Abstract
Under the aegis of the National Infectious Diseases Act, the virus transitioned from a category 2 menace to category 5, analogous to seasonal influenza. For this classification to be appropriate, a comprehensive assessment of the immune status of the Japanese population must be conducted to ensure adequacy. The current study has investigated the protracted immune responses of healthcare workers (HCWs) to SARS-CoV-2 in Japan. One year subsequent to the systematically implemented SARS-CoV-2 vaccination campaign among HCWs, humoral and cellular immune responses were sustained at levels as high as or higher than those immediately following the third booster vaccination. Persisting immunity has highlighted the resilience and lasting memory exhibited in HCW defense against the virus, suggesting that the classification of SARS-CoV-2 infection as a category 5 in Japan has appeared judicious.
1. Introduction
In Japan, as of 8 May 2023, pursuant to the Japanese National Infectious Diseases Act, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) underwent a reclassification from category 2, akin to SARS or highly pathogenic influenza, with profound implications for human well-being and public health, to category 5, aligning it with seasonal influenza. Within the ambit of category 5, no administrative measures or imperatives for vaccination against or prevention of SARS-CoV-2 infection exist, leaving the onus on each individual to safeguard their own health [1]. To arrive at this juncture, coronavirus disease 2019 (COVID-19) must, by implication, be equated to a common cold or seasonal flu. For this classification of SARS-CoV-2 as category 5 to be appropriate, a comprehensive assessment of the immune status of the Japanese population must be conducted to ensure adequacy. However, the evaluation, particularly regarding the maintenance of cellular immunity, remains incomplete.
Previously, in the course of our surveys, the initial one transpired just prior to the commencement of the SARS-CoV-2 vaccination initiative for healthcare workers (HCWs). Consequently, the serological antibody levels of the participants remained unaffected by the vaccine [2]. The subsequent survey occurred seven to eight months subsequent to the inaugural vaccination campaign, i.e., antecedent to the administration of the third dose of the vaccine and amidst the progression of the sixth and seventh waves (Omicron strain-predominant epidemics) in Japan. This survey provided insights into humoral and cellular immune sustenance following repeated vaccinations in HCWs, up to 4 months after the third vaccination [3]. Then, the current study, conducted at the end of October 2023, transpired 20 months subsequent to the third booster dose and approximately 1 year following the SARS-CoV-2 vaccination initiative, systematically implemented in HCWs until October in the autumn of 2022.
2. Materials and Methods
2.1. Study Design, Participants, and Data Collection
We have delineated the assessment of humoral and cellular immunities among 48 HCWs under continual observation. Each consenting participant was provided a self-administered questionnaire (Supplementary Material S1) to obtain information about their health conditions and history of exposure to SARS-CoV-2. From each participant, 5 mL of heparinized peripheral venous blood samples was collected for the serological testing of SARS-CoV-2 antibodies, and the peripheral blood mononuclear cells (PBMCs) were also collected for evaluating cellular immunity.
2.2. Serological Tests for SARS-CoV-2 Antibodies
The detection of SARS-CoV-2 antibodies was conducted using the Abbott ARCHITECT® i2000SR PLUS (Abbott, Chicago, IL, USA) platforms at the Department of Clinical Laboratory Medicine, Shiga University of Medical Science. Abbott ARCHITECT® assays were used to measure specific immunoglobulin (Ig) M to the spike protein (SARS-CoV-2 IgM; Spike-IgM), IgG to the nucleocapsid (SARS-CoV-2 IgG; N-IgG), and IgG to the RBD of the spike protein (SARS-CoV-2 IgG II Quant; Spike-IgG). The measured values of Spike-IgM, N-IgG, and Spike-IgG, adjusted with the manufacturers’ calibrators/standards, were interpreted as positive values, with a cut-off index of ≥1.0, ≥1.4, and ≥50.0 AU/mL, respectively.
2.3. Evaluation of SARS-CoV-2 T-Cell Responses in PBMCs
To measure SARS-CoV-2-specific T-cell reactivity, PBMCs were isolated from a heparinized whole-blood sample using a Ficoll density gradient (Leucosep, #163288, Greiner, Germany). After quantification and dilution of the recovered cells, 250,000 PBMCs were plated into each well of a T-SPOT® Discovery SARS-CoV-2 kit (#DISCOVERY.432, Oxford Immunotec, Abingdon, UK). The kit is designed to measure responses to four different but overlapping peptide pools to cover protein sequences of four different SARS-CoV-2 antigens, including spike, nucleocapsid, and membrane proteins, without human leukocyte antigen restriction, and includes negative and positive controls. The fourth peptide pools showed a high degree of homology with endemic coronaviruses. The plated cells were incubated at 37 °C and 5% CO2 in a humidified chamber overnight, and we could detect circulating interferon-γ-secreting T cells, such as CD4+ helper T, CD8+ cytotoxic T, and innate lymphocyte 1 cells. The sum of the T-SPOT immune response values was calculated (T-SPOT value), and <20 spot-forming units per 1,000,000 PBMCs (SFU/106 cells) were recognized as insufficient counts.
