Abstract
Real-world effectiveness studies of remdesivir in hospitalized patients with COVID-19 conducted to date have produced conflicting findings which may be due, in part, to treatment heterogeneity within standard of care comparison groups. Our objective was to evaluate the comparative effectiveness and safety of remdesivir in a cohort of patients all treated with corticosteroids. We conducted a retrospective cohort study in the National Veterans Affairs Healthcare System. We included hospitalized patients (>18 years old) with positive COVID-19 PCR tests and COVID-19 diagnosis codes, and corticosteroid treatment within 2 days of admission, from 1 May 2020 to 30 November 2021. Time-to-event outcomes included time to inpatient mortality (primary), discharge, mortality after discharge, readmission, and acute kidney injury and bacterial infection after treatment initiation. Propensity score (PS)-adjusted, PS-matched, and inverse probability of treatment weighted (IPTW) Cox proportional hazards regression models controlled for study timeframe, supplemental oxygen, vaccination status, and other important confounders. We observed significantly lower inpatient mortality, 90-day post-discharge mortality, 30-day post-discharge readmission, and significantly longer hospital stays in the remdesivir group (n = 14,509) compared with the non-remdesivir group (n = 4365). Higher rates of bacterial infections were observed in the remdesivir group. Acute kidney injury was lower in subgroup analyses restricting the study population to index dates in 2021, on supplemental oxygen, and fully vaccinated, and higher in those without baseline supplemental oxygen. When comparing the effectiveness and safety of remdesivir plus corticosteroids to a homogenous comparison group, all also treated with corticosteroids, mortality and readmission were significantly lower in the remdesivir group. Longer length of stay corresponds with duration of remdesivir treatment and may impact the risk of developing infections during the hospitalization, which requires further study.
1. Introduction
The novel coronavirus SARS-CoV-2, the virus responsible for the coronavirus-19 (COVID-19) pandemic, continues to have a significant burden, causing severe infection, hospitalization, and death worldwide. There is an ongoing need for treatment options to improve clinical outcomes in patients with COVID-19. Remdesivir, a novel nucleotide analog that inhibits SARS-CoV-2 viral replication, is fully approved by the United States (U.S.) Food and Drug Administration (FDA) for adults and pediatric patients with COVID-19 regardless of severity [1]. The World Health Organization has a conditional recommendation for the use of remdesivir in patients with severe COVID-19 but not in patients with critical COVID-19, such as those on mechanical ventilation (invasive or non-invasive) or vasopressor therapy [2]. The Infectious Diseases Society of America (IDSA) and National Institutes of Health (NIH) similarly recommend remdesivir in hospitalized patients with severe COVID-19 but not in those who require mechanical ventilation or extracorporeal membrane oxygenation (ECMO) [3,4]. Data from clinical trials have not consistently demonstrated a major clinical benefit for all hospitalized patients treated with remdesivir [5,6,7,8].
Real-world effectiveness studies have also generated conflicting results regarding the benefits of remdesivir in patients hospitalized with COVID-19, with and without supplemental oxygen, with several studies showing reduced time to clinical improvement [9,10,11,12] and another showing shorter duration of mechanical ventilation and shorter length of stay among critically ill patients requiring mechanical ventilation [13]. Other studies show no association or longer duration of hospitalization [14,15] and no impact on mortality with remdesivir [10,14,15,16]. There are several limitations to previous studies, including completion early in the pandemic (i.e., ended March 2020) [17] and before widespread vaccination in the U.S. (i.e., ended December 2020–March 2021) [10,14,16,18], smaller study populations (53–1200 patients) [14,15,16,17,19], short duration of follow-up (up to 28–30 days or discharge) [10,14,16,17,18], missing data before and after hospitalization [10,14,16,17,18], and single hospital analyses [14,16].
Another major limitation of previous studies is that the standard of care for COVID-19 continues to evolve [20], and most studies to date have not required standard of care with corticosteroids, monoclonal antibodies, immunomodulating agents, antivirals, and/or any other therapies found to be effective against COVID-19 [10,14,15,16,17]. The comparison groups for these studies have been simply those “not receiving remdesivir”, with no minimal requirement for any other COVID-19 treatments [10,14,15,16,17]. These studies have therefore assumed that patients not receiving remdesivir are receiving a shared standard of care. However, shared standard of care was not verified, resulting in treatment heterogeneity within the comparison groups utilized in each of these studies, as well as the inclusion of patients not receiving appropriate treatment [10,14,15,16,17].
International recommendations for corticosteroids in patients with COVID-19 and progressive deterioration of oxygenation were set in March 2020, and in the U.S. by August (NIH) and September 2020 (IDSA) for critically ill patients who require mechanical ventilation or supplemental oxygenation [3,4,21,22]. Regardless of changing recommendations, corticosteroids have remained a mainstay in hospitalized patients with COVID-19, with or without oxygenation. A literature review and meta-analysis of 52 clinical trials found that 27.9% of patients with severe COVID-19 were treated with corticosteroids, and another study of hospitalized patients with COVID-19 revealed that 42.1% received corticosteroids [23,24]. Only one sensitivity analysis compared patients who received both remdesivir and dexamethasone to matched patients who received dexamethasone alone, which showed a statistically significant benefit in clinical improvement associated with remdesivir, particularly for those on room air and low-flow oxygen [10]. Additionally, few studies have assessed safety outcomes [5] and secondary outcomes associated with remdesivir, such as secondary bacterial infections. One retrospective cohort study among patients with COVID-19 from a single hospital revealed that patients treated with remdesivir had a lower likelihood of acute kidney injury but not acute liver injury [16].
