Harnessing Antiviral Peptides as Means for SARS-CoV-2 Control
Abstract
1. Introduction
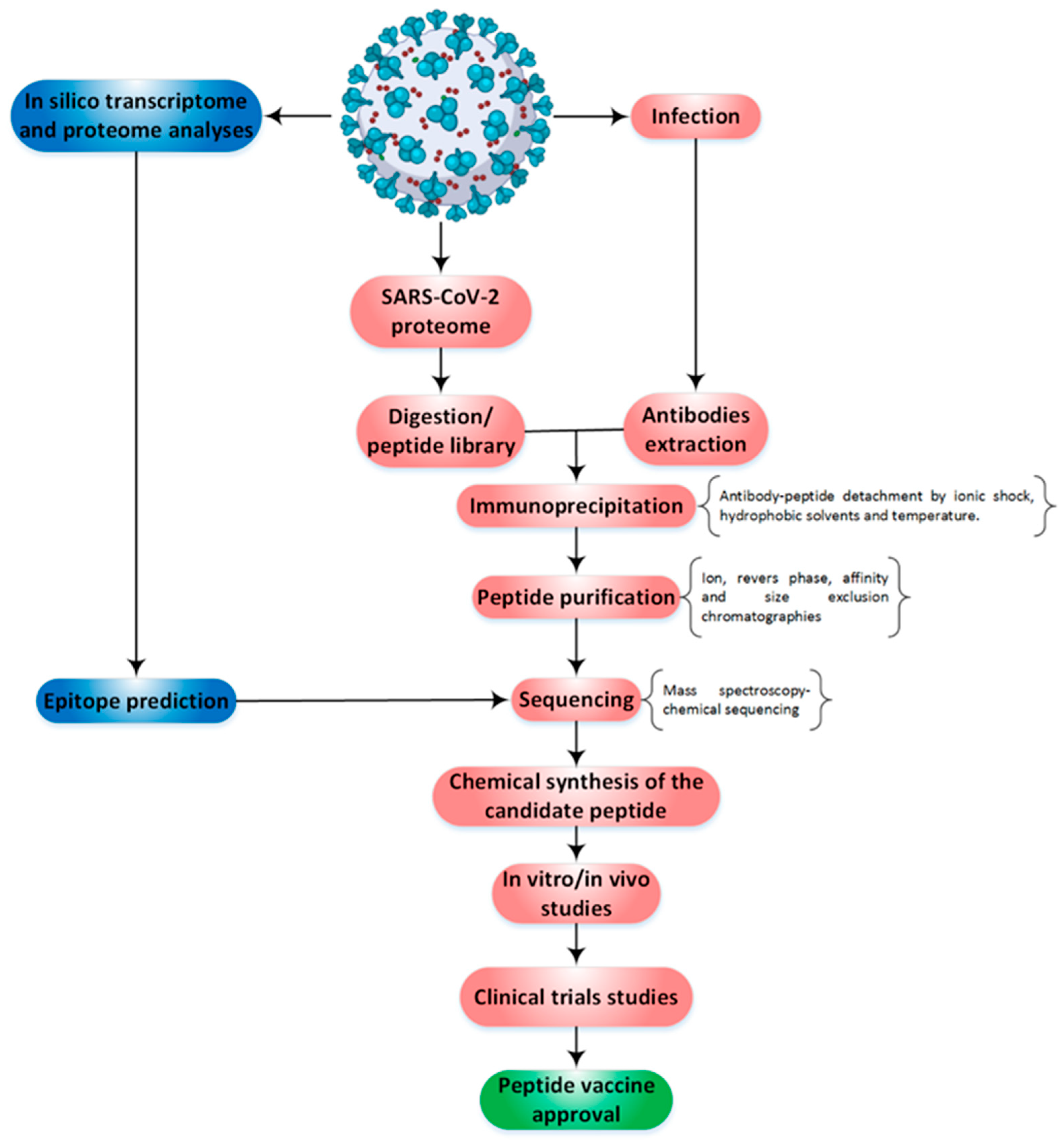
2. Methodological Routes to Discover and Finalize a Peptide as a Protective Agent
3. Landscape of SARS-CoV-2 Peptide Vaccines on Clinical Trials
4. Monoclonal Antibodies (Mabs) as Potential Candidates for SARS-CoV-2 Treatment
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Epidemiology of viral infections. Fenner White’s Med. Virol. 2017, 185–203. [Google Scholar] [CrossRef]
- Zarkesh, K.; Entezar-Almahdi, E.; Ghasemiyeh, P.; Akbarian, M.; Bahmani, M.; Roudaki, S.; Fazlinejad, R.; Mohammadi-Samani, S.; Firouzabadi, N.; Hosseini, M.; et al. Drug-based therapeutic strategies for COVID-19-infected patients and their challenges. Future Microbiol. 2021, 16, 1415–1451. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Coronaviruses Methods Protoc. 2015, 1–23. [Google Scholar] [CrossRef]
- Zhang, S.F.; Tuo, J.L.; Huang, X.B.; Zhu, X.; Zhang, D.M.; Zhou, K.; Yuan, L.; Luo, H.J.; Zheng, B.J.; Yuen, K.Y.; et al. Epidemiology characteristics of human coronaviruses in patients with respiratory infection symptoms and phylogenetic analysis of HCoV-OC43 during 2010–2015 in Guangzhou. PLoS ONE 2018, 13, e0191789. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.S.; Liu, D.X. Human coronavirus: Host-pathogen interaction. Annu. Rev. Microbiol. 2019, 73, 529–557. [Google Scholar] [CrossRef]
- Peiris, M.; Poon, L.L.M. Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) (Coronaviridae). Encycl. Virol. 2021, 814–824. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 4 July 2020).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Dal Negro, R.W.; Nisini, R. The first, holistic immunological model of COVID-19: Implications for prevention, diagnosis, and public health measures. Pediatr. Allergy Immunol. 2020, 31, 454–470. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, J.; Ma, X.; Tan, J.; Chen, L.; Liu, S.; Xin, Y.; Zhuang, L. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed. Pharmacother. 2020, 131, 110678. [Google Scholar] [CrossRef]