3. Results
3.1. Patient Characteristics
This HCW cohort exhibited a 60.4% female predominance (19/29) and was characterized by an average age of 46.6 ± 11.3 years and a body mass index (BMI) of 22.0 ± 2.5 kg/m2. In this investigation, the HCW population received vaccination on an average of 4.2 ± 0.9 occasions, with the blood collection timeframe being 12.1 ± 5.3 months from the administration of the last dose of the mRNA vaccine, which was a bivalent formulation (Omicron BA.4 or BA.5 + Wuhan-Hu-1 strain). Twenty-six cases (54.2%) had a prior diagnosis of COVID-19, and the analyzed timing from the diagnosis was 12.2 ± 5.8 months (Supplementary Materials S1 and S2).
3.2. Anti-Spike Antibodies
Humoral immunities, measured by Abbott Spike-IgG values, recorded immediately before, after, and 4 months subsequent to the administration of the third booster dose, and for up to approximately 20 months after, corresponding to about 1 year after the fourth vaccination, in the current investigation were as follows: 921.7 ± 845.4, 22,903.1 ± 17,820.8, 11,494.4 ± 14,570.4, and 26,457.1 ± 28,270.3 (AU/mL), respectively. Even a year after the systematic vaccination campaign, many HCWs were observed to maintain humoral immunity at levels comparable to those immediately following the third booster vaccination (Figure 1A).
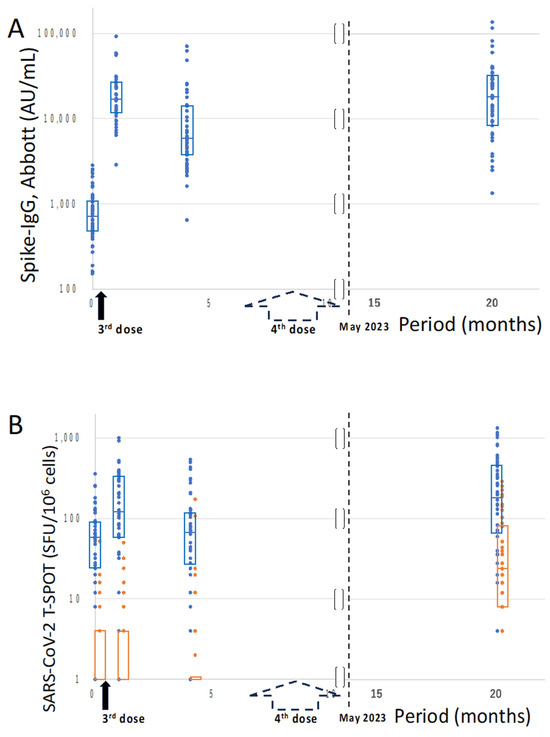
Figure 1.
Time-elapsed values of Spike-IgG and those of T-SPOT for SARS-CoV-2. The timings of the 3rd and 4th vaccination dose are indicated on the X-axis (months). The black and dotted arrows indicate the 3rd and 4th vaccines, respectively. On the Y-axis, the Spike-IgG values (SARS-CoV-2 IgG II Quant, Abbott; AU/mL) and T-SPOT values for SARS-CoV-2 peptides (Oxford Immunotec; SFU/106 cells) are indicated in (A,B), respectively. The 48 healthcare workers (blue dots) were periodically followed up immediately before, after, and 4 months subsequent to the administration of the 3rd booster dose, and for up to 20 months, corresponding to about 1 year after the 4th vaccination. In the T-SPOT evaluation (B), the values (orange dots) for non-vaccine-derived nucleocapsid and/or membrane proteins were also analyzed. Each dot represents the measured values of individual cases, and the rectangle boxes in each dot section indicate the interquartile ranges. Note: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IgG, immunoglobulin G.
