuous endpoints (orange for psychosomatic endpoints, green for respiratory endpoints, and blue for neurocognitive endpoints). In each plot, red points and dashed gray lines indicate mean values and clinical cut-off points, respectively.
Our sample size of 17 patients provided an actual statistical power of 32% based on a post hoc power analysis with Gpower software for a simple linear regression and an expected medium effect size (Cohen’s F2) of 0.15 [32]. Based on univariate linear, logistic, and ordinal regression analyses, we identified several variables associated with long-term outcomes. However, these findings did not remain statistically significant after Bonferroni adjustment for multiple testing. Figure 2 and Figure 3 show two heatmaps representing p-values and effect sizes in order to illustrate health clusters between predictors and associated endpoints. The following two examples illustrate how to read the figures: A high CRP was positively correlated with a higher score in the FSS questionnaire, indicating worse fatigue in a statistically relevant way, with a medium effect size (adjusted R2). Extracorporeal membrane oxygenation (ECMO) was negatively associated with total lung capacity (TLC) in a statistically relevant way and was therefore associated with a smaller lung capacity, with a medium effect size (adjusted R2).
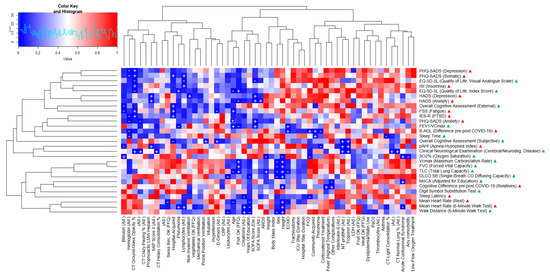
Figure 2.
Variables associated with clinical outcomes at 12 months (p-values, univariate regressions). Heatmap consisting of predictor variables (x-axis), endpoints (y-axis), and p-values (blue for p-values close to 0, red for p-values close to +1) for all calculated predictions, whereas a plus (+; positive regression coefficient) and minus (−; negative regression coefficient) both indicate statistical significance. The terms “Ad.” and “Dis.” next to a predictor stand for “ICU admission” and “ICU discharge”, respectively, indicating the time point of data collection. The five yes/no FFQ predictors are based on the Food Frequency Questionnaire where an “OK” indicates that they fulfilled Swiss nutritional recommendations [21]. Next to the endpoints, green and red triangles show that higher scores indicate better or worse outcomes, respectively.
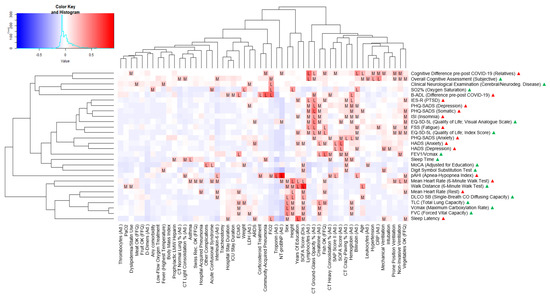
Figure 3.
Variables associated with clinical outcomes at 12 months (effect sizes, univariate regressions). Heatmap consisting of predictor variables (x-axis), endpoints (y-axis), and effect sizes (blue for effect sizes close to −1, red for effect sizes close to +1) for all calculated predictions, whereas “L” and “M” indicate large and medium effect sizes of adjusted R2 or pseudo-McFadden R2 ≥ 0.26. The terms “Ad.” and “Dis.” next to a predictor stand for “ICU admission” and “ICU discharge”, respectively, indicating the time point of data collection. The five yes/no FFQ predictors are based on the Food Frequency Questionnaire where an “OK” indicates that they fulfilled Swiss nutritional recommendations [21]. Next to the endpoints, green and red triangles show that higher scores indicate better or worse outcomes, respectively.
Inflammatory or infectious (CRP, lymphocytes), cardiac (troponin, NT-proBNP), renal (creatinine), and hematologic (hemoglobin) markers were among the most promising predictors. Other relevant predictors consisted of ICU clinical severity (SOFA, SAPS II, need for mechanical ventilation), complications (ARDS), and lung CT data (ground-glass opacity). The main endpoints associated with these predictors were psychosomatic outcome measures (fatigue, depression, and anxiety symptoms) and sleep problems (sleep apnea, insomnia). In particular, higher levels of inflammation (CRP, lymphocytes) predicted worse outcomes of fatigue, depression, and anxiety. The presence of ARDS and worse ICU severity scores (SOFA, SAPS II) were further predictors for worse depression and anxiety symptoms. The need for mechanical ventilation was significantly associated with higher depression scores. Concerning these affective symptoms, high hemoglobin and creatinine levels were associated with a better outcome. Cardiac markers (troponin, NT-proBNP) were further positively associated with sleep apnea. Ground-glass opacity in the lung CT scans was positively associated with depression, anxiety, fatigue, and insomnia levels, as well as an overall impairment of quality of life.