- Akbarian, M.; Lundstrom, K.; Redwan, E.M.; Uversky, V.N. Vaccine Development Strategies and the Current Status of COVID-19 Vaccines. In COVID-19: From Bench to Bedside; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Lucchese, G. Epitopes for a 2019-nCoV vaccine. Cell. Mol. Immunol. 2020, 17, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xu, Y.; Bao, L.; Zhang, L.; Yu, P.; Qu, Y.; Zhu, H.; Zhao, W.; Han, Y.; Qin, C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses 2019, 11, 59. [Google Scholar] [CrossRef]
- Purcell, A.W.; McCluskey, J.; Rossjohn, J. More than one reason to rethink the use of peptides in vaccine design. Nat. Rev. Drug Discov. 2007, 6, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Nelde, A.; Rammensee, H.G.; Walz, J.S. The peptide vaccine of the future. Mol. Cell. Proteom. 2021, 20, 100022. [Google Scholar] [CrossRef] [PubMed]
- Kianpour, M.; Akbarian, M.; Uversky, V.N. Nanoparticles for coronavirus control. Nanomaterials 2022, 12, 1602. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, J.S.; Bilich, T.; Tandler, C.; Nelde, A.; Maringer, Y.; Marconato, M.; Reusch, J.; Jäger, S.; Denk, M.; Richter, M.; et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature 2022, 601, 617–622. [Google Scholar] [CrossRef]
- Bartlam, M.; Xu, Y.; Rao, Z. Structural proteomics of the SARS coronavirus: A model response to emerging infectious diseases. J. Struct. Funct. Genom. 2007, 8, 85–97. [Google Scholar] [CrossRef]
- Stukalov, A.; Girault, V.; Grass, V.; Bergant, V.; Karayel, O.; Urban, C.; Haas, D.A.; Huang, Y.; Oubraham, L.; Wang, A.; et al. Multi-level proteomics reveals host-perturbation strategies of SARS-CoV-2 and SARS-CoV. bioRxiv 2020, 1, 1. [Google Scholar]
- Dudek, N.L.; Croft, N.P.; Schittenhelm, R.B.; Ramarathinam, S.H.; Purcell, A.W. A systems approach to understand antigen presentation and the immune response. Proteom. Syst. Biol. Methods Protoc. 2016, 1394, 189–209. [Google Scholar]
- Faridi, P.; Purcell, A.W.; Croft, N.P. In immunopeptidomics we need a sniper instead of a shotgun. Proteomics. 2018, 18, 1700464. [Google Scholar] [CrossRef]
- Ihling, C.; Tanzler, D.; Hagemann, S.; Kehlen, A.; Huttelmaier, S.; Arlt, C.; Sinz, A. Mass spectrometric identification of SARS-CoV-2 proteins from gargle solution samples of COVID-19 patients. J. Proteome Res. 2020, 19, 4389–4392. [Google Scholar] [CrossRef] [PubMed]
