Evusheld Prophylaxis Improves Social Interactions, Anxiety, Depression, Agoraphobia, and Quality of Life in Blood Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
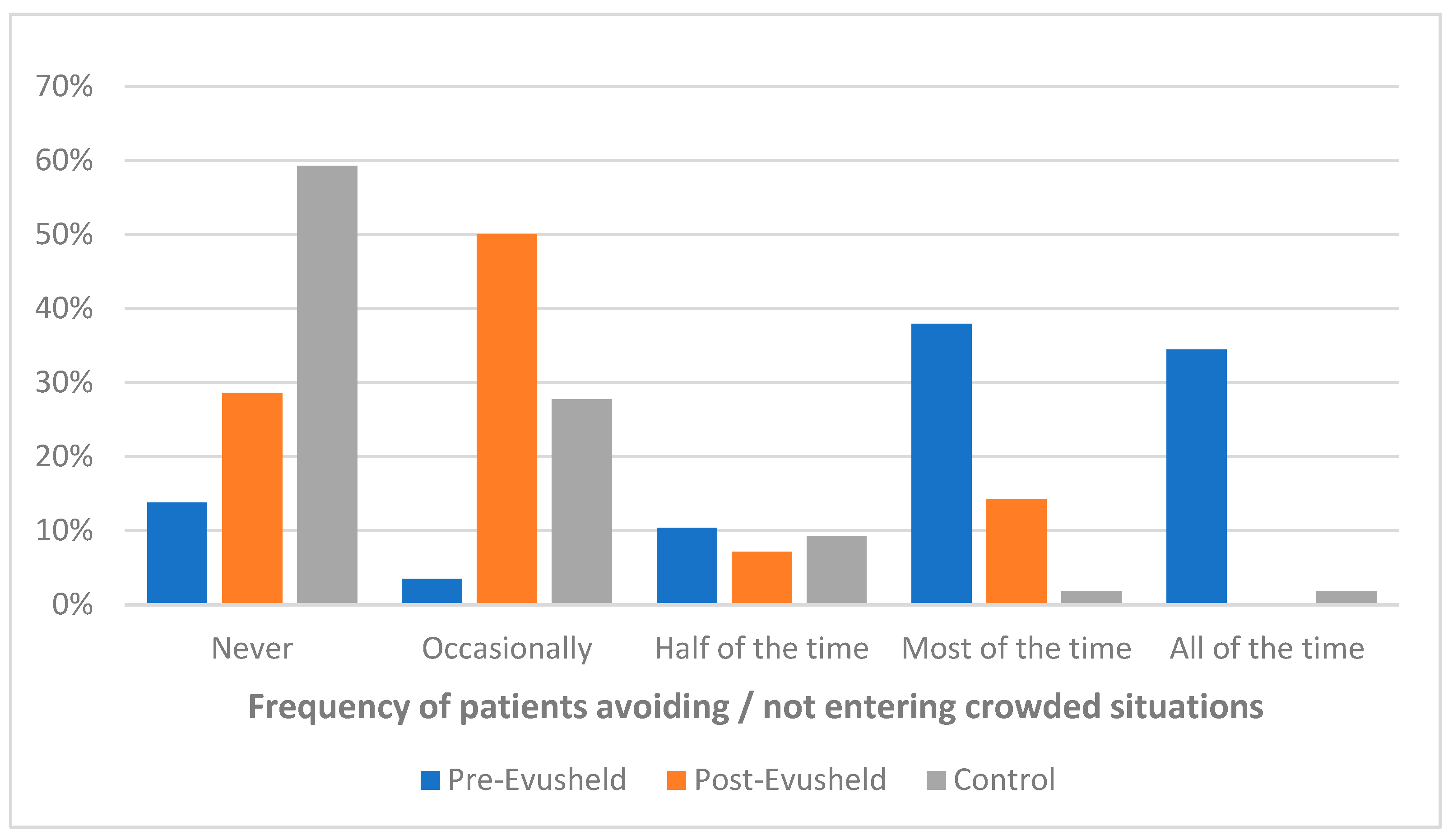
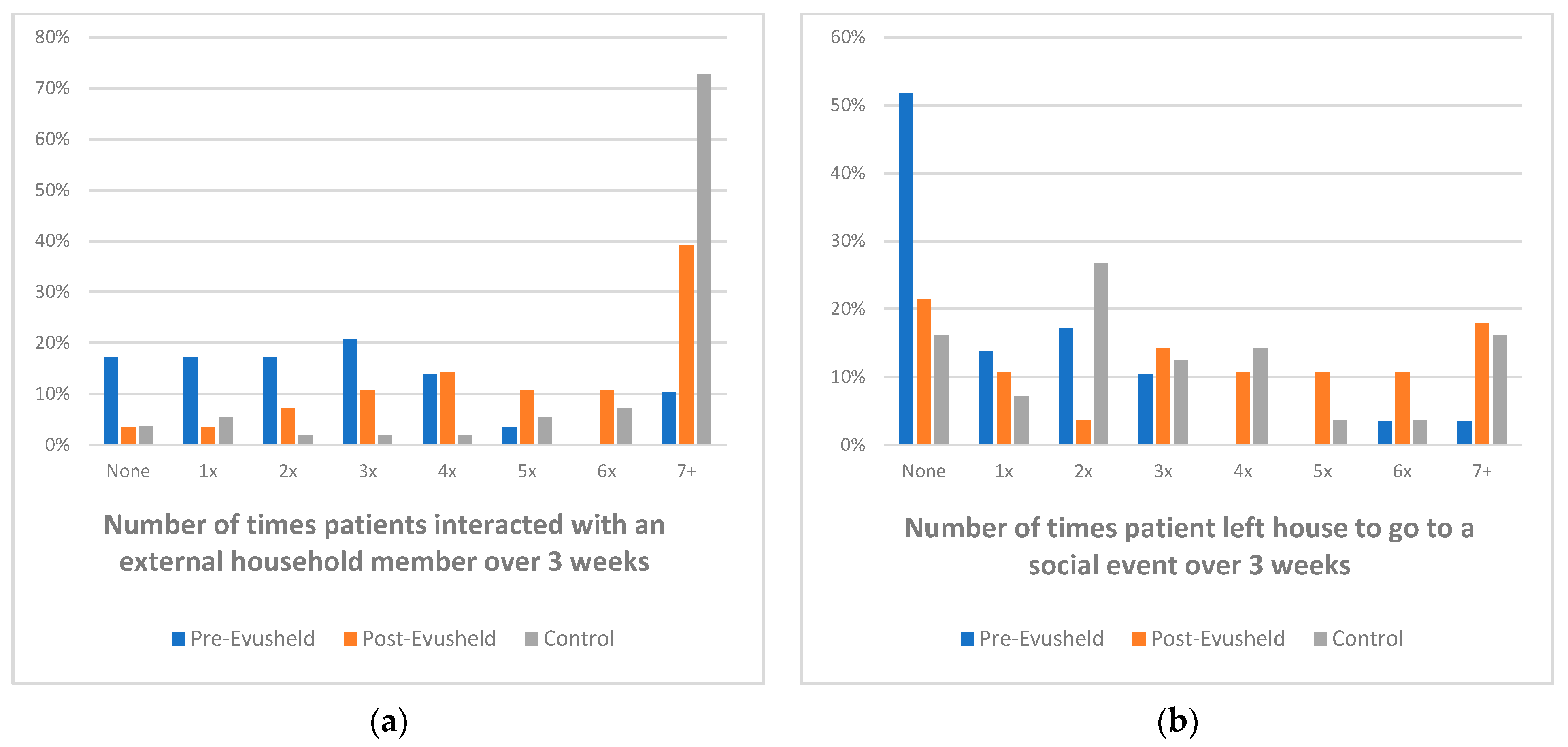
3. Results
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medicines and Healthcare Products Regulatory Agency. Evusheld Approved to Prevent COVID-19 in People Whose Immune Response Is Poor. Available online: https://www.gov.uk/government/news/evusheld-approved-to-prevent-covid-19-in-people-whose-immune-response-is-poor (accessed on 17 March 2022).