4. Discussion
Our study provides several main results. The higher the disease severity in the acute phase of COVID-19, the more likely patients needing ICU treatment will suffer from long-term sequelae. Higher levels of inflammatory markers, higher ICU severity scores, and markers of organ dysfunction—e.g., the need for mechanical ventilation, more ground-glass opacities in the lung CT scan, presence of ARDS, and elevated cardiac biomarkers—were associated with worse outcomes of fatigue and affective symptoms such as depression and anxiety, while higher hemoglobin and creatinine levels seemed to be protective factors.
Multiple neurological, cardiopulmonary, gastrointestinal, and dermatological symptoms, described as the multiorgan phenotype (MOP) of acute COVID-19, were linked to an elevated risk of protracted recovery [13]. When hospitalization was required, Evans et al. [11] identified female sex, obesity, and invasive mechanical ventilation in COVID-19 patients as associated with a lower likelihood of recovering full quality of life, with a substantial deficit in median EQ-5D-5L index score at one year after discharge. They further found a correlation between increased inflammatory mediators (including interleukin-6) and cognitive impairment at 5 months, emphasizing the idea of persistent systemic inflammation and its consequences on cognitive performance [11]. In our study, we confirmed the association of inflammatory markers (CRP, lymphocytes) with fatigue and affective symptoms. Irwin et al. [39] concluded that there is a growing body of evidence linking sleep disturbances to the risk of inflammatory diseases and all-cause mortality, possibly through the effects of sleep disturbances on two systemic inflammatory markers (CRP, interleukin-6). Our results showed the same relationship, as CRP (but not interleukin-6) positively predicted fatigue, however with a small effect size. Recently a correlation was shown between higher CRP in the general population and reduced sleep quality and increased fatigue, corroborating our findings [40,41,42,43,44]. Al-Hakeim et al. [45] mentioned the significant role of activated immune-inflammatory and oxidative and nitrosative stress (IO&NS) pathways in determining the long-term outcome of a COVID-19 infection. Chronic fatigue syndrome (CFS), major depression (MD), and generalized anxiety disorder (GAD) are all characterized by activated IO&NS pathways and increased levels of inflammatory mediators, including CRP [41]. Six months or more after SARS-CoV-2 infection, elevated CRP levels are significantly associated with concomitant fatigue severity [46]. Using a similar approach to our study, Saito et al. [47] analyzed 12-month post-infection outcomes in Long COVID patients with CFS. They found an association between Long COVID and elevated levels of plasma pro-inflammatory cytokines, chemokines, galectin-9, and artemin, reflecting an underlying chronic inflammation in these patients. In this context, an increased artemin concentration correlated with cognitive impairment. Further, Long COVID patients showed significantly elevated CRP levels 12 months after acute infection [47]. In contrast to the positive correlation observed between elevated lymphocyte levels and fatigue in our study, Swanink et al. [48] did not find any significant changes in absolute lymphocyte counts among patients with chronic fatigue syndrome (CFS) and the control group. However, aligning with our findings, Zheng et al. [49] and Saito et al. [47] described low levels of lymphocytes in acutely infected COVID-19 patients and Long COVID patients 12 months, respectively, after acute infection.
Similar to our study, Naudé et al. [50] described a correlation between elevated CRP levels and depression as well as generalized anxiety disorder. Furthermore, Azevedo et al. [51] demonstrated that patients who suffered from both SARS-CoV-2 infection and major depression showed notably higher CRP levels compared to COVID-19 patients without concurrent depression. It is plausible that inflammatory markers may help predict the long-term mental health outcome of COVID-19 and identify patients with fatigue and affective disorders.
One severe complication of the SARS-CoV-2 virus is acute respiratory distress syndrome (ARDS), which affected more than half of our patients. In our analysis, the presence of ARDS predicted 12-month anxiety. A multi-site, longitudinal cohort study found that ARDS survivors experienced persistent psychiatric issues, anxiety, depression, and posttraumatic stress disorder [52]. Palakshappa et al. [53] reported similar outcomes involving 629 patients from three different trials, with a significant proportion of ARDS survivors having substantial mental health symptoms. The prevalence of substantial symptoms of depression, anxiety, and PTSD at 6 months reached 36%, 42%, and 24%, respectively. Our study revealed a significant association between mechanical ventilation, the presence of ARDS, and depressive symptoms. Pre-pandemic, depressive symptoms one year after discharge had been reported in ICU survivors of mechanical ventilation but, to our knowledge, not previously described in COVID-19 [54].