As such, real-world evidence is still urgently needed for hospitalized patients with COVID-19, with varied oxygen requirements, particularly comparisons of remdesivir and verified minimal standard of care treatments. In light of the limited existing literature, our study sought to assess the impact of remdesivir on inpatient mortality and secondary effectiveness and safety outcomes in the national Veterans Affairs (VA) Healthcare System among a cohort of patients hospitalized with COVID-19 and all treated with corticosteroids.
2. Materials and Methods
2.1. Data Sources
We conducted a retrospective cohort study in the national VA Healthcare System. We utilized the Veterans Health Administration (VHA) Corporate Data Warehouse and VHA COVID-19 Shared Data Resource, which contain health information from electronic health records and other administrative systems, including data on hospitalizations and outpatient visits, inpatient and outpatient pharmacy data (including barcode administration data, pharmacy dispensing data, and non-VA medications), inpatient and outpatient diagnoses (International Statistical Classification of Diseases, Tenth Revision, Clinical Modification codes), laboratory and microbiology results, vital signs and vital status, and other health factors, such as COVID-19 symptoms and smoking status. This study was approved by the Institutional Review Board and Research and Development Committee of the VA Providence Healthcare System.
2.2. Study Population
We included hospitalized patients >18 years old with positive COVID-19 PCR tests between 1 May 2020 and 30 November 2021 in the national VA Healthcare System. We excluded non-veterans and patients testing positive more than 2 days after admission or admitted more than 10 days after a positive PCR test. The index date was defined as the date of the first positive PCR test or inpatient admission date, whichever occurred first. Patients with index dates which did not correspond with the test or admission date were excluded. Further exclusions included transfer from another hospital, death or discharge in the first 2 days of admission, pregnancy, length of stay >100 days, no primary or secondary diagnosis of COVID-19, no baseline supplemental oxygen information, no record of any medications dispensed/administered during the hospitalization, and clinical trial patients. Only patients receiving corticosteroids in the first 2 days of admission were included in our study. For those with more than one admission meeting these inclusion and exclusion criteria during the study period, only the first admission was selected for inclusion. Those initiating treatment with remdesivir in the first 2 days of admission were selected for the remdesivir treatment group and those not receiving remdesivir treatment during the admission made up the comparison group (Figure 1).
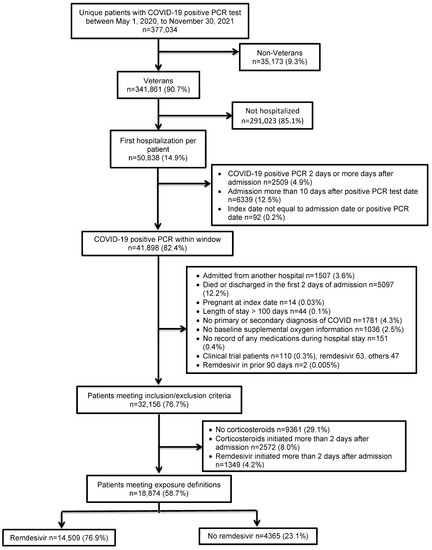
Figure 1.
Study population.
2.3. Outcomes
The primary outcome was time to inpatient mortality. The secondary time-to-event effectiveness and safety outcomes assessed included time to intensive care unit (ICU) discharge, acute kidney injury (AKI), bacterial infection, fungal infection, hospital discharge, 30-day, 60-day, and 90-day mortality from discharge, and readmission. For AKI, baseline serum creatinine was assessed in the seven days prior to admission until remdesivir initiation for the remdesivir group or corticosteroid initiation for the corticosteroid group. The highest value during that time period was selected as the baseline. If baseline serum creatinine was missing or >1.3 mg/dL, then patients were excluded from the assessment of this outcome. Follow-up serum creatinine was assessed from one day after remdesivir initiation for the remdesivir group or corticosteroid initiation for the corticosteroid group until discharge. AKI was defined as a serum creatinine increase of 1.5 times the baseline serum creatinine or an absolute value >1.5 mg/dL. Bacterial infection and fungal infections were defined as positive clinical cultures collected from one day after remdesivir initiation for the remdesivir group or corticosteroid initiation for the corticosteroid group until discharge. Patients were followed until 31 December 2021 (allowing for at least 30 days of follow-up from inclusion end date of 30 November 2021) or their date of death, whichever occurred sooner.
2.4. Variables
We assessed patient demographics including age, sex, race, body mass index, and treating facility. Symptoms in the 30 days prior to initial clinical presentation included abdominal pain, chills, cold, cough, diarrhea, dyspnea, fatigue, fever, headache, loss of smell, loss of taste, myalgia, rhinorrhea, and sore throat. Medical history over the previous two years included conditions of the Elixhauser and Charlson comorbidity score, and other important medical history, including smoking and alcohol/drug dependence and previous infections, such as pneumonia and influenza. Exposure mapping methods were utilized to identify other medications received by the patient prior to the index date and during the admission.
2.5. Statistical Analyses
Patient characteristics and comorbidities were assessed to identify potentially confounding baseline characteristics that may differ between the treatment groups. Categorical data were analyzed using X2 or Fisher’s exact test. Student’s t-test or Mann–Whitney U test was used for continuous variables of interest.
