Association of ABO Blood Type with Infection and Severity of COVID-19 in Inpatient and Longitudinal Cohorts
Abstract
:1. Introduction
2. Methods
2.1. Retrospective Study Design and Participants
2.2. Inpatient Cohort Statistical Analysis
2.3. Longitudinal Cohort Study Design and Participants
2.4. Longitudinal Cohort Statistical Analysis
3. Results
3.1. Inpatient Cohort Results
3.2. Longitudinal Cohort Results: Cohort Characteristics
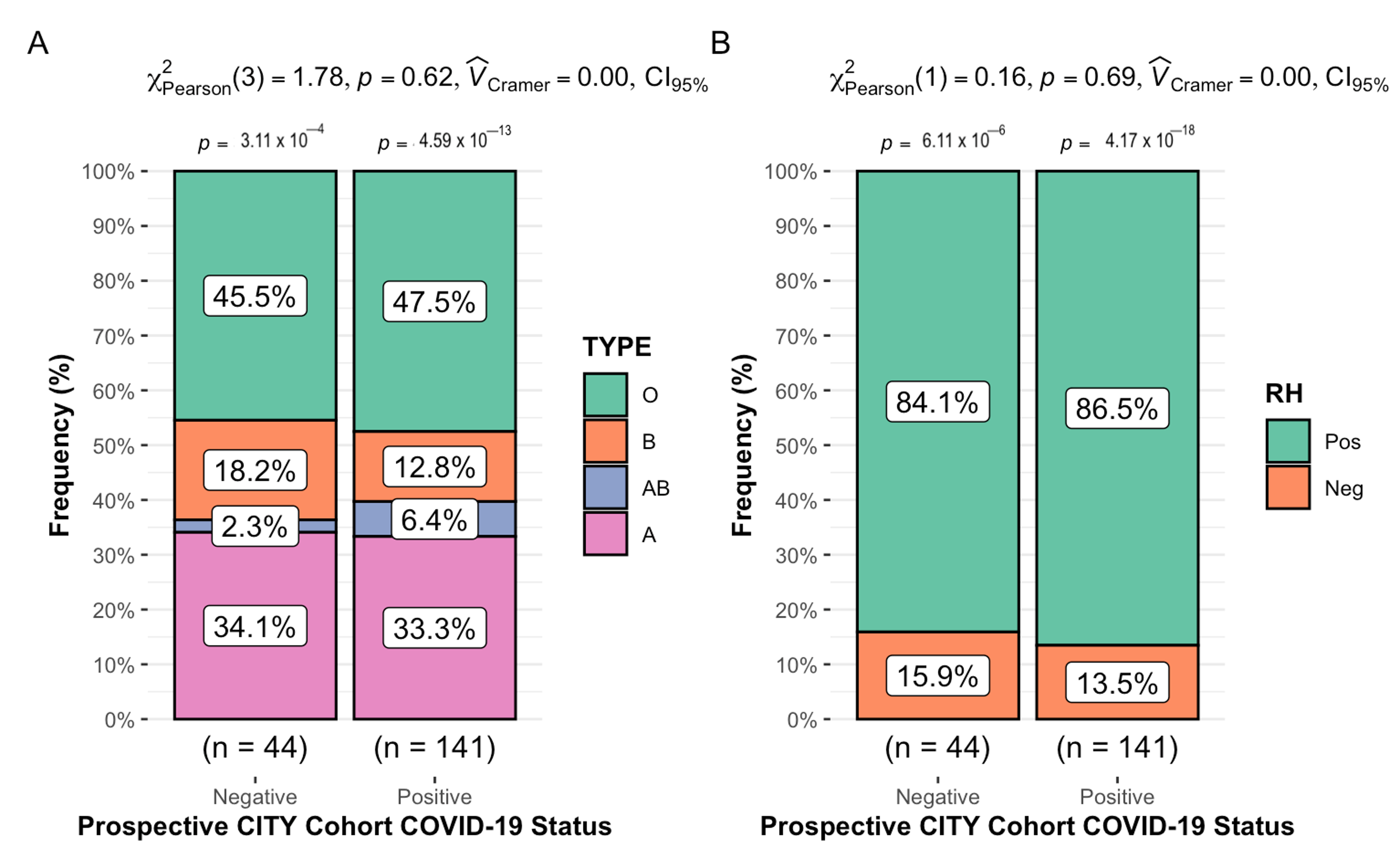
3.3. Longitudinal Cohort Results: ABO/Rh Blood Typing and COVID-19
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Huang, J.Y.; Wang, R.; Gao, Y.T.; Yuan, J.M. ABO blood type and the risk of cancer—Findings from the Shanghai Cohort Study. PLoS ONE 2017, 12, e0184295. [Google Scholar] [CrossRef] [PubMed]
- Dentali, F.; Sironi, A.P.; Ageno, W.; Turato, S.; Bonfanti, C.; Frattini, F.; Crestani, S.; Franchini, M. Non-O blood type is the commonest genetic risk factor for VTE: Results from a meta-analysis of the literature. Semin. Thromb. Hemost. 2012, 38, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Alkebsi, L.; Ideno, Y.; Lee, J.-S.; Suzuki, S.; Nakajima-Shimada, J.; Ohnishi, H.; Sato, Y.; Hayashi, K. Gastroduodenal Ulcers and ABO Blood Group: The Japan Nurses’ Health Study (JNHS). J. Epidemiol. 2018, 28, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Cooling, L. Blood Groups in Infection and Host Susceptibility. Clin. Microbiol. Rev. 2015, 28, 801–870. [Google Scholar] [CrossRef]
- Wang, D.S.; Chen, D.L.; Ren, C.; Wang, Z.-Q.; Qiu, M.-Z.; Luo, H.-Y.; Zhang, D.-S.; Wang, F.-H.; Li, Y.-H.; Xu, R.-H. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int. J. Cancer 2012, 131, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Garratty, G. Blood groups and disease: A historical perspective. Transfus. Med. Rev. 2000, 14, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Lindesmith, L.; Moe, C.; Marionneau, S.; Ruvoen, N.; Jiang, X.; Lindblad, L.; Stewart, P.; LePendu, J.; Baric, R. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 2003, 9, 548–553. [Google Scholar] [CrossRef]
- Rowe, J.A.; Handel, I.G.; Thera, M.A.; Deans, A.-M.; Lyke, K.E.; Koné, A.; Diallo, D.A.; Raza, A.; Kai, O.; Marsh, K.; et al. Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proc. Natl. Acad. Sci. USA 2007, 104, 17471–17476. [Google Scholar] [CrossRef]