- Wise, J. COVID-19: Evusheld is approved in UK for prophylaxis in immunocompromised people. BMJ 2022, 376, o722. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Tixagevimab–Cilgavimab for Preventing COVID-19 [ID6136]. Available online: https://www.nice.org.uk/guidance/indevelopment/gid-ta11102 (accessed on 16 December 2022).
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Stein, N.; Saliba, W. Effectiveness of Evusheld in Immunocompromised Patients: Propensity Score–Matched Analysis. Clin. Infect. Dis. 2022, 76, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Psychiatry. COVID-19 and mental health. Lancet Psychiatry 2021, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Weich, S. Mental health after COVID-19. BMJ 2022, 376, o326. [Google Scholar] [CrossRef] [PubMed]
- Heesen, G.; Heinemann, S.; Müller, F.; Dopfer-Jablonka, A.; Mikuteit, M.; Niewolik, J.; Klawonn, F.; Vahldiek, K.; Hummers, E.; Schröder, D. Social participation and mental health of immunocompromised individuals before and after COVID-19 vaccination–Results of a longitudinal observational study over three time points. Front. Psychiatry 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.Y.; Vidot, D.C.; Camacho-Rivera, M. Evaluating Mental Health–Related Symptoms Among Cancer Survivors During the COVID-19 Pandemic: An Analysis of the COVID Impact Survey. JCO Oncol. Pract. 2021, 17, e1258–e1269. [Google Scholar] [CrossRef]
- The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- George, L.K.; Blazer, D.G.; Hughes, D.C.; Fowler, N. Social Support and the Outcome of Major Depression. Br. J. Psychiatry 1989, 154, 478–485. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Koltai, J.; Raifman, J.; Bor, J.; McKee, M.; Stuckler, D. COVID-19 Vaccination and Mental Health: A Difference-In-Difference Analysis of the Understanding America Study. Am. J. Prev. Med. 2022, 62, 679–687. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Follows, A.M.; Clark, C.; Dye, C.; King, L.; Skillings, G.; Byrne, G.; Tinkler, V.; Follows, G.A. Evusheld Prophylaxis Improves Social Interactions, Anxiety, Depression, Agoraphobia, and Quality of Life in Blood Cancer Patients. COVID 2023, 3, 664-670. https://doi.org/10.3390/covid3050048
Follows AM, Clark C, Dye C, King L, Skillings G, Byrne G, Tinkler V, Follows GA. Evusheld Prophylaxis Improves Social Interactions, Anxiety, Depression, Agoraphobia, and Quality of Life in Blood Cancer Patients. COVID. 2023; 3(5):664-670. https://doi.org/10.3390/covid3050048
Chicago/Turabian StyleFollows, Annabel M., Charlotte Clark, Catherine Dye, Lorraine King, Gail Skillings, Grace Byrne, Vicki Tinkler, and George A. Follows. 2023. "Evusheld Prophylaxis Improves Social Interactions, Anxiety, Depression, Agoraphobia, and Quality of Life in Blood Cancer Patients" COVID 3, no. 5: 664-670. https://doi.org/10.3390/covid3050048
APA StyleFollows, A. M., Clark, C., Dye, C., King, L., Skillings, G., Byrne, G., Tinkler, V., & Follows, G. A. (2023). Evusheld Prophylaxis Improves Social Interactions, Anxiety, Depression, Agoraphobia, and Quality of Life in Blood Cancer Patients. COVID, 3(5), 664-670. https://doi.org/10.3390/covid3050048