We utilized propensity score methods to balance baseline covariates predictive of treatment with corticosteroids and remdesivir vs. corticosteroids without remdesivir. We built an unconditional logistic regression model to derive propensity scores using manual backward elimination modeling [25,26]. Variables which differed significantly between the treatment groups or between those with or without the study outcomes were considered as potential confounders and assessed for inclusion in the propensity score model, including demographics and clinical characteristics, medical history, symptoms, as well as previous medications in the 90 days prior to the index date, baseline medications administered on the day of admission or day after admission, and concomitant medications administered during remdesivir treatment for the remdesivir group or during corticosteroids treatment for the corticosteroid group. Clinically important variables were forced into the model and included age, facility, obesity/severe obesity, month and year of index date, race, ethnicity, sex, admission source, treating specialty, vaccination status, smoking status/history, rural residence, baseline oxygen status, and Charlson and Elixhauser comorbidity indices in the 2 years prior to the index date [25]. Propensity score assumptions were assessed along with model fit, model discrimination, and multicollinearity [25,27,28,29]. Covariate balance was assessed with standardized differences [30,31].
Separate Cox proportional hazards regression models were developed to quantify differences in time-to-event effectiveness and safety outcomes of the treatment approaches for each of the aforementioned outcomes. Cox proportional hazards model assumptions, including that of proportionality, were evaluated with formal tests and graphical displays [32]. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were calculated using propensity score adjustment (quintiles), propensity score matching (nearest neighbor matching within a caliper of 0.0001), and inverse probability of treatment weighting [26,27,33,34,35].
Sensitivity analyses included assessment of time-to-event outcomes additionally controlling for specific concomitant corticosteroids, anticoagulants, and antibiotics, and corticosteroid duration in Cox proportional hazard models. Subgroup analyses included assessment of effectiveness and safety outcomes by timeframe of the index date (1 May 2020–31 December 2020 and 1 January 2021–30 November 2021), by baseline oxygenation status (no supplemental oxygen, supplemental oxygen, non-invasive ventilation, mechanical ventilator or extracorporeal membrane oxygenation (ECMO)), and by vaccination status (fully vaccinated, partially vaccinated, boostered, unvaccinated). All analyses were conducted using SAS (Version 9.2, SAS Institute Inc., Cary, NC, USA).
3. Results
We included 18,874 patients from 120 hospitals (Figure 1).
Demographics and clinical characteristic differences between groups are shown in Table 1. Patients in the remdesivir group were younger (mean age 66.7 years, standard deviation (SD) 13.8 vs. mean 68.9 years, SD 13.5) than those in the non-remdesivir group. Patients receiving remdesivir were more likely to be obese (42.4% vs. 36.3%) and severely obese (10.7% vs. 8.2%), with a higher mean body mass index (BMI) in the remdesivir group (31.3, SD 7.2 vs. 29.7, SD 7.2). Those in the remdesivir group were more likely to be treated in the ICU during admission (4.4% vs. 5.7%) and to receive supplemental oxygen at baseline (76.0% vs. 66.3%). Patients in the remdesivir group were less likely to be fully vaccinated for COVID-19 (11.7% vs. 15.2%) and more likely to have had no prior COVID-19 vaccination (85.5% vs. 82.2%) than those in the non-remdesivir group. Mean duration of corticosteroids differed by only a day between the remdesivir and non-remdesivir groups (mean 7.5 days, SD 5.5 vs. 6.2 days, SD 5.5) in matched analyses.

Table 1.
Baseline demographics and clinical characteristics among patients treated with remdesivir-based regimens vs. non-remdesivir based regimens, overall and propensity score-matched groups.
Medical history differences between groups are shown in Table 2. Patients in the remdesivir group had a lower Charlson comorbidity index (median 2, interquartile range (IQR) 0–4 vs. 3, IQR 1–5) but higher Elixhauser score (median 4, IQR 0–14 vs. 9, IQR 0–21) than those in the in the non-remdesivir group. Those in the remdesivir group were less likely to have a history of diabetes (45.7% vs. 48.5%), hypertension (72.6% vs. 78.1%), cardiovascular disease (44.0% vs. 53.7%), chronic liver disease (8.2% vs. 9.2%), chronic lung disease (39.4% vs. 43.5%), chronic kidney disease (30.2% vs. 45.8%), dementia (6.1% vs. 9.4%), and cancer (23.9% vs. 26.5%) than those in the non-remdesivir group.

Table 2.
Medical history among patients treated with remdesivir-based regimens vs. non-remdesivir based regimens, overall and propensity score-matched groups.
Symptoms differences between groups are shown in Table 3. Patients in the remdesivir group were more likely to have new onset cough (61.4% vs. 52.9%), dyspnea (64.4% vs. 58.3%), and fever (55.0% vs. 47.8%) than those in the non-remdesivir group. Medications before hospitalization and during hospitalization are shown in Table 4. Patients in the remdesivir group were less likely to have received previous, baseline, and concomitant immunosuppressants and antibiotics than those in the non-remdesivir group. Patients in the remdesivir group were less likely to have received anticoagulant/antiplatelets (19.1% vs. 25.1%), while baseline and concomitant anticoagulant/antiplatelets were more common in the remdesivir vs. non-remdesivir group.

Table 3.
COVID-19 symptoms among patients treated with remdesivir-based regimens vs. non-remdesivir based regimens, overall and propensity score-matched groups.

Table 4.
Previous, baseline, and concomitant medications among patients treated with remdesivir-based regimens vs. non-remdesivir based regimens, overall and propensity score-matched groups.
Propensity score matching eliminated significant differences in the remdesivir and non-remdesivir matched groups, as shown in Table 1, Table 2, Table 3 and Table 4 (all standardized differences <0.10; PS model C-statistic 0.71, Hosmer–Lemeshow goodness-of-fit test p = 0.48). Only five variables differed significantly in chi-square, Fisher’s exact, or t-tests after PS-matching; however, all of these standardized differences were less than 10%. Utilization rates of other medications that have been used to treat COVID-19, such as monoclonal antibodies, interleukin-6 inhibitors, janus kinase inhibitors, and hydroxychloroquine, were low and well balanced after matching.