It is widely recognized that anemia can lead to symptoms such as fatigue, irritability, and concentration problems, which are often associated with depression components. However, findings from a large cohort study conducted by Lever van Milligen et al. [55] and another study by Chen et al. [56] failed to provide significant evidence of a direct connection between depression, anxiety, and hemoglobin levels. In a study by Jackowsa et al. [57] a positive relationship between hemoglobin levels and sleep duration was observed. Our data showed that higher hemoglobin levels were associated with lower scores in depression and anxiety questionnaires. Concerning protective factors against depression and anxiety, our data further identified higher levels of creatinine being associated with lower depression scores. The scientific evidence on the correlation between creatinine and affective symptoms such as depression or anxiety is sparse and incongruent. One study by Ibrahim et al. [58] reported no association between creatinine and depression, while a study by Ogrizovic et al. [59] found a positive correlation between creatinine and depression. The findings of Bossola et al. [60] showed that patients with lower creatinine were more depressed.
Concerning the sequential-related organ failure assessment (SOFA) score at ICU admission, we observed elevated scores for both HADS scores, assessing anxiety and depression, along with a low self-assessment of current health in our patients. This correlation is plausible, as the prolonged ICU stay, poor and critical condition, as well as the uncertainty and anxiety experienced by the patients directly impact mental health [61]. Similar findings emerged in evaluating the Simplified Acute Physiology Score (SAPS) II, even outside of COVID-19 [62]. The lung diffusion capacity 12 months after their ICU stays was reduced in patients with an elevated SOFA score at ICU discharge. This is likely multifactorial including pulmonary edema, which decreases the gas exchange surface area and consequently lowers carbon monoxide diffusing capacity (DLCO), inflammation, and subsequent fibrosis [62].
In our study, both heart failure markers, troponin and NT-proBNP, positively predicted higher Apnea–Hypopnea Index (pAHI) scores with large effect sizes. Sasaki et al. [63] demonstrated a correlation between elevated NT-proBNP levels and sleep difficulties, as well as short sleep duration, which aligns with our findings. Several studies have reported that poor sleep is a risk factor for coronary heart disease and stroke [64,65,66]. However, the data are limited regarding the association between poor sleep and heart failure. Laugsand et al. [67] stated a positive relationship between the number of insomnia symptoms, namely the difficulty initiating or maintaining sleep and having nonrestorative sleep, and heart failure. Concerning sleep apnea, Hübner et al. [68] and Tasci et al. [69] found no correlation between changes in NT-proBNP and the pAHI as measured by the WatchPAT, which contrasts with our findings of a positive prediction. In contrast, Vartany et al. [70] found no correlations between the evening baseline or post-sleep NT-proBNP levels and obstructive sleep apnea syndrome (OSAS). Lazzarino et al. [71] reported that certain individuals when exposed to intense stress or exhibiting exaggerated stress responses release both inflammatory factors and cardiac troponin in response. This observation mirrors the elevated troponin levels in stress-induced Takotsubo cardiomyopathy [72,73]. Several studies have further demonstrated an association between sleep apnea and increased troponin levels. However, this association lost statistical significance after adjusting for cardiovascular risk factors [74,75].
In analyzing our lung CT data, ground-glass opacity showed notable associations with some endpoints. Patients with pulmonary ground-glass opacity scored worse in depression, anxiety, fatigue, and insomnia questionnaires. In addition, these patients experienced an overall lower quality of life. Compared with initial lung CT scans, 78% of the COVID-19 hospital survivors still had some pulmonary ground-glass opacity showing up on lung scans at a one-year follow-up [76]. These residual lesions are further associated with lower peripheral oxygen saturation, as was the case in our patients [77]. To our knowledge, our study is the first to describe pulmonary ground-glass opacity as a predictor of depression, anxiety, fatigue, and insomnia scores.
This study has several limitations. Most importantly, the small sample size of 17 patients resulted in low statistical power. Therefore, the results must be interpreted cautiously. Secondly, despite having pre-post COVID-19 difference scores for several neurocognitive endpoints (e.g., B-ADL, external cognitive assessment), other endpoints lacked a pre-COVID-19 reference value. For instance, we had no data on psychosomatic or respiratory health prior to the COVID-19 and ICU stay, e.g., depressive symptoms or 6 min walk test performance. Although there are generally more male than female ICU COVID-19 patients, the male sex was overrepresented in our study [4,5]. As this study investigates PASC in a 12-month follow-up, it should be mentioned that most patients suffering from PASC are of female sex and usually have a mild course of acute disease without the need for ICU admission [78]. Conversely, the strengths of our study consisted of a broad range of interdisciplinary predictors and endpoints using detailed validated questionnaires as well as the identification of novel associations between ICU factors and relevant long-term health outcomes in COVID-19.