- Abegaz, S.B.; Human, A.B.O. Blood Groups and Their Associations with Different Diseases. Biomed. Res. Int. 2021, 2021, 6629060. [Google Scholar] [CrossRef]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef]
- Zhang, Y.; Garner, R.; Salehi, S.; La Rocca, M.; Duncan, D. Association between ABO blood types and coronavirus disease 2019 (COVID-19), genetic associations, and underlying molecular mechanisms: A literature review of 23 studies. Ann. Hematol. 2021, 100, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Jia, L.; Ai, J.; Yu, Y.; Wang, M.; Li, P. ABO blood group influence COVID-19 infection: A meta-analysis. J. Infect. Dev. Ctries. 2021, 15, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Schull, M.J.; Vermeulen, M.J.; Park, A.L. Association Between ABO and Rh Blood Groups and SARS-CoV-2 Infection or Severe COVID-19 Illness: A Population-Based Cohort Study. Ann. Intern. Med. 2021, 174, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; May, H.T.; Knight, S.; Bair, T.L.; Horne, B.D.; Knowlton, K.U. Association of Rhesus factor blood type with risk of SARS-CoV-2 infection and COVID-19 severity. Br. J. Haematol. 2022, 197, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Miotto, M.; Di Rienzo, L.; Gosti, G.; Milanetti, E.; Ruocco, G. Does blood type affect the COVID-19 infection pattern? PLoS ONE 2021, 16, e0251535. [Google Scholar] [CrossRef]
- Kim, Y.; Latz, C.A.; DeCarlo, C.S.; Lee, S.; Png, C.Y.M.; Kibrik, P.; Sung, E.; Alabi, O.; Dua, A. Relationship between blood type and outcomes following COVID-19 infection. Semin. Vasc. Surg. 2021, 34, 125–131. [Google Scholar] [CrossRef]
- Ishaq, U.; Malik, A.; Malik, J.; Mehmood, A.; Qureshi, A.; Laique, T.; Zaidi, S.M.J.; Javaid, M.; Rana, A.S. Association of ABO blood group with COVID-19 severity, acute phase reactants and mortality. PLoS ONE 2021, 16, e0261432. [Google Scholar] [CrossRef]
- Kabrah, S.M.; Abuzerr, S.S.; Baghdadi, M.A.M.; Kabrah, A.M.; Flemban, A.F.; Bahwerth, F.S.M.; Assaggaf, H.M.; Alanazi, E.A.; Alhifany, A.A.; Al-Shareef, S.A.; et al. Susceptibility of ABO blood group to COVID-19 infections: Clinico-hematological, radiological, and complications analysis. Medicine 2021, 100, e28334. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A.; Hu, D.; Chereshnev, V. Problems of Pathogenesis and Pathogenetic Therapy of COVID-19 from the Perspective of the General Theory of Pathological Systems (General Pathological Processes). Int. J. Mol. Sci. 2021, 22, 7582. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int. J. Mol. Sci. 2022, 23, 1716. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Renia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Overmyer, K.A.; Shishkova, E.; Miller, I.J.; Balnis, J.; Bernstein, M.N.; Peters-Clarke, T.M.; Meyer, J.G.; Quan, Q.; Muehlbauer, L.K.; Trujillo, E.A.; et al. Large-Scale Multi-omic Analysis of COVID-19 Severity. Cell Syst. 2021, 12, 23–40.e27. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Huang, Y.; Guo, Y.; Yin, M.; Chen, X.; Xiao, L.; Deng, G. Association of inflammatory markers with the severity of COVID-19: A meta-analysis. Int. J. Infect. Dis. 2020, 96, 467–474. [Google Scholar] [CrossRef]
- Team, R. RStudio: Integrated Development for, R; RStudio PBC: Boston, MA, USA, 2020. [Google Scholar]
- Simon, V.; Kota, V.; Bloomquist, R.F.; Hanley, H.B.; Forgacs, D.; Pahwa, S.; Pallikkuth, S.; Miller, L.G.; Schaenman, J.; Yeaman, M.R.; et al. PARIS and SPARTA: Finding the Achilles’ Heel of SARS-CoV-2. mSphere 2022, 7, e0017922. [Google Scholar] [CrossRef]
- Cleveland Clinic. Blood Types. Available online: https://my.clevelandclinic.org/health/articles/21213-blood-types (accessed on 14 March 2023).
- Zietz, M.; Zucker, J.; Tatonetti, N.P. Associations between blood type and COVID-19 infection, intubation, and death. Nat. Commun. 2020, 11, 5761. [Google Scholar] [CrossRef]
- Göker, H.; Aladağ-Karakulak, E.; DemiÏroğlu, H.; Ayaz, C.M.; Büyükaşik, Y.; Inkaya, A.C.; Aksu, S.; Sayinalp, N.; Haznedaroğlu, I.C.; Uzun, Ö.; et al. The effects of blood group types on the risk of COVID-19 infection and its clinical outcome. Turk. J. Med. Sci. 2020, 50, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, G.; Chui, C.H.; Lau, F.Y.; Chan, P.K.S.; Ng, M.H.L.; Sung, J.J.Y.; Wong, R.S.M. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA 2005, 293, 1450–1451. [Google Scholar] [PubMed]
- Wu, Y.; Feng, Z.; Li, P.; Yu, Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin. Chim. Acta 2020, 509, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, Y.; Huang, H.; Li, D.; Gu, D.; Lu, X.; Zhang, Z.; Liu, L.; Liu, T.; Liu, Y.; et al. Relationship Between the ABO Blood Group and the Coronavirus Disease 2019 (COVID-19) Susceptibility. Clin. Infect. Dis. 2021, 73, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Chen, J.; Cai, Y.; Deng, A.; Yang, M. Association between ABO blood groups and risk of SARS-CoV-2 pneumonia. Br. J. Haematol. 2020, 190, 24–27. [Google Scholar] [CrossRef]