3.1. Time-to-Event Outcomes
We observed significantly lower inpatient mortality (PS-quintile adjusted HR 0.79, 95% CI 0.72–0.87; PS-matched HR 0.70, 95% CI 0.55–0.88; IPTW HR 0.79, 95% CI 0.73–0.85), 90-day post-discharge mortality (PS-quintile adjusted HR 0.77, 95% CI 0.67–0.88; PS-matched HR 0.72, 95% CI 0.58–0.89; IPTW HR 0.82, 95% CI 0.73–0.92), and 30-day post-discharge readmission (PS-quintile adjusted HR 0.80, 95% CI 0.72–0.89; PS-matched HR 0.83, 95% CI 0.71–0.97; IPTW HR 0.86, 95% CI 0.78–0.94) in the remdesivir group (Figure 2; PS model variables in Supplementary Table S1). Remdesivir was also associated with significantly longer hospital stays (PS-quintile adjusted HR 0.86, 95% CI 0.83–0.90; PS-matched HR 0.74, 95% CI 0.69–0.79; IPTW HR 0.86, 95% CI 0.83–0.89, i.e., decreased probability of the event occurring sooner in the remdesivir group compared with the non-remdesivir group) and intensive care stays (PS-matched HR 0.77, 95% CI 0.62–0.95; IPTW HR 0.93, 95% CI 0.88–0.98). In the PS-matched analysis, the median length of stay in the remdesivir group was 5 days (IQR 4–10) vs. 4 days (IQR 3–9) in the non-remdesivir group. Among those admitted to the ICU, the median length of ICU stay in the remdesivir group was 7 days (IQR 4–13) vs. 6 days (IQR 3–12) in the non-remdesivir group in the PS-matched analysis. Higher rates of bacterial infections were also observed in the remdesivir group (PS-quintile adjusted HR 1.15, 95% CI 1.02–1.29; PS-matched HR 1.45, 95% CI 1.17–1.80; IPTW HR 1.10, 95% CI 1.01–1.20, i.e., higher probability of positive bacterial culture after treatment initiation occurring sooner in the remdesivir group compared with the non-remdesivir group). No differences were observed in time to fungal infection or acute kidney injury.
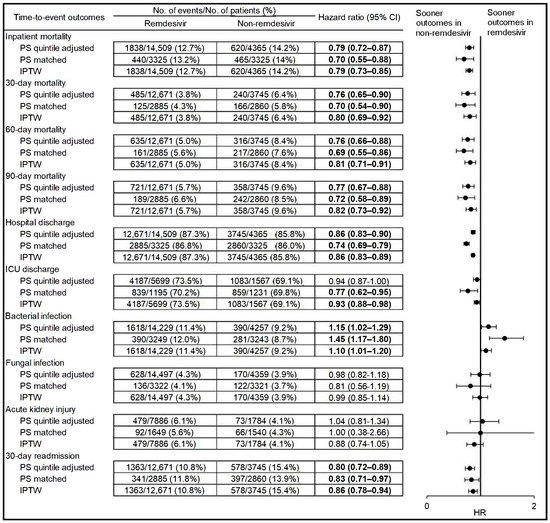
Figure 2.
Time-to-event effectiveness and safety outcomes among hospitalized patients with COVID-19 and treated with remdesivir-based regimens vs. non-remdesivir-based regimens. HR = hazard ratio; ICU = intensive care unit; IPTW = inverse probability of treatment weighted; PS = propensity score. Bold indicates statistically significant (p < 0.05). The propensity score was derived from an unconditional logistic regression model and controlled for the variables listed below and in Supplementary Table S1. Variables in the propensity score model included age at index date, baseline supplemental oxygen, body mass index, Charlson comorbidity index, current intensive care admission, Elixhauser score, ethnicity, facility indicator, fungal infection 90 days prior to index date, gender, history of acute kidney injury, acute liver injury, alcohol dependence, any diabetes, arrhythmia, Bell’s palsy, bipolar disorder, bronchitis, cancer, cardiomyopathy, Cheyne stokes breathing pattern, chronic kidney disease, chronic kidney failure, chronic lung disease, chronic rheumatic heart disease, cirrhosis, congestive heart failure, coronary atherosclerosis and other heart disease, dementia, emphysema, hyperlipidemia, hypertension, major depressive disorder, mild liver disease, nephrosis, non-alcohol drug dependency, obesity hypoventilation, obstructive sleep apnea, other heart disease/ill-defined heart disease, pneumonia, pulmonary fibrosis, schizophrenia, septic shock, sickle cell disease, sleep apnea, sleep related non-obstructive hypoventilation, thalassemia, urinary stones/kidney stones, and ventilator-associated pneumonia 2 years prior to index date, marital status, medications during admission concomitant with remdesivir in the remdesivir group or concomitant with corticosteroids in the corticosteroid group (angiotensin receptor blockers inhibitors use, angiotensin-converting-enzyme’s inhibitors, antibiotics, anticoagulant/antiplatelets, antiparasitic, antivirals, interleukin-6 inhibitors, janus kinase inhibitors, monoclonal antibodies (bamlanivimab/etesevimab, casirivimab/imdevimab), non-steroidal anti-inflammatory drugs and statins), medications during admission prior to remdesivir initiation in the remdesivir group or prior to corticosteroids initiation in the corticosteroid group (angiotensin-converting-enzyme’s inhibitors, antibiotics, anticoagulant/antiplatelets, h2 blocker, immunosuppressants, interleukin-6 inhibitors, janus kinase inhibitors, monoclonal antibodies (bamlanivimab/etesevimab, casirivimab/imdevimab), non-steroidal anti-inflammatory drugs and statins), medications used 90 days prior to index date (angiotensin receptor blockers inhibitors, angiotensin-converting-enzyme’s inhibitors, antibiotics, corticosteroids, immunosuppressants, interleukin-6 inhibitors, and janus kinase inhibitors), month and year of index date, race, rurality, smoking status, source of admission, symptoms 30 days prior to index date (abdominal pain, chills, cough new onset, diarrhea, fever >100.4 F, headache, loss of smell, malaise/fatigue, and muscle aches/myalgia), treating specialty, and vaccination status.