- Worl Health Organization. Draft Landscape of COVID-19 Candidate Vaccines. 2 July 2020. Available online: https://www.who.int/publications/m/item/draft-landscape-of-COVID-19-candidate-vaccines (accessed on 4 July 2020).
- Fares, S.; Elmnyer, M.M.; Mohamed, S.S.; Elsayed, R. COVID-19 vaccination perception and attitude among healthcare workers in Egypt. J. Prim. Care Community Health 2021, 12, 21501327211013303. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.P.; Yang, M.; Lai, C.L. COVID-19 vaccines: A review of the safety and efficacy of current clinical trials. Pharmaceuticals 2021, 14, 406. [Google Scholar] [CrossRef] [PubMed]
- OSE Immunotherapeutics. A Randomized, Open Label, Phase 1 Study to Evaluate the Safety, Reactogenicity and Immunogenicity of OSE-13E, a Multiepitope-Based Vaccine Candidate against COVID-19, in Healthy Adults (COVEPIT-3). Clinical Trial Registration NCT04885361. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04885361 (accessed on 26 June 2023).
- Belayachi, J.; Obtel, M.; Mhayi, A.; Razine, R.; Abouqal, R. Long term effectiveness of inactivated vaccine BBIBP-CorV (Vero Cells) against COVID-19 associated severe and critical hospitalization in Morocco. PLoS ONE 2022, 17, e0278546. [Google Scholar] [CrossRef]
- Garnett, L.; Tran, K.; Chan, M.; Tierney, K.; Schiffman, Z.; Audet, J.; Lew, J.; Meilleur, C.; Chan, M.; Manguiat, K.; et al. An S1 subunit vaccine and combination adjuvant (COVAC-1) elicits robust protection against SARS-CoV-2 challenge in African green monkeys. bioRxiv 2022, 6. [Google Scholar] [CrossRef]
- Luo, D.; Pan, H.; He, P.; Yang, X.; Li, T.; Ning, N.; Fang, X.; Yu, W.; Wei, M.; Gao, H. A randomized, double-blind, placebo-controlled phase 1 and phase 2 clinical trial to evaluate efficacy and safety of a SARS-CoV-2 vaccine SCoK in adults. Clin. Transl. Med. 2022, 12, e1016. [Google Scholar] [CrossRef] [PubMed]
- Richmond, P.; Hatchuel, L.; Dong, M.; Ma, B.; Hu, B.; Smolenov, I.; Li, P.; Liang, P.; Han, H.H.; Liang, J.; et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: A phase 1, randomised, double-blind, placebo-controlled trial. Lancet 2021, 397, 682–694. [Google Scholar] [CrossRef] [PubMed]
- A Controlled Phase 2/3 Study of Adjuvanted Recombinant SARS-CoV-2 Trimeric S-protein Vaccine (SCB-2019) for the Prevention of COVID-19. NCT04672395. Available online: www.ClinicalTrials.gov (accessed on 26 June 2023).