5. Conclusions
The cumulative prevalence of critically ill COVID-19 survivors reporting at least one persisting symptom four months after recovery was as high as 45%, particularly for fatigue and weakness [79]. High levels of inflammatory or cardiac injury parameters, the need for mechanical ventilation, and the presence of ARDS or ground-glass opacity may identify subgroups of COVID-19 patients who deserve close follow-up regarding long-term psychological well-being and quality of life. Our results contribute towards a better understanding of the mechanisms underlying the long-term risk profile of COVID-19 but—given the small sample size—should be interpreted as hypothesis-generating.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/covid4080082/s1, Table S1: Assessment of clinical outcomes 12 (±1) months after ICU stay; Table S2: Dietary behavior assessed with the FFQ questionnaire.
Author Contributions
Conceptualization, W.C.A., D.A.S., and C.R.K.; methodology, W.C.A., N.G., K.G.-F., T.F. (Tim Fischer), G.-R.K., M.F. (Manuel Frischknecht), M.H.B., and D.A.S.; software, N.G.; validation, G.-R.K., U.P., M.F. (Manuel Frischknecht), M.H.B., D.A.S., and W.C.A.; formal analysis, N.G. and D.A.; investigation, D.A., K.G.-F., T.F. (Tim Fischer), and T.F. (Thomas Frauenfelder); resources, W.C.A., G.-R.K., U.P., M.F. (Miodrag Filipovic), M.H.B., T.F. (Thomas Frauenfelder), M.H.B., and D.A.S.; data curation, N.G. and D.A.; writing—original draft preparation, N.G., D.A., and W.C.A.; writing—review and editing, W.C.A., D.A.S., M.F. (Manuel Frischknecht), M.H.B., and T.F. (Tim Fischer); visualization, N.G.; supervision, W.C.A. and D.A.S.; project administration, W.C.A. and D.A.S.; funding acquisition, W.C.A., G.-R.K., U.P., M.H.B., and D.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by intramural grants from the Cantonal Hospital St. Gallen (20/13 and 21/18; to W.C.A.).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Eastern Switzerland (EKOS) (BASEC Nr. EKOS 20/058).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request due to restrictions for ethical reasons.
Acknowledgments
First, we thank all the patients, relatives, and healthcare workers involved in the treatment of COVID-19 patients during the course of the study and the pandemic as a whole, including Cornelia Knapp, Susanne Nigg, Thomas Egger, Andrea Blöchlinger, Tia Wisser, Melanie Gätzi, Patrick Münger, and Gianina Toller (Cantonal Hospital St. Gallen). We further thank Natacha Noël and Julien Lamour for their support regarding the Food Frequency Questionnaire (FFQ).
Conflicts of Interest
W.C.A. received funding from the Swiss National Science Foundation (33IC30_201300), Cantonal Hospital St. Gallen, OM Pharma, FUNGINOS, and Gilead and payments to his institution for lectures and presentations from Pfizer, GSK, MSD, and Gilead; travel to meetings from Pfizer, GSK, and Gilead; and participation on Advisory Boards from MSD, Sanofi, Pfizer, GSK, OM Pharma, and Janssen.
References
- Eythorsson, E.; Helgason, D.; Ingvarsson, R.F.; Bjornsson, H.K.; Olafsdottir, L.B.; Bjarnadottir, V.; Runolfsdottir, H.L.; Bjarnadottir, S.; Agustsson, A.S.; Oskarsdottir, K.; et al. Clinical Spectrum of Coronavirus Disease 2019 in Iceland: Population Based Cohort Study. BMJ 2020, 371, m4529. [Google Scholar] [CrossRef]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive Care Management of Coronavirus Disease 2019 (COVID-19): Challenges and Recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Hajjar, L.A.; Costa, I.B.S.D.S.; Rizk, S.I.; Biselli, B.; Gomes, B.R.; Bittar, C.S.; De Oliveira, G.Q.; De Almeida, J.P.; De Oliveira Bello, M.V.; Garzillo, C.; et al. Intensive Care Management of Patients with COVID-19: A Practical Approach. Ann. Intensive Care 2021, 11, 36. [Google Scholar] [CrossRef]
- Anderegg, N.; Panczak, R.; Egger, M.; Low, N.; Riou, J. Survival among People Hospitalized with COVID-19 in Switzerland: A Nationwide Population-Based Analysis. BMC Med. 2022, 20, 164. [Google Scholar] [CrossRef]
- Roelens, M.; Martin, A.; Friker, B.; Sousa, F.M.; Thiabaud, A.; Vidondo, B.; Buchter, V.; Gardiol, C.; Vonlanthen, J.; Balmelli, C.; et al. Evolution of COVID-19 Mortality over Time: Results from the Swiss Hospital Surveillance System (CH-SUR). Swiss Med. Wkly 2021, 151, w30105. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Qin, E.S.; Hough, C.L.; Andrews, J.; Bunnell, A.E. Intensive Care Unit-Acquired Weakness and the COVID-19 Pandemic: A Clinical Review. PMR 2022, 14, 227–238. [Google Scholar] [CrossRef]