3.2. Sensitivity and Subgroup Analyses
The results were consistent in sensitivity analyses controlling for concomitant treatment with cefepime, dexamethasone, enoxaparin, heparin, hydrocortisone, meropenem, methylprednisolone, piperacillin-tazobactam, prednisone, and vancomycin (Supplementary Table S2), with two exceptions. Readmission in the 30 days after discharge was only significant in PS-adjusted analyses. Additionally, fungal infection rates were higher in the remdesivir group when controlling for specific antibiotics, anticoagulants, and corticosteroids (PS-adjusted HR 1.42, 95% CI 1.16–1.73; IPTW 1.38, 95% CI 1.18–1.62).
Results in the subgroup analysis by timeframe of the index date, 1 May 2020–31 December 2020 (Supplementary Table S3, n = 7438, 74.4% remdesivir, 25.6% non-remdesivir) and 1 January 2021–30 November 2021 (Supplementary Table S4, n = 11,436, 78.5% remdesivir, 21.5% non-remdesivir), were mostly similar to the main analyses (Figure 2). Exceptions were non-significance of duration of ICU stay and significantly lower AKI (IPTW HR 0.74, 95% CI 0.60–0.92) in the remdesivir group in 2021.
Significantly lower AKI (IPTW HR 0.65, 95% CI 0.52–0.81) in the remdesivir group was also observed among those with baseline supplemental oxygen (Supplementary Table S5, n = 13,922, 79.2% remdesivir, 20.8% non-remdesivir), while higher AKI (IPTW HR 2.20, 95% CI 1.09–4.43) was observed in the remdesivir group among a smaller subgroup of patients with no baseline supplemental oxygen (Supplementary Table S6, n = 2500, 61.2% remdesivir, 38.8% non-remdesivir).
Fewer significant differences were observed in subgroup analyses among those not requiring supplemental oxygen (Supplementary Table S6, n = 2500, 61.2% remdesivir, 38.8% non-remdesivir; significantly lower HR for hospital and ICU discharge and readmission, higher HR for bacterial infection, no differences in mortality), requiring non-invasive mechanical ventilation (Supplementary Table S7, n = 1678, 81.2% remdesivir, 18.8% non-remdesivir; significantly lower HR for hospital discharge, no differences in mortality) or invasive mechanical ventilation/ECMO (Supplementary Table S8, n = 774, 75.7% remdesivir, 24.3% non-remdesivir; significantly lower HR for inpatient mortality and readmission) due to small numbers.
As 84.7% of the study population was unvaccinated, results in the subgroup analysis among the unvaccinated (Supplementary Table S9, n = 15,989, 77.6% remdesivir, 22.4% non-remdesivir) were similar to the main analysis (Figure 2). Among the fully vaccinated (Supplementary Table S10, n = 2355, 71.8% remdesivir, 28.2% non-remdesivir), there were only significant differences in inpatient mortality (IPTW HR 0.62, 95% CI 0.50–0.78), AKI (IPTW HR 0.54, 95% CI 0.31–0.92), length of hospital stay (IPTW HR 0.80, 95% CI 0.73–0.87), and length of ICU stay (IPTW HR 0.82, 95% CI 0.69–0.96).
Results were also similar in sensitivity analyses controlling for corticosteroid duration (Supplementary Table S11), except inpatient mortality, hospital discharge, and ICU discharge results were only significant in the PS-adjusted and IPTW analyses. Bacterial infection results were only significant in the PS-adjusted and matched analyses.
4. Discussion
In our large, national, multicenter, retrospective cohort study among almost 15,000 hospitalized patients with COVID-19, all treated with corticosteroids, mortality and readmission rates were significantly lower among patients treated with remdesivir-based regimens vs. non-remdesivir regimens. Remdesivir inhibits the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp), which is essential for viral replication [36]. Remdesivir is a prodrug that is metabolized to the pharmacologically active remdesivir triphosphate [36]. Remdesivir triphosphate is an adenosine triphosphate analogue that competes for incorporation into RNA chains by the SARS-CoV-2 RdRp, resulting in delayed chain termination during viral RNA replication. Remdesivir triphosphate also can inhibit viral RNA synthesis by incorporation into the viral RNA template [36].
In our study, hospital and ICU stays were longer in the remdesivir group, as were rates of bacterial infection compared to those not treated with remdesivir. Importantly, these effects persisted in patients requiring supplemental oxygen and in the unvaccinated, two groups that are especially vulnerable to more serious manifestations of COVID-19. While previous studies have been limited by treatment heterogeneity within the comparison groups and inclusion of patients not receiving appropriate treatment [10,14,15,16,17], a major strength of our study is the requirement for a shared standard of care with corticosteroids among patients receiving and not receiving remdesivir. While no differences in AKI rates were observed in the overall population, remdesivir was associated with significantly lower AKI among several subgroups, including those hospitalized in 2021, those fully vaccinated, and those with baseline supplemental oxygen. Alternatively, higher AKI rates were observed among the subgroup of patients without baseline supplemental oxygen, a patient population in which concomitant remdesivir and corticosteroid treatment is not routinely recommended [3,4].