- Solmaz, İ.; Araç, S. ABO blood groups in COVID-19 patients; Cross-sectional study. Int. J. Clin. Pract. 2021, 75, e13927. [Google Scholar] [CrossRef]
- Muñiz-Diaz, E.; Llopis, J.; Parra, R.; Roig, I.; Ferrer, G.; Grifols, J.; Millán, A.; Ene, G.; Ramiro, L.; Maglio, L.; et al. Relationship between the ABO blood group and COVID-19 susceptibility, severity and mortality in two cohorts of patients. Blood Transfus. 2021, 19, 54–63. [Google Scholar]
- Ellinghaus, D.; Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Invernizzi, P.; Fernández, J.; Prati, D.; Baselli, G.; Asselta, R.; et al. Genomewide Association Study of Severe COVID-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Hoque, M.M.; Adnan, S.D.; Karim, S.; Al-Mamun, M.A.; Faruki, M.A.; Islam, K.; Nandy, S. Relationship between Serum Iron Profile and Blood Groups among the Voluntary Blood Donors of Bangladesh. Mymensingh Med. J. 2016, 25, 340–348. [Google Scholar]
- Menshawey, R.; Menshawey, E.; Alserr, A.H.K.; Abdelmassih, A.F. Low iron mitigates viral survival: Insights from evolution, genetics, and pandemics—A review of current hypothesis. Egypt. J. Med Hum. Genet. 2020, 21, 75. [Google Scholar] [CrossRef]
- Gérard, C.; Maggipinto, G.; Minon, J.M. COVID-19 and ABO blood group: Another viewpoint. Br. J. Haematol. 2020, 190, e93–e94. [Google Scholar] [CrossRef] [PubMed]
- Guillon, P.; Clément, M.; Sébille, V.; Rivain, J.G.; Chou, C.F.; Ruvoën-Clouet, N.; Le Pendu, J. Inhibition of the interaction between the SARS-CoV spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology 2008, 18, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Sang, L.; Ye, F.; Ruan, S.; Zhong, B.; Song, T.; Alshukairi, A.N.; Chen, R.; Zhang, Z.; et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J. Clin. Investig. 2020, 130, 5235–5244. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lan, Y.; Yuan, X.; Deng, X.; Li, Y.; Cai, X.; Li, L.; He, R.; Tan, Y.; Deng, X.; et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg. Microbes. Infect. 2020, 9, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, J.C.; Melo, C.G.F.; Oliveira, J.L. The influence of ABO blood groups on COVID-19 susceptibility and severity: A molecular hypothesis based on carbohydrate-carbohydrate interactions. Med. Hypotheses 2020, 144, 110155. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: First Full Regulatory Approval of a COVID-19 Vaccine, the BNT162b2 Pfizer-BioNTech Vaccine, and the Real-World Implications for Public Health Policy. Med. Sci. Monit. 2021, 27, e934625. [Google Scholar] [CrossRef]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar]