- Carola, V.; Vincenzo, C.; Morale, C.; Pelli, M.; Rocco, M.; Nicolais, G. Psychological Health in COVID-19 Patients after Discharge from an Intensive Care Unit. Front. Public Health 2022, 10, 951136. [Google Scholar] [CrossRef]
- Aguila, E.J.T.; Lontok, M.A.D.; Francisco, C.P.D. Follow Your Gut: Challenges in Nutritional Therapy During the COVID-19 Pandemic. Clin. Gastroenterol. Hepatol. 2020, 18, 2638–2639. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P.; Endorsed by the ESPEN Council. ESPEN Expert Statements and Practical Guidance for Nutritional Management of Individuals with SARS-CoV-2 Infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef]
- PHOSP-COVID Collaborative Group. Clinical Characteristics with Inflammation Profiling of Long COVID and Association with 1-Year Recovery Following Hospitalisation in the UK: A Prospective Observational Study. Lancet Respir. Med. 2022, 10, 761–775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zang, C.; Xu, Z.; Zhang, Y.; Xu, J.; Bian, J.; Morozyuk, D.; Khullar, D.; Zhang, Y.; Nordvig, A.S.; et al. Data-Driven Identification of Post-Acute SARS-CoV-2 Infection Subphenotypes. Nat. Med. 2023, 29, 226–235. [Google Scholar] [CrossRef]
- Sahanic, S.; Tymoszuk, P.; Ausserhofer, D.; Rass, V.; Pizzini, A.; Nordmeyer, G.; Hüfner, K.; Kurz, K.; Weber, P.M.; Sonnweber, T.; et al. Phenotyping of Acute and Persistent Coronavirus Disease 2019 Features in the Outpatient Setting: Exploratory Analysis of an International Cross-Sectional Online Survey. Clin. Infect. Dis. 2022, 75, e418–e431. [Google Scholar] [CrossRef] [PubMed]
- CDC. Post-COVID Conditions. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 12 May 2024).
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V.; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A Clinical Case Definition of Post-COVID-19 Condition by a Delphi Consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Coronavirus Disease (COVID-19): Post COVID-19 Condition. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition (accessed on 12 May 2024).
- Pfaff, E.R.; Girvin, A.T.; Bennett, T.D.; Bhatia, A.; Brooks, I.M.; Deer, R.R.; Dekermanjian, J.P.; Jolley, S.E.; Kahn, M.G.; Kostka, K.; et al. Who Has Long-COVID? A Big Data Approach. medRxiv 2021. [Google Scholar] [CrossRef]
- Albrich, W.C.; Ghosh, T.S.; Ahearn-Ford, S.; Mikaeloff, F.; Lunjani, N.; Forde, B.; Suh, N.; Kleger, G.-R.; Pietsch, U.; Frischknecht, M.; et al. A High-Risk Gut Microbiota Configuration Associates with Fatal Hyperinflammatory Immune and Metabolic Responses to SARS-CoV-2. Gut Microbes 2022, 14, 2073131. [Google Scholar] [CrossRef]
- Fischer, T.; Baz, Y.E.; Scanferla, G.; Graf, N.; Waldeck, F.; Kleger, G.-R.; Frauenfelder, T.; Bremerich, J.; Kobbe, S.S.; Pagani, J.-L.; et al. Comparison of Temporal Evolution of Computed Tomography Imaging Features in COVID-19 and Influenza Infections in a Multicenter Cohort Study. Eur. J. Radiol. Open 2022, 9, 100431. [Google Scholar] [CrossRef]
- Goyal, N.; Chung, M.; Bernheim, A.; Keir, G.; Mei, X.; Huang, M.; Li, S.; Kanne, J.P. Computed Tomography Features of Coronavirus Disease 2019 (COVID-19): A Review for Radiologists. J. Thorac. Imaging 2020, 35, 211–218. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, D.; Guessous, I.; Vaucher, J.; Preisig, M.; Waeber, G.; Vollenweider, P.; Marques-Vidal, P. Low Compliance with Dietary Recommendations for Food Intake among Adults. Clin. Nutr. 2013, 32, 783–788. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef]
- Valko, P.O.; Bassetti, C.L.; Bloch, K.E.; Held, U.; Baumann, C.R. Validation of the Fatigue Severity Scale in a Swiss Cohort. Sleep 2008, 31, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W.; Löwe, B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: A Systematic Review. Gen. Hosp. Psychiatry 2010, 32, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Wilner, N.; Alvarez, W. Impact of Event Scale: A Measure of Subjective Stress. Psychosom Med. 1979, 41, 209–218. [Google Scholar] [CrossRef] [PubMed]
- EQ-5D-5L—EQ-5D. Available online: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (accessed on 4 December 2023).