- Lopez, P.; Bravo, L.; Buntinx, E.; Borja-Tabora, C.; Velasquez, H.; Rodriguez, E.J.; Rodriguez, C.A.; Carlos, J.; Montellano, M.E.; Alberto, E.R. Safety and immunogenicity of SCB-2019, an adjuvanted, recombinant SARS-CoV-2 trimeric S-protein subunit COVID-19 vaccine in healthy 12–17 year-old adolescents. medRxiv 2023, 2, 23286317. [Google Scholar] [CrossRef]
- Tabarsi, P.; Anjidani, N.; Shahpari, R.; Roshanzamir, K.; Fallah, N.; Andre, G.; Petrovsky, N.; Barati, S. Immunogenicity and safety of SpikoGen®, an adjuvanted recombinant SARS-CoV-2 spike protein vaccine as a homologous and heterologous booster vaccination: A randomized placebo-controlled trial. Immunology 2022, 167, 340–353. [Google Scholar] [CrossRef]
- Tabarsi, P.; Anjidani, N.; Shahpari, R.; Mardani, M.; Sabzvari, A.; Yazdani, B.; Kafi, H.; Fallah, N.; Ebrahimi, A.; Taheri, A.; et al. Evaluating the efficacy and safety of SpikoGen®, an Advax-CpG55. 2–adjuvanted severe acute respiratory syndrome coronavirus 2 spike protein vaccine: A phase 3 randomized placebo-controlled trial. Clin. Microbiol. Infect. 2023, 29, 215–220. [Google Scholar] [CrossRef]
- Mallory, R.M.; Formica, N.; Pfeiffer, S.; Wilkinson, B.; Marcheschi, A.; Albert, G.; McFall, H.; Robinson, M.; Plested, J.S.; Zhu, M.; et al. Safety and immunogenicity following a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373): A secondary analysis of a randomised, placebo-controlled, phase 2 trial. Lancet Infect. Dis. 2022, 22, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Medicine UNLo. Evaluation of the Safety and Immunogenicity of a SARS-CoV-2 rS (COVID-19) Nanoparticle Vaccine with/without Matrix-M. Adjuvant. Available online: https://clinicaltrials.gov/ct2/show/NCT04368988 (accessed on 26 June 2023).
- Chavda, V.P.; Bezbaruah, R.; Dolia, S.; Shah, N.; Verma, S.; Savale, S.; Ray, S. Convalescent plasma (hyperimmune immunoglobulin) for COVID-19 management: An update. Process Biochem. 2023, 127, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Pan, C.; Chen, H.; Xie, J.; Huang, Y.; Yang, Y.; Du, B.; Qiu, H. The Efficiency of Convalescent Plasma Therapy in the Management of Critically Ill Patients Infected With COVID-19: A Matched Cohort Study. Front. Med. 2022, 9, 822821. [Google Scholar] [CrossRef]
- Hachem, H.; Godara, A.; Schroeder, C.; Fein, D.; Mann, H.; Lawlor, C.; Marshall, J.; Klein, A.; Poutsiaka, D.; Breeze, J.L.; et al. Rapid and sustained decline in CXCL-10 (IP-10) annotates clinical outcomes following TNFα-antagonist therapy in hospitalized patients with severe and critical COVID-19 respiratory failure. J. Clin. Transl. Sci. 2021, 5, e146. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Chua, J.V.; Talwani, R.; Bentzen, S.M.; Baddley, J. Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19, A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 897. [Google Scholar] [CrossRef]