- Rosenblum, H.G.; Hadler, S.C.; Moulia, D.; Shimabukuro, T.T.; Su, J.R.; Tepper, N.K.; Ess, K.C.; Woo, E.J.; Mba-Jonas, A.; Alimchandani, M.; et al. Use of COVID-19 Vaccines after Reports of Adverse Events among Adult Recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 Vaccines (Pfizer-BioNTech and Moderna): Update from the Advisory Committee on Immunization Practices—United States, July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1094–1099. [Google Scholar]
| Blood Type | Overall Inpatient Study Cohort Distribution | Female Inpatient Study Cohort Distribution | Male Inpatient Study Cohort Distribution | Rh Positive | Rh Negative |
|---|---|---|---|---|---|
| O | 52.6% (n = 352) | 52.6% (n = 185) | 47.4% (n = 167) | 86.6% (n = 305) | 13.4% (n = 47) |
| A | 35.3% (n = 236) | 50.8% (n = 120) | 49.2% (n = 116) | 88.6% (n = 209) | 11.4% (n = 27) |
| B | 10.8% (n = 72) | 51.4% (n = 37) | 48.6% (n = 35) | 100% (n = 72) | 0% (n = 0) |
| AB | 1.3% (n = 9) | 66.7% (n = 6) | 33.3% (n = 3) | 100% (n = 9) | 0% (n = 0) |
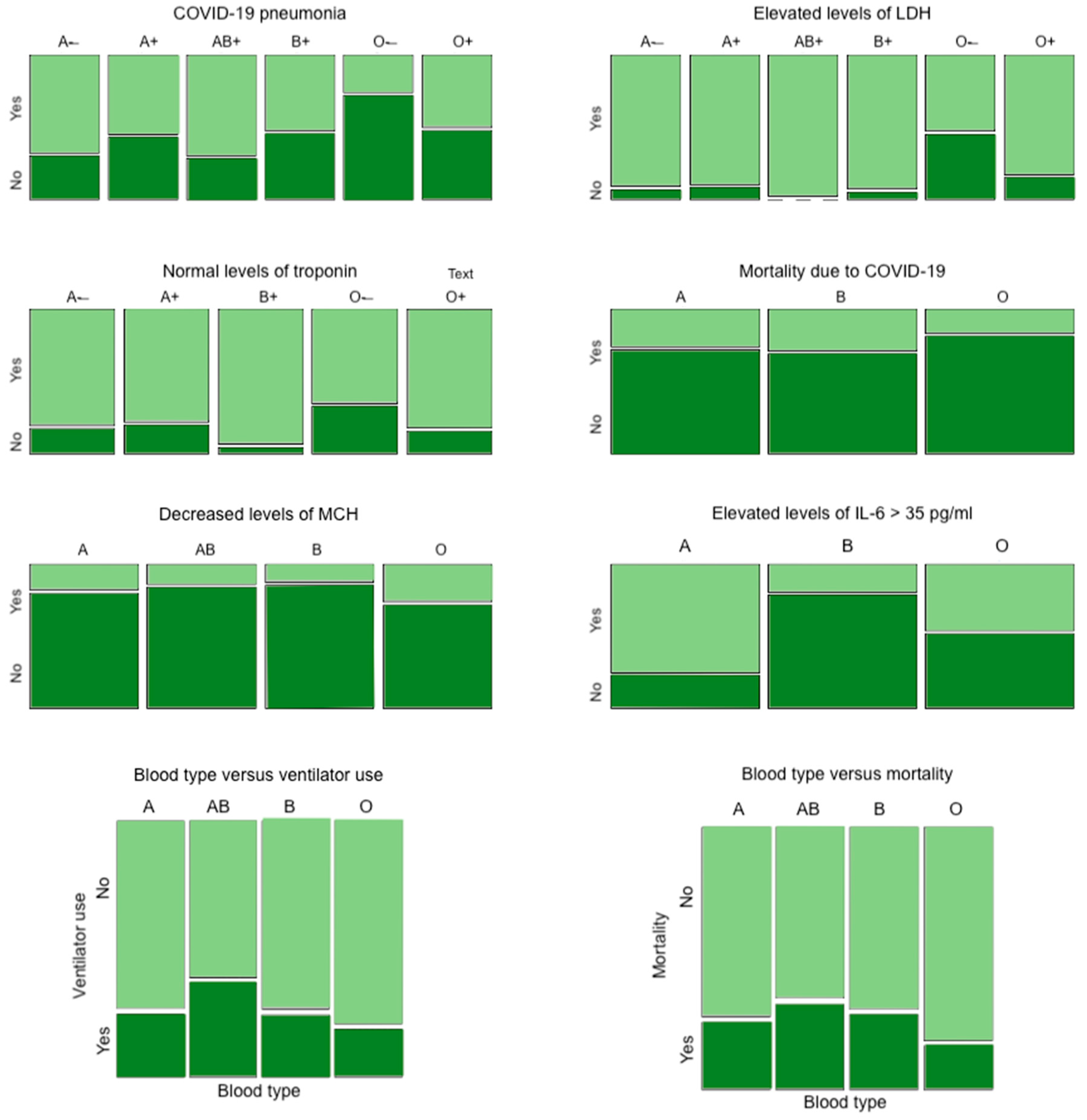
| Condition | A− | A+ | AB+ | B+ | O− | O+ | p-Value |
|---|---|---|---|---|---|---|---|
| COVID-19 pneumonia (n = 642) | 69.2% | 56.5% | 71.4% | 53.7% | 26.7% | 51.5% | 0.003 |
| Elevated levels of LDH (n = 376) | 93.3% | 91.5% | 100% | 95.1% | 54.2% | 85.0% | 0.001 |
| Normal levels of troponin (n = 389) | 82.4% | 79.8% | N/A | 95.6% | 66.7% | 83.8% | 0.026 |
| A | AB | B | O | ||||
| Mortality due to COVID-19 (n = 669) | 26.3% | N/A | 29.2% | 17.0% | 0.012 | ||
| Decreased levels of MCH (n = 564) | 18.4% | 14.3% | 13.1% | 27.0% | 0.006 | ||
| Elevated levels of IL-6 > 35 pg/mL (n = 110) | 77.1% | N/A | 20.0% | 47.1% | <0.001 |
| Frequency (%) | |
|---|---|
| N | 185 |
| Group | |
| HCW | 98 (53%) |
| CTL | 87 (47%) |
| Age (Median ± SD; Range) | 51 ± 16.54; 20–93 |
| Sex | |
| Male | 81 (43.8%) |
| Female | 104 (56.2%) |
| Race | |
| White | 157 (84.9%) |
| Asian | 11 (5.9%) |
| Black/African American | 10 (5.4%) |
| Other | 7 (3.8%) |
| Ethnicity | |
| Hispanic | 117 (63.2%) |