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Hindmarch, I.; Lehfeld, H.; de Jongh, P.; Erzigkeit, H. The Bayer Activities of Daily Living Scale (B-ADL). Dement. Geriatr. Cogn. Disord. 1998, 9 (Suppl. 2), 20–26. [Google Scholar] [CrossRef]
- Jaeger, J. Digit Symbol Substitution Test: The Case for Sensitivity Over Specificity in Neuropsychological Testing. J. Clin. Psychopharmacol. 2018, 38, 513–519. [Google Scholar] [CrossRef]
- Pang, K.P.; Gourin, C.G.; Terris, D.J. A Comparison of Polysomnography and the WatchPAT in the Diagnosis of Obstructive Sleep Apnea. Otolaryngol. Head Neck Surg. 2007, 137, 665–668. [Google Scholar] [CrossRef]
- Erdfelder, E.; Faul, F.; Buchner, A. GPOWER: A General Power Analysis Program. Behav. Res. Methods Instrum. Comput. 1996, 28, 1–11. [Google Scholar] [CrossRef]
- Buuren, S.V.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Soft. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- von Hippel, P.T. 4. Regression with Missing Ys: An Improved Strategy for Analyzing Multiply Imputed Data. Sociol. Methodol. 2007, 37, 83–117. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. “Package ‘Gplots’.” Various R Programming Tools for Plotting Data. R Package Version 2009, 2, 1. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; MSOR Connect; R Core Team: Vienna, Austria, 2014. [Google Scholar]
- Armstrong, R.A. When to Use the Bonferroni Correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Olmstead, R.; Carroll, J.E. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Gaines, J.; Vgontzas, A.N.; Fernandez-Mendoza, J.; He, F.; Calhoun, S.L.; Liao, D.; Bixler, E.O. Increased Inflammation from Childhood to Adolescence Predicts Sleep Apnea in Boys: A Preliminary Study. Brain Behav. Immun. 2017, 64, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Jokela, M.; Virtanen, M.; Batty, G.D.; Kivimäki, M. Inflammation and Specific Symptoms of Depression. JAMA Psychiatry 2016, 73, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Orre, I.J.; Reinertsen, K.V.; Aukrust, P.; Dahl, A.A.; Fosså, S.D.; Ueland, T.; Murison, R. Higher Levels of Fatigue Are Associated with Higher CRP Levels in Disease-Free Breast Cancer Survivors. J. Psychosom. Res. 2011, 71, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Seeman, T.E.; Bower, J.E.; Kiefe, C.I.; Irwin, M.R. Prospective Association between C-Reactive Protein and Fatigue in the Coronary Artery Risk Development in Young Adults Study. Biol. Psychiatry 2009, 66, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Karshikoff, B.; Sundelin, T.; Lasselin, J. Role of Inflammation in Human Fatigue: Relevance of Multidimensional Assessments and Potential Neuronal Mechanisms. Front. Immunol. 2017, 8, 21. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Long-COVID Post-Viral Chronic Fatigue and Affective Symptoms Are Associated with Oxidative Damage, Lowered Antioxidant Defenses and Inflammation: A Proof of Concept and Mechanism Study. Mol. Psychiatry 2023, 28, 564–578. [Google Scholar] [CrossRef]
- Hartung, T.J.; Neumann, C.; Bahmer, T.; Chaplinskaya-Sobol, I.; Endres, M.; Geritz, J.; Haeusler, K.G.; Heuschmann, P.U.; Hildesheim, H.; Hinz, A.; et al. Fatigue and Cognitive Impairment after COVID-19: A Prospective Multicentre Study. eClinicalMedicine 2022, 53, 101651. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Shahbaz, S.; Osman, M.; Redmond, D.; Bozorgmehr, N.; Rosychuk, R.J.; Lam, G.; Sligl, W.; Cohen Tervaert, J.W.; Elahi, S. Diverse Immunological Dysregulation, Chronic Inflammation, and Impaired Erythropoiesis in Long COVID Patients with Chronic Fatigue Syndrome. J. Autoimmun. 2024, 147, 103267. [Google Scholar] [CrossRef] [PubMed]
- Swanink, C.M.; Vercoulen, J.H.; Galama, J.M.; Roos, M.T.; Meyaard, L.; van der Ven-Jongekrijg, J.; de Nijs, R.; Bleijenberg, G.; Fennis, J.F.; Miedema, F.; et al. Lymphocyte Subsets, Apoptosis, and Cytokines in Patients with Chronic Fatigue Syndrome. J. Infect. Dis. 1996, 173, 460–463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional Exhaustion of Antiviral Lymphocytes in COVID-19 Patients. Cell. Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef]