- Atmowihardjo, L.; Schippers, J.R.; Bartelink, I.H.; Bet, P.M.; van Rein, N.; Purdy, K.; Cavalla, D.; Comberiati, V.; McElroy, A.; Snape, S.D.; et al. The invent COVID trial: A structured protocol for a randomized controlled trial investigating the efficacy and safety of intravenous imatinib mesylate (Impentri®) in subjects with acute respiratory distress syndrome induced by COVID-19. Trials 2022, 23, 158. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Sehrawat, T.S.; Simonetto, D.A.; Verma, V.K.; Feng, D.; Tang, T.; Dreyer, K.; Yan, X.; Daley, W.L.; Sanyal, A.; et al. An open-label, dose-escalation study to assess the safety and efficacy of il-22 agonist f-652 in patients with alcohol-associated hepatitis. Hepatology 2020, 72, 441–453. [Google Scholar] [CrossRef]
- Tang, K.-Y.; Lickliter, J.; Huang, Z.-H.; Xian, Z.-S.; Chen, H.-Y.; Huang, C.; Xiao, C.; Wang, Y.-P.; Tan, Y.; Xu, L.-F.; et al. Safety, pharmacokinetics, and biomarkers of F-652, a recombinant human interleukin-22 dimer, in healthy subjects. Cell. Mol. Immunol. 2018, 16, 473–482. [Google Scholar] [CrossRef]
- Rosas, I.O.; Bräu, N.; Waters, M.; Go, R.C.; Malhotra, A.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Savic, S.; Douglas, I.S.; et al. Tocilizumab in patients hospitalised with COVID-19 pneumonia: Efficacy, safety, viral clearance, and antibody response from a randomised controlled trial (COVACTA). Eclinicalmedicine 2022, 47, 101409. [Google Scholar] [CrossRef]


| Vaccine | Trial Registries | Developer | Enrollment | Phase |
|---|---|---|---|---|
| KBP-COVID-19 (RBD-based) | NCT04473690 | Kentucky Bioprocessing Inc. (Owensboro, KY, USA) | 101 | Phase ½ |
| VAT00008: SARS-CoV-2 S protein with adjuvant (1) CoV2 preS dTM monovalent D614 antigen, (2) Bivalent (2-antigen) vaccine comprising spike protein of D614 and spike protein of the SARS-CoV-2 Beta variant (B.1.351) | NCT04537208 | Sanofi Pasteur (Lyon, France) + GSK (Brentford, UK) | 442 | Phase 3 |
| CpG 1018/Alum-adjuvanted Recombinant SARS-CoV-2 Trimeric S-protein Subunit Vaccine (SCB-2019) | NCT05193279 | Clover Biopharmaceuticals Inc. (Chengdu, China)/Dynavax (Emeryville, CA, USA) | 1000 | Phase 3 |
| COVAX-19® Recombinant spike protein + adjuvant SPIKOGEN | NCT05175625 | Vaxine Pty Ltd. (Adelaide, Australia)/CinnaGen Co. (Tehran, Iran) | 300 | Phase 3 |
| MF59 adjuvanted SARS-CoV-2 Sclamp vaccine | NCT04806529 | CSL Ltd. (Melbourne, Australia) + Seqirus (Holly Springs, NC, USA) + University of Queensland | - | Phase 2/3 |
| FINLAY-FR1 anti-SARS-CoV-2 Vaccine (RBD + adjuvant) | RPCEC00000366 | Instituto Finlay de Vacunas (La Habana, Cuba) | 450 | Phase 2 |
| FINLAY-FR-2 anti-SARS-CoV-2 Vaccine (RBD chemically conjugated to tetanus toxoid plus adjuvant) | RPCEC00000354 | Instituto Finlay de Vacunas (La Habana, Cuba) | 44,031 | Phase 3 |