Our findings related to significantly lower inpatient mortality (PS-matched HR 0.70) and lower 30-day mortality (PS-matched HR 0.70) in the remdesivir group are consistent with one previous study, with effect estimates of a similar magnitude. A previous large, multicenter, retrospective cohort study among hospitalized patients diagnosed with COVID-19 between August and November 2020 similarly demonstrated that remdesivir was associated with lower in-hospital mortality at 14 days (PS-matched HR 0.76, 95% CI 0.70–0.83) and 29 days (PS-matched HR 0.89, 95% CI 0.82–0.96) [18]. Though corticosteroid treatment was not an inclusion criterion of this study, after matching, 96.0% of patients in the remdesivir group and 96.8% in the non-remdesivir had received steroids.
Alternatively, another large, multicenter, retrospective cohort study among individuals hospitalized with laboratory-confirmed COVID-19 from February 2020 to February 2021 did not identify a survival benefit with remdesivir in the overall study population [10]. However, in the subgroup of patients on low-flow oxygen, concomitant remdesivir and dexamethasone treatment was associated with significantly lower 28-day mortality rates (PS-matched HR 0.83, 95% CI 0.76–0.91), as compared with dexamethasone alone [10].
In contrast to our findings, another previous multicenter VA study among hospitalized patients with laboratory-confirmed COVID-19 from May to October 2020 found that remdesivir was not associated with lower 30-day mortality (PS-matched HR 1.06, 95% CI 0.83–1.36) [15]. Interestingly, in this study, less than half of the cohort in the matched analysis had received dexamethasone (47.7% in remdesivir recipients and matched controls). In subgroup analyses by dexamethasone treatment, concomitant remdesivir and dexamethasone treatment was not associated with significantly lower 30-day mortality (PS-matched HR 0.93, 95% CI 0.64–1.35; non-dexamethasone PS-matched HR 1.19, 95% CI 0.84–1.69) [15].
A strength of our study is that all patients were treated with standard of care corticosteroids, including dexamethasone and methylprednisolone. Previous studies of remdesivir only assessed dexamethasone use in the remdesivir and comparison groups [10,15]. While dexamethasone is recommended as the agent of choice in COVID-19 patients, methylprednisolone is recommended when dexamethasone is not available [37]. Additionally, current evidence suggests that methylprednisolone has similar beneficial effects as dexamethasone, with some studies demonstrating lower mortality and mechanical ventilation with methylprednisolone compared with dexamethasone [38]. Differential effectiveness and safety with dexamethasone and methylprednisolone is an important area of future study.
Our work also demonstrated improved survival at 90 days post-discharge. As most previous studies have only assessed mortality at 28–30 days, ours is among the first to show an extended survival benefit with remdesivir. Further, remdesivir was associated with a 17% lower readmission rate (PS-matched HR 0.83) in our study. This finding is important, as in the previous national VA cohort of 2179 hospitalizations for COVID-19 early in the pandemic, 27% of survivors were readmitted or died by 60 days after discharge [39]. Our findings are supported by a smaller study among 2062 patients hospitalized with laboratory-confirmed COVID-19 from April to December 2020 in Rhode Island, which found that remdesivir was associated with a 19% decrease in risk of 30-day readmission [40].
Though remdesivir is not currently recommended for patients requiring mechanical ventilation/ECMO, it has been utilized in those patient populations. Our study demonstrated the effectiveness of remdesivir in subgroups of patients with less severe disease; however, we could not assess endpoints in subgroups of patients with more severe disease, such as those on ECMO or invasive mechanical ventilation, due to insufficient sample size. In our study, only 1678 (8.9%) patients had non-invasive mechanical ventilation and 774 (4.1%) had invasive mechanical ventilation or ECMO. As other studies also had few patients with more severe disease and advanced respiratory needs, conflicting findings have been reported in these subgroups of patients. One study suggested that patients with less severe disease (not on oxygen or on low-flow oxygen) may be more likely to benefit from remdesivir than those with more severe disease past the point where anti-viral therapies may be helpful [10]. In the multicenter, retrospective cohort study among individuals hospitalized with laboratory-confirmed COVID-19 from February 2020 to February 2021, higher rates of clinical improvement were observed with remdesivir in the subgroups of patients on room air (PS-matched HR 1.30, 95% CI 1.23–1.41) and low-flow oxygen (PS-matched HR 1.24, 95% CI 1.20–1.28) but not in subgroups with more advanced respiratory support [10]. Importantly, the survival benefit was only observed in those on low-flow oxygen [10]. However, in another study among hospitalized patients diagnosed with COVID-19 (not laboratory-confirmed), remdesivir was associated with improved survival in patients without oxygen (14-day PS-matched HR 0.69, 95% CI 0.57–0.83; 28-day PS-matched HR 0.80, 95% CI 0.68–0.94), on low flow oxygen (14-day PS-matched HR 0.67, 95% CI 0.59–0.77; 28-day PS-matched HR 0.76, 95% CI 0.68–0.86), and in patients with invasive mechanical ventilation/ECMO (14-day PS-matched HR 0.70, 95% CI 0.58–0.84; 28-day PS-matched HR 0.81, 95% CI 0.69–0.94) [18]. In patients on high-flow oxygen or non-invasive ventilation, a lower risk or morality was only found at 14 days (PS-matched HR 0.81, 95% CI 0.70–0.93) and not at 28 days.