- Naudé, P.J.W.; Roest, A.M.; Stein, D.J.; de Jonge, P.; Doornbos, B. Anxiety Disorders and CRP in a Population Cohort Study with 54,326 Participants: The LifeLines Study. World J. Biol. Psychiatry 2018, 19, 461–470. [Google Scholar] [CrossRef]
- de Azevedo Cardoso, T.; Silva, R.H.; Fernandes, J.L.; Arent, C.O.; Amboni, G.; Borba, L.A.; Padilha, A.P.Z.; Botelho, M.E.M.; Maciel, A.L.; Barichello, T.; et al. Stress Levels, Psychological Symptoms, and C-Reactive Protein Levels in COVID-19: A Cross-Sectional Study. J. Affect. Disord. 2023, 330, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, O.J.; Friedman, L.A.; Colantuoni, E.; Dinglas, V.D.; Sepulveda, K.A.; Mendez-Tellez, P.; Shanholz, C.; Pronovost, P.J.; Needham, D.M. Psychiatric Symptoms after Acute Respiratory Distress Syndrome: A 5-Year Longitudinal Study. Intensive Care Med. 2018, 44, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Palakshappa, J.A.; Krall, J.T.W.; Belfield, L.T.; Files, D.C. Long-Term Outcomes in Acute Respiratory Distress Syndrome: Epidemiology, Mechanisms, and Patient Evaluation. Crit. Care Clin. 2021, 37, 895–911. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.; Tomlinson, G.; Chu, L.; Robles, P.; Matte, A.; Burns, S.; Thomas, C.; Lamontagne, F.; Adhikari, N.K.J.; Ferguson, N.; et al. Determinants of Depressive Symptoms at 1 Year Following ICU Discharge in Survivors of ≥7 Days of Mechanical Ventilation: Results From the RECOVER Program, a Secondary Analysis of a Prospective Multicenter Cohort Study. Chest 2019, 156, 466–476. [Google Scholar] [CrossRef]
- Lever-van Milligen, B.A.; Vogelzangs, N.; Smit, J.H.; Penninx, B.W.J.H. Hemoglobin Levels in Persons with Depressive and/or Anxiety Disorders. J. Psychosom. Res. 2014, 76, 317–321. [Google Scholar] [CrossRef]
- Chen, H.-H.; Yeh, H.-L.; Tsai, S.-J. Association of Lower Hemoglobin Levels with Depression, Though Not with Cognitive Performance, in Healthy Elderly Men. Psychiatry Clin. Neurosci. 2012, 66, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Jackowska, M.; Kumari, M.; Steptoe, A. Sleep and Biomarkers in the English Longitudinal Study of Ageing: Associations with C-Reactive Protein, Fibrinogen, Dehydroepiandrosterone Sulfate and Hemoglobin. Psychoneuroendocrinology 2013, 38, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; El Salamony, O. Depression, Quality of Life and Malnutrition-Inflammation Scores in Hemodialysis Patients. Am. J. Nephrol. 2008, 28, 784–791. [Google Scholar] [CrossRef]
- Simic Ogrizovic, S.; Jovanovic, D.; Dopsaj, V.; Radovic, M.; Sumarac, Z.; Bogavac, S.N.; Stosovic, M.; Stanojevic, M.; Nesic, V. Could Depression Be a New Branch of MIA Syndrome? Clin. Nephrol. 2009, 71, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Bossola, M.; Ciciarelli, C.; Di Stasio, E.; Conte, G.L.; Vulpio, C.; Luciani, G.; Tazza, L. Correlates of Symptoms of Depression and Anxiety in Chronic Hemodialysis Patients. Gen. Hosp. Psychiatry 2010, 32, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ringdal, M.; Plos, K.; Lundberg, D.; Johansson, L.; Bergbom, I. Outcome after Injury: Memories, Health-Related Quality of Life, Anxiety, and Symptoms of Depression after Intensive Care. J. Trauma 2009, 66, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Mazeraud, A.; Polito, A.; Sivanandamoorthy, S.; Porcher, R.; Heming, N.; Stoclin, A.; Hissem, T.; Antona, M.; Blot, F.; Gaillard, R.; et al. Groupe d’Explorations Neurologiques en Réanimation (GENER). Association Between Anxiety and New Organ Failure, Independently of Critical Illness Severity and Respiratory Status: A Prospective Multicentric Cohort Study. Crit. Care Med. 2020, 48, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Yamamoto, H.; Ozono, R.; Maeda, R.; Kihara, Y. Sleeping Difficulty and Subjective Short Sleep Duration Are Associated with Serum N-Terminal Pro-Brain Natriuretic Peptide Levels in the Elderly Population. Intern. Med. 2020, 59, 2213–2219. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep Duration Predicts Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Studies. Eur. Heart J. 2011, 32, 1484–1492. [Google Scholar] [CrossRef]