| EpiVacCorona (EpiVacCorona vaccine based on peptide antigens for the prevention of COVID-19) | NCT04780035 | Federal Budgetary Research Institution State Research Center of Virology and Biotechnology “Vector” | 3000 | Phase 3 |
| RBD (baculovirus production expressed in Sf9 cells) Recombinant SARS-CoV-2 vaccine (Sf9 Cell) | NCT04904471 | West China Hospital + Sichuan University WestVac Biopharma Co., Ltd. (Chengdu, China) | 40,000 | Phase 3 |
| UB-612 (Multitope peptide based S1-RBD-protein based vaccine) | NCT04683224 | Vaxxinity (Dallas, TX, USA) | 60 | Phase2/3 |
| CIGB-66 (RBD+Aluminium hydroxide) | RPCEC00000359 | Center for Genetic Engineering and Biotechnology (CIGB) Cuba | 21,146 | Phase 3 |
| Recombinant SARS-CoV-2 Spike protein, Aluminum adjuvanted (Nanocovax) | NCT04922788 | Nanogen Pharmaceutical Biotechnology, Ho Chi Minh City, Vietnam | 13,000 | Phase 3 |
| Protein Subunit Recombinant Vaccine (Adjuvanted With Alum+CpG 1018) | NCT05525208 | PT Bio Farma, Kota Bandung, Indonesia | 900 | Phase 2 |
| Protein Subunit Recombinant Vaccine | NCT05546502 | PT Bio Farma, Kota Bandung, Indonesia | 1050 (Healthy Children) | Phase 3 |
| RBD-based protein subunit vaccine (ZF2001) | NCT04833101 | Jiangsu Province Centers for Disease Control and Prevention, Nanjing, China | 120 | Phase 4 |
| Subunit recombinant vaccine | NCT05726084 | St. Petersburg Research Institute of Vaccines and Sera, St. Petersburg, Russia | 16,304 | Phase 3 |
| CpG 1018/Alum-adjuvanted SCB-2019 vaccine | NCT04672395 | Clover Biopharmaceuticals AUS Pty Ltd., Altona North, Australia | 31,454 | Phase 3 |
| PIKA COVID-19 vaccine | NCT05463419 | Yisheng Biopharma (Singapore) Pte. Ltd. | 9300 | Phase 3 |
| ZR-202-CoV | NCT05313022 | Shanghai Zerun Biotechnology Co.,Ltd., Shanghai, China | 84 | Phase 2 |
| Booster dose of VidPrevtyn® Beta, Sanofi | NCT05749926 | Assistance Publique-Hôpitaux de Paris, Paris, France | 236 | Phase 3 |
| Monoclonal Antibody | Trial Registries | Clinical Phase | Sponsor | Enrollment |
|---|---|---|---|---|
| Anti CD14 (CaTT) | NCT04391309 | Phase 2 | National Institute of Allergy and Infectious Diseases (NIAID) | 40 hospitalized patients |
| LY3819253 (LY-CoV555) and LY3832479 (LY-CoV016) | NCT04427501 | Phase 3 | Eli Lilly and Company (Indianapolis, IN, USA) | 3360 Mild to Moderate patients |
| CONDIVIDIAMO (Only Bamlanivimab Combination with Etesevimab | NCT05268601 | ----- | University of Milano Bicocca, Italy | 1000 patients with Severe form |
| Casirivimab+Imdevimab REGN10933+REGN10987 | NCT04425629 | Phase 3 | Regeneron Pharmaceuticals (Rensselaer, NY, USA) | 10078 mild and Ambulatory patients |
| Canakinumab | NCT04362813 | Phase 3 | Novartis Pharmaceuticals (Basel, Switzerland) | 454 patients |
| BRII-196 and BRII-198 | NCT04770467 | Phase 2 | Brii Biosciences, Inc. (Beijing, China) | 17,495 mild and moderate patients |