We also observed longer hospital stays and ICU stays among patients with remdesivir-based regimens. The median length of stay was 1 day longer in the PS-matched population that received remdesivir as compared with the population that did not. Previous work in the VA has demonstrated significant differences in length of stay, but of a greater magnitude than the difference seen in our study [15]. In the previous VA cohort study from earlier in the pandemic (May to October 2020), remdesivir recipients had a longer median length of hospital stay compared with matched controls (6 days, IQR 4–12 vs. 3 days, IQR 1–7, p < 0.001) [15]. This difference in length of stay may be explained by the duration of remdesivir, which is administered intravenously generally for 5 days but may extend up to 10 days based on clinical response and in certain patient populations, particularly among hospitalized patients with less severe disease not requiring invasive mechanical ventilation or ECMO [1,3,4,15]. We observed significantly longer length of hospital stay in subgroups of patients with and without supplemental oxygen and with non-invasive mechanical ventilation but not in the subgroup with invasive mechanical ventilation/ECMO, which may be due to low numbers. Alternatively, a single-center French study of 325 hospitalized patients with less severe laboratory confirmed COVID-19 pneumonia receiving low-flow oxygen and dexamethasone found no difference in length of stay [18]. The median duration of hospitalization was 9 days in both the remdesivir and non-remdesivir groups (p = 0.37). Another small, single-hospital study found no difference in length of stay between the remdesivir and dexamethasone group compared with a historical control group (median 7 vs. 6 days, p = 0.55) [14].
In our study, higher rates of culture-confirmed bacterial infections after treatment initiation were observed in the remdesivir group. A previous retrospective cohort study demonstrated a higher frequency of bacteremia in COVID-19 patients treated with remdesivir as compared with matched control patients [41]. Remdesivir is a nucleoside prodrug of an adenosine analog. Adenosine plays a central role in the control of inflammation and might attenuate the host’s antimicrobial response, promote bacterial virulence, and consequently facilitate bacterial superinfection [42]. As such, a proposed mechanism for the higher rates of culture-confirmed bacterial infection we observed after remdesivir treatment may be through the alteration of innate and specific immunity by adenosine analogue metabolites of remdesivir, in a similar manner as adenosine [41,43]. However, as infection was a secondary outcome of interest, we did not assess type of organism (e.g., Gram-positive, Gram-negative), type of infection (e.g., bacteremia, pneumonia), type of antibiotic/s (e.g., Gram-negative coverage, Gram-positive coverage), or time to antibiotic initiation in our study. These will be important factors to assess in future research on bacterial infections in patients with COVID-19.
It is also possible that the extended length of hospital and ICU stay observed in the remdesivir group may increase the risk of secondary bacterial infections; however, the difference in median length of stay was only one day in our study. Previous work suggests that secondary bacterial infections in patients with COVID-19 are associated with worse outcomes, including prolonged length of stay [44]. It is also possible that our results are related to the corticosteroid exposure in each group, as mean duration was 1 day longer in the remdesivir group. However, in sensitivity analyses controlling for corticosteroid duration, the results were similar (PS-matched HR 1.37, 95% CI 1.09–1.71). Previous work has shown that the use of corticosteroids is associated with bacterial and fungal infections associated with COVID-19 in ICU patients [45]. Moreover, other work has shown that each day of treatment with steroids increased the odds of BSI by 13% (adjusted odds ratio 1.13, 95% CI 1.04–1.25) in patients with COVID-19 requiring intensive care [46]. Future research will need to further assess this observed association and whether longer length of stay (~1 day) and longer corticosteroid duration (~1 day) affect the risk of secondary bacterial infection in hospitalized patients with COVID-19.
Our study is one of the few real-world comparative effectiveness and safety studies to assess AKI. We found that in the overall population, remdesivir treatment was not associated with AKI. A small, single-center study which included patients hospitalized with COVID-19 in New York City from June 2020 to March 2021 found that patients treated with remdesivir had a significantly lower likelihood of AKI (odds ratio 0.40, 95% CI 0.24–0.67, p < 0.001) compared to the non-remdesivir group in the overall cohort [16]. However, this association was not significant in the PS-matched analysis. It appears that corticosteroid use was not controlled for in this study. A multicenter, retrospective chart review assessing remdesivir safety in patients with baseline estimated creatinine clearance (eCrCl) <30 mL/min compared with patients with baseline eCrCl > 30 mL/min found there was no difference in the frequency of AKI (5% vs. 2.5%) [47]. Interestingly, our work suggests that the impact of remdesivir on AKI rates may vary by oxygenation and vaccination status. We are the first to find lower AKI among those with baseline supplemental oxygen and those fully vaccinated, but higher AKI among those with no baseline oxygen supplementation. Future research is warranted to continue to assess AKI rates in different subgroups of patients with COVID-19.
There are limitations to our observational comparative effectiveness analysis. We used propensity score methods to control for many confounders, but residual confounding may be present due to unmeasured confounders. The utilization of other COVID-19 therapies was low, but well balanced after matching. Next, we only captured medications received outside of the VA system if they were entered into the medication record as non-VA medications. Moreover, we only captured secondary effectiveness and safety outcomes if they occurred in the VA system. We defined bacterial infection and fungal infections as subsequent positive clinical cultures after treatment initiation, but it is possible that positive cultures represent colonization/contamination vs. a true clinical infection. The generalizability of our study may be limited to the VA population, which included mostly older white males. Finally, we implemented three analytic approaches, including PS quintile adjustment, PS matching, and IPTW, with agreement in effect estimates across the three approaches in the overall analysis. However, in several subgroup analyses, the significance of PS-adjusted, PS-matched, and IPTW results may have varied due to small numbers. Our study was conducted prior to authorization of the COVID-19 antivirals, and therefore, they were not assessed in this study.