- Laugsand, L.E.; Vatten, L.J.; Platou, C.; Janszky, I. Insomnia and the Risk of Acute Myocardial Infarction. Circulation 2011, 124, 2073–2081. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Winkelman, J.W.; Redline, S.; Hu, F.B.; Stampfer, M.; Ma, J.; Gao, X. Association Between Insomnia Symptoms and Mortality: A Prospective Study of US Men. Circulation 2014, 129, 737–746. [Google Scholar] [CrossRef]
- Laugsand, L.E.; Strand, L.B.; Platou, C.; Vatten, L.J.; Janszky, I. Insomnia and the Risk of Incident Heart Failure: A Population Study. Eur. Heart J. 2014, 35, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Hübner, R.-H.; El Mokhtari, N.E.; Freitag, S.; Rausche, T.; Göder, R.; Tiroke, A.; Lins, M.; Simon, R.; Bewig, B. NT-proBNP Is Not Elevated in Patients with Obstructive Sleep Apnoea. Respir. Med. 2008, 102, 134–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tasci, S.; Manka, R.; Scholtyssek, S.; Lentini, S.; Troatz, C.; Stoffel-Wagner, B.; Lüderitz, B. NT-pro-BNP in Obstructive Sleep Apnea Syndrome Is Decreased by Nasal Continuous Positive Airway Pressure. Clin. Res. Cardiol. 2006, 95, 23–30. [Google Scholar] [CrossRef]
- Vartany, E.; Imevbore, M.; O’Malley, M.; Manfredi, C.; Pasquarella, C.; Scinto, L.; Fine, J. N-terminal Pro-brain Natriuretic Peptide for Detection of Cardiovascular Stress in Patients with Obstructive Sleep Apnea Syndrome. J. Sleep Res. 2006, 15, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, A.I.; Hamer, M.; Gaze, D.; Collinson, P.; Rumley, A.; Lowe, G.; Steptoe, A. The Interaction between Systemic Inflammation and Psychosocial Stress in the Association with Cardiac Troponin Elevation: A New Approach to Risk Assessment and Disease Prevention. Prev. Med. 2016, 93, 46–52. [Google Scholar] [CrossRef]
- Lazzarino, A.I.; Hamer, M.; Gaze, D.; Collinson, P.; Steptoe, A. The Association Between Cortisol Response to Mental Stress and High-Sensitivity Cardiac Troponin T Plasma Concentration in Healthy Adults. J. Am. Coll. Cardiol. 2013, 62, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.; Solh, T. Takotsubo Cardiomyopathy: Review of Broken Heart Syndrome. JAAPA 2020, 33, 24–29. [Google Scholar] [CrossRef]
- Raut, S.; Gupta, G.; Narang, R.; Ray, A.; Pandey, R.M.; Malhotra, A.; Sinha, S. The Impact of Obstructive Sleep Apnoea Severity on Cardiac Structure and Injury. Sleep Med. 2021, 77, 58–65. [Google Scholar] [CrossRef]
- Einvik, G.; Røsjø, H.; Randby, A.; Namtvedt, S.K.; Hrubos-Strøm, H.; Brynildsen, J.; Somers, V.K.; Omland, T. Severity of Obstructive Sleep Apnea Is Associated with Cardiac Troponin I Concentrations in a Community-Based Sample: Data from the Akershus Sleep Apnea Project. Sleep 2014, 37, 1111–1116. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-Year Outcomes in Hospital Survivors with COVID-19: A Longitudinal Cohort Study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Jassas, H.K.; Morris, G.; Maes, M. Increased ACE2, sRAGE, and Immune Activation, but Lowered Calcium and Magnesium in COVID-19. Recent Adv. Inflamm. Allergy Drug Discov. 2022, 16, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Martín-Guerrero, J.D.; Pellicer-Valero, Ó.J.; Navarro-Pardo, E.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Arias-Navalón, J.A.; Cigarán-Méndez, M.; Hernández-Barrera, V.; Arendt-Nielsen, L. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. J. Clin. Med. 2022, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- The Prevalence and Long-Term Health Effects of Long Covid Among Hospitalised and Non-Hospitalised Populations: A Systematic Review and Meta-Analysis—eClinicalMedicine. Available online: https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(22)00491-6/fulltext (accessed on 17 May 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).