| Not Hispanic | 68 (36.8%) |
| COVID-19 Vaccination Status | |
| Vaccinated | 176 (95.1%) |
| Unvaccinated | 9 (4.9%) |
| Primary Vaccination | |
| Pfizer-BioNTech (mRNA) | 107 (57.8%) |
| Moderna (mRNA) | 61 (33.0%) |
| Unvaccinated | 9 (4.9%) |
| Johnson & Johnson (viral vector) | 8 (4.3%) |
| Booster Vaccination | 132 (71.4%) |
| Pfizer-BioNTech (mRNA) | 77 (41.6%) |
| Moderna (half) (mRNA) | 34 (18.4%) |
| Moderna (full) (mRNA) | 21 (11.4%) |
| Group | COVID Positive | COVID Negative | Total (N = 185) |
|---|---|---|---|
| HCW | 98 (53%) | ||
| A | 19 (65.5%) | 10 (34.5%) | 29 |
| AB | 7 (100%) | 0 (0%) | 7 |
| B | 12 (70.6%) | 5 (29.5%) | 17 |
| O | 25 (61.0%) | 16 (39.0%) | 41 |
| Rh+ | 55 (67.1%) | 27 (32.0%) | 82 |
| Rh− | 8 (66.7%) | 4 (33.3%) | 12 |
| CTL | 87 (47%) | ||
| A | 28 (84.8%) | 5 (15.2%) | 33 |
| AB | 2 (66.7%) | 1 (33.3%) | 3 |
| B | 6 (66.7%) | 4 (8.7%) | 9 |
| O | 42 (91.3%) | 10 (34.5%) | 46 |
| Rh+ | 67 (87.0%) | 10 (13.0%) | 77 |
| Rh− | 11 (78.6%) | 3 (21.4%) | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eatz, T.; Mantero, A.M.A.; Williams, E.; Cash, C.J.; Perez, N.; Cromar, Z.J.; Hernandez, A.; Cordova, M.; Godbole, N.; Le, A.; et al. Association of ABO Blood Type with Infection and Severity of COVID-19 in Inpatient and Longitudinal Cohorts. COVID 2023, 3, 1429-1439. https://doi.org/10.3390/covid3090098
Eatz T, Mantero AMA, Williams E, Cash CJ, Perez N, Cromar ZJ, Hernandez A, Cordova M, Godbole N, Le A, et al. Association of ABO Blood Type with Infection and Severity of COVID-19 in Inpatient and Longitudinal Cohorts. COVID. 2023; 3(9):1429-1439. https://doi.org/10.3390/covid3090098
Chicago/Turabian StyleEatz, Tiffany, Alejandro Max Antonio Mantero, Erin Williams, Charles J. Cash, Nathalie Perez, Zachary J. Cromar, Adiel Hernandez, Matthew Cordova, Neha Godbole, Anh Le, and et al. 2023. "Association of ABO Blood Type with Infection and Severity of COVID-19 in Inpatient and Longitudinal Cohorts" COVID 3, no. 9: 1429-1439. https://doi.org/10.3390/covid3090098
APA StyleEatz, T., Mantero, A. M. A., Williams, E., Cash, C. J., Perez, N., Cromar, Z. J., Hernandez, A., Cordova, M., Godbole, N., Le, A., Lin, R., Luo, S., Patel, A., Abu, Y., Pallikkuth, S., & Pahwa, S. (2023). Association of ABO Blood Type with Infection and Severity of COVID-19 in Inpatient and Longitudinal Cohorts. COVID, 3(9), 1429-1439. https://doi.org/10.3390/covid3090098