| VIR-7831/GSK418236 | NCT04545060 | Phase 3 | Vir Biotechnology, Inc. (San Francisco, CA, USA) | 1057 Non-hospitalized Patients |
| Cizanlizumab | NCT04435184 | Phase 2 | Johns Hopkins University Novartis, USA | 45 patients with vasculopathy |
| JS016 | NCT04780321 | Phase 2 | Shanghai Junshi Bioscience Co., Ltd. (Shanghai, China) | 62 patients with mild and moderate COVID-19 |
| BGB-DXP593 | NCT04551898 | Phase 2 | BeiGene (Cambridge, UK) | 181 mild to moderate Patients |
| STI-1499 (COVI-GUARD) | NCT04454398 | Phase 1 | Sorrento Therapeutics, Inc. (San Diego, CA, USA) | ----- |
| MAD0004J08 | NCT04932850 | Phase 1 | Toscana Life Sciences Sviluppo s.r.l. | 30 Healthy Adults |
| MANTICO Bamlanivimb + Etesevimab | NCT05205759 | Phase 3 | Azienda Ospedaliera Universitaria Integrata Verona | 319 Mild or Moderate Patients |
| BRII-196 and BRII-198 | NCT04787211 | Phase 2 | Brii Biosciences Limited (Beijing, China) | 48 mild to moderate Patients |
| MAD0004J08 | NCT04952805 | Phase 2, Phase 3 | Toscana Life Sciences Sviluppo s.r.l. | 800 moderate’s patients |
| Bamlanivimab or Casirivimab + Imdevimab | NCT04840459 | Phase 2 | Sohail Rao | 1000 Non-Hospitalized Patients |
| SCTA01 | NCT04644185 | Phase 3 | Sinocelltech Ltd. (Beijing, China) | 795 Hospitalized Patients |
| DZIF-10c | NCT04631666 | Phase 2 | University of Cologne | 57 mild to moderate Patients |
| CSL312 Garadacimab | NCT04409509 | Phase 2 | CSL Behring (King of Prussia, PA, USA) | 124 patients |
| Inhalation DZIF-10c | NCT04631705 | Phase 2 | University of Cologne | 45 healthy volunteers |
| AZD7442 (Tixagevimab [AZD8895] + Cilgavimab [AZD1061]) | NCT04625725 | Phase 3 | AstraZeneca (Gaithersburg, MD, USA) | 5254 healthy adults |
| Ravulizumab + Baricitinib | NCT04390464 | Phase 4 | Cambridge University Hospitals NHS Foundation Trust | 1167 Hospitalized Patients |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarkesh, K.; Akbarian, M.; Tayebi, L.; Uversky, V.N.; Rubio-Casillas, A.; Redwan, E.M. Harnessing Antiviral Peptides as Means for SARS-CoV-2 Control. COVID 2023, 3, 975-986. https://doi.org/10.3390/covid3070070
Zarkesh K, Akbarian M, Tayebi L, Uversky VN, Rubio-Casillas A, Redwan EM. Harnessing Antiviral Peptides as Means for SARS-CoV-2 Control. COVID. 2023; 3(7):975-986. https://doi.org/10.3390/covid3070070
Chicago/Turabian StyleZarkesh, Khatereh, Mohsen Akbarian, Lobat Tayebi, Vladimir N. Uversky, Alberto Rubio-Casillas, and Elrashdy M. Redwan. 2023. "Harnessing Antiviral Peptides as Means for SARS-CoV-2 Control" COVID 3, no. 7: 975-986. https://doi.org/10.3390/covid3070070
APA StyleZarkesh, K., Akbarian, M., Tayebi, L., Uversky, V. N., Rubio-Casillas, A., & Redwan, E. M. (2023). Harnessing Antiviral Peptides as Means for SARS-CoV-2 Control. COVID, 3(7), 975-986. https://doi.org/10.3390/covid3070070