3.3. T-SPOT Immune Response
On the other hand, cellular immunities, measured by T-SPOT values, for SARS-CoV-2 peptides at the respective time points were as follows: 85.0 ± 84.2, 219.4 ± 230.4, 111.1 ± 133.6, and 310.0 ± 337.2 (SFU/106 cells), respectively. Cellular immunity in the current HCW cohort was seemingly sustained at levels as high as or higher than those immediately following the third booster vaccination. Additional T-SPOT values for nucleocapsid and membrane proteins appeared to have occurred through natural infection of SARS-CoV-2 both pre- and post-vaccinations (Figure 1B). Although 54.2% (26/48) of the cases had received a diagnosis of SARS-CoV-2 infection in alignment with the questionnaire results, 81.3% (39/48) of the cases exhibited reactivity to nucleocapsid and/or membrane proteins in the T-SPOT evaluation. The duration until the present study for 10.4% (5/48) of those with common cold symptoms, possibly indicative of COVID-19 without a formal diagnosis, was 2.6 ± 1.5 months (Supplementary Material S2), suggesting a continuous occurrence of repeated coronavirus exposure in the post-vaccinated HCW population, possibly due to SARS-CoV-2 subclinical infection and/or common cold-like coronaviruses.
4. Discussion
Anti-spike antibody titer evaluation and natural infection rate evaluation using the positivity of anti-nucleocapsid antibodies have been analyzed in each individual and/or Japanese population, and just under half are reported to have spontaneously contracted SARS-CoV-2 [4]. Indeed, in the current investigation, IgG values in response to the nucleocapsid were positive in only 27% (13/48) of cases (Supplementary Material S3), which underestimated the actual number of diagnosed COVID-19 cases (26/48). This discrepancy may be due to the fact that N-IgG becomes negative within several months [5,6]. To accurately identify SARS-CoV-2-infected individuals, analyses should employ methods that can assess longer-term positivity, such as evaluating cellular immune memory. For the classification of SARS-CoV-2 as category 5 to be appropriate, a comprehensive evaluation of immunocompetence of the Japanese population must be conducted to ensure adequacy. However, the evaluation, particularly encompassing the maintenance of cellular immunity, remains incomplete.
The current investigation has indicated that one year subsequent to the systematically implemented SARS-CoV-2 vaccination campaign among HCWs in Japan, humoral and cellular immune responses were sustained at levels as high as or higher than those immediately following the third booster vaccination. The extent to which elevated spike-IgG values contribute to neutralizing antibodies against concurrently circulating omicron subvariants and the extent of their efficacy in preventing infectivity remain questionable. Nonetheless, the HCW population with consistently high spike-IgG values likely achieved a certain level of herd immunity. In fact, 9 out of the 48 HCWs exhibited no reactivity to either nucleocapsid or membrane proteins in the T-SPOT evaluation, suggesting that their acquired immunity to coronaviruses was solely from vaccination. In these cases, during the current investigation, Abbott Spike-IgG values were 7720.0 ± 4898.2 (AU/mL), and T-SPOT values were 89.8 ± 158.6 (SFU/106 cells) (Supplementary Material S4), both of which were approximately 30% of the population averages. On the other hand, more than 80% of the HCWs exhibited reactivity to nucleocapsid and/or membrane proteins in the T-SPOT evaluation, indicating recurring exposure to coronaviruses among the HCW population. This group showed humoral and cellular immune properties three times stronger or more than those caused solely by vaccination, and has contributed to form a state of herd immunity with additional cellular immune memories responsive to the nucleocapsid and membrane proteins of SARS-CoV-2.
Over the 20-month follow-up of 48 HCWs, none of the documented COVID-19 cases (26/48) progressed to severe illness, and no instances of long-COVID were reported. This includes individuals with common cold symptoms (5/48), with only 14.6% (7/48) requiring more than 3 days off work, and the majority experiencing mild illness. Only 2 cases out of 48 HCWs with T-SPOT <20 and Spike-IgG <6000, whose immune status could not be affirmed as sufficient, involved one with renal failure and peritoneal dialysis and another with asthma (Supplementary Material S2). In light of the aforementioned findings, it is posited that SARS-CoV-2 infection itself, among the Japanese HCW population, has evolved into a condition akin to that of the common cold or seasonal influenza. However, it is crucial to note that the actual Omicron variant infection carries a higher mortality rate than seasonal influenza or Respiratory Syncytial Virus infections [7]. Furthermore, a recent subvariant of Omicron, BA.2.86, appears to possess a heightened ability to infect human lung cells compared to other variants, thereby elevating the likelihood of more severe lung involvement [8]. Thus, repeated vaccination should be recommended in high-risk cases among HCWs.
5. Conclusions
In conclusion, one year following the meticulously executed SARS-CoV-2 vaccination initiative among HCWs, both humoral and cellular immune responses have sufficiently remained the same or more in the Japanese HCW population. The enduring immunity underscores the resilience and enduring recollection demonstrated in the immune defense of HCWs against the virus. This implies that the classification of SARS-CoV-2 infection as category 5 in Japan, where no administrative measures, nor mandates for vaccination or prevention, are enforced, has appeared judicious.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/covid4070060/s1, Supplementary Material S1. Individual questionnaire for assessing the health conditions of participants, before and after SARS-CoV-2 vaccination. (xlsx); Supplementary Material S2. Individual data regarding T-SPOT values representing cellular immunity against SARS-CoV-2 and the antibodies and health conditions of 48 healthcare workers, followed up periodically for up to 20 months after the 3rd dose, corresponding to about 1 year after the 4th vaccination in Shiga Prefecture. (xlsx); Supplementary Material S3. Time-elapsed values of nucleocapsid-IgG for SARS-CoV-2. (pdf); Supplementary Material S4. Individual data regarding T-SPOT values and the antibodies against SARS-CoV-2 and health conditions of 9 healthcare workers, exhibiting no reactivity to either nucleocapsid or membrane proteins in the T-SPOT evaluation, and suggesting that their acquired immunity to coronaviruses was solely from vaccination. (xlsx).
Author Contributions
T.C. conceptualized the study and participated in the process of designing the study with T.I., H.K., T.Y., H.F. and T.I. were involved in blood collection and data collection. T.C., H.K., T.Y., H.F. and T.I. performed the laboratory tests. T.C. and T.I. were involved in data management and performed the statistical analysis. T.C. was involved in the interpretation of data and drafted the original draft, and T.C. and T.I. reviewed and edited the manuscript. All authors had full access to all of the data in the study and accept the responsibility to submit the data for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded mainly by the budget of Department of Clinical Laboratory Medicine, Shiga University of Medical Science, and partly supported by JSPS KAKENHI, Grant number JP22K07250 (T.C.).
Institutional Review Board Statement
The Research Review Board of the Shiga University of Medical Science checked all of the processes and contents of this study in accordance with relevant laws and regulations, and approved this as an administrative investigation (No. R2021-039; approved on 18 November 2021).
Informed Consent Statement
Written informed consent was obtained from all study participants. During the process of obtaining the consent, all participants were informed of the need to publish the results. All participants’ personal information was anonymized, and no participant is identifiable in the report.
Data Availability Statement
The data are contained within the article and Supplementary Materials.
Acknowledgments
We would like to thank the 48 HCWs undergoing long-term continuous observation in Japan for more than 2 years regarding SARS-CoV-2 for the purpose of the present study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Available online: https://www.mhlw.go.jp/file/05-Shingikai-10601000-Daijinkanboukouseikagakuka-Kouseikagakuka/0000040509.pdf (accessed on 23 October 2023).
- Chano, T.; Morita, S.-Y.; Suzuki, T.; Yamashita, T.; Fujimura, H.; Yuri, T.; Menju, M.; Tanaka, M.; Kakuno, F. Serology suggests adequate safety measures to protect healthcare workers from COVID-19 in Shiga Prefecture, Japan. PLoS ONE 2022, 17, e0270334. [Google Scholar] [CrossRef] [PubMed]
- Chano, T.; Yamashita, T.; Fujimura, H.; Kita, H.; Ikemoto, T.; Kume, S.; Morita, S.-Y.; Suzuki, T.; Kakuno, F. Effectiveness of COVID-19 vaccination in healthcare workers in Shiga Prefecture, Japan. Sci. Rep. 2022, 12, 17621. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.mhlw.go.jp/content/001146809.pdf (accessed on 20 November 2023).
- Schallier, A.; De Baets, S.; De Bruyne, D.; Dauwe, K.; Herpol, M.; Couck, P. Assay dependence of long-term kinetics of SARS-CoV-2 antibodies. Diagn. Microbiol. Infect. Dis. 2021, 100, 115403. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.A.; Rassen, J.A.; Kabelac, C.A.; Turenne, W.; Leonard, S.; Klesh, R.; Meyer, W.A.; Kaufman, H.W.; Anderson, S.; Cohen, O.; et al. Association of SARS-CoV-2 Seropositive Antibody Test With Risk of Future Infection. JAMA Intern. Med. 2021, 181, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, P.; Karlsson Valik, J.; Abdel-Halim, L.; Alfvén, T.; Nauclér, P. Outcomes of SARS-CoV-2 Omicron Variant Infections Compared With Seasonal Influenza and Respiratory Syncytial Virus Infections in Adults Attending the Emergency Department: A Multicenter Cohort Study. Clin. Infect. Dis. 2024, 78, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kempf, A.; Nehlmeier, I.; Cossmann, A.; Richter, A.; Bdeir, N.; Graichen, L.; Moldenhauer, A.-S.; Dopfer-Jablonka, A.; Stankov, M.V.; et al. SARS-CoV-2 BA.2.86 enters lung cells and evades neutralizing antibodies with high efficiency. Cell 2024, 187, 596–608.e17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).