5. Conclusions
In our large, national, multicenter, retrospective cohort study among almost 15,000 hospitalized patients with COVID-19, laboratory-confirmed with primary/secondary diagnosis, and all treated with corticosteroids, mortality and readmission rates were significantly lower among patients treated with remdesivir-based regimens vs. non-remdesivir regimens, including in high-risk subgroups who were unvaccinated or required baseline supplemental oxygen. Hospital and ICU stays were longer in the remdesivir group, which corresponded with the duration of remdesivir treatment, as were rates of bacterial infection compared to those not treated with remdesivir. While no differences in AKI rates were observed in the overall population, remdesivir was associated with significantly lower AKI among several subgroups, including those hospitalized in 2021, those fully vaccinated, and those with baseline supplemental oxygen. Alternatively, higher AKI rates were observed among the subgroup of patients without baseline supplemental oxygen. Future work in larger cohorts is needed to better understand the impact of remdesivir among those with invasive mechanical ventilation or ECMO. Further research is also needed to determine whether higher rates of culture-confirmed infections after treatment initiation are observed in other patient populations, and whether these differences are related to longer durations of hospital and intensive care stays, longer corticosteroid treatment, and/or other patient and healthcare system factors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/covid3020015/s1, Tables S1–S11. Table S1: Variables included in the propensity score model, Table S2: Sensitivity analyses of time-to-event outcomes among hospitalized patients with COVID-19 and treated with remdesivir-based regimens and non-remdesivir-based regimens, Table S3: Subgroup analyses of time-to-event outcomes among hospitalized patients with COVID-19 and treated with remdesivir-based regimens and non-remdesivir-based regimens, index dates from 1 May 2020 to 31 December 2020 (n = 7438), Table S4: Subgroup analyses of time-to-event outcomes among hospitalized patients with COVID-19 and treated with remdesivir-based regimens and non-remdesivir-based regimens, index dates from 1 January 2021 to 30 November 2021 (n = 11,436), Table S5: Subgroup analyses of time-to-event outcomes among hospitalized patients with COVID-19 and treated with remdesivir-based regimens and non-remdesivir-based regimens, supplemental oxygen at baseline (n = 13,922), Table S6: Subgroup analyses of time-to-event outcomes among hospitalized patients with COVID-19 and treated with remdesivir-based regimens and non-remdesivir-based regimens, no supplemental oxygen at baseline (n = 2500), Table S7: Subgroup analyses of time-to-event outcomes among hospitalized patients with COVID-19 and treated with remdesivir-based regimens and non-remdesivir-based regimens, non-invasive mechanical ventilation at baseline (n = 1678), Table S8: Subgroup analyses of time-to-event outcomes among hospitalized patients with COVID-19 and treated with remdesivir-based regimens and non-remdesivir-based regimens, invasive mechanical ventilation/ECMO at baseline (n = 774), Table S9: Subgroup analyses of time-to-event outcomes among hospitalized patients with COVID-19 and treated with remdesivir-based regimens and non-remdesivir-based regimens, unvaccinated (n = 15,989), Table S10: Subgroup analyses of time-to-event outcomes among hospitalized patients with COVID-19 and treated with remdesivir-based regimens and non-remdesivir-based regimens, fully vaccinated (n = 2355), Table S11: Sensitivity analyses of time-to-event outcomes among hospitalized patients with COVID-19 and treated with remdesivir-based regimens and non-remdesivir-based regimens, controlling for corticosteroid duration.
Author Contributions
Conception and design of the study: A.R.C. Data generation: A.R.C. and V.V.L. Analysis and/or interpretation of the data: A.R.C., J.X.L., V.V.L., K.L.L. and H.J.A. Preparation or critical revision of the manuscript: A.R.C., J.X.L., V.V.L., K.L.L. and H.J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded, in part, by the Gilead COMMIT™ (COVID-19 unMet MedIcal needs and associated research exTension) Program.
Institutional Review Board Statement
This study was exempted by the Institutional Review Board and approved by the Research and Development Committee of the VA Providence Healthcare System (RDC-2020-038).
Informed Consent Statement
Patient consent was waived as this was a retrospective study of existing health records.
Data Availability Statement
The study data may be made available upon reasonable request and approval by the Department of Veterans Affairs.
Acknowledgments
Views expressed are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs. This material is based upon work supported, in part, by the Office of Research and Development, Department of Veterans Affairs.
Conflicts of Interest
A.R.C. has received research funding from AbbVie Inc., and Merck & Co. Inc., and has been a speaker/advisor for Merck & Co. Inc. on topics unrelated to COVID-19. K.L.L. has received research funding from AbbVie Inc., Merck & Co. Inc., Pfizer Inc., and acted as a consultant for Ferring Pharmaceuticals Inc., Melinta Therapeutics, AbbVie Inc., and Seres Therapeutics on topics unrelated to COVID-19. A.R.C. and K.L.L. received funding by the Gilead COMMIT™ (COVID-19 unMet MedIcal needs and associated research exTension) Program for this study. There are no other conflict to report.
References
- Gilead Sciences I. Veklury® (Remdesivir) Prescribing Information. Available online: https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf (accessed on 4 August 2022).
- WHO. Therapeutics and COVID-19: Living Guideline, 16 September 2022. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.5 (accessed on 11 October 2022).
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Available online: https://covid19treatmentguidelines.nih.gov/ (accessed on 8 August 2022).
- Infectious Diseases Society of America Guidelines. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Available online: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (accessed on 8 August 2022).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Preliminary report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Peto, R.; Henao-Restrepo, A.M.; WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Ader, F.; Bouscambert-Duchamp, M.; Hites, M.; Peiffer-Smadja, N.; Poissy, J.; Belhadi, D.; Diallo, A.; Lê, M.-P.; Peytavin, G.; Staub, T.; et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 2022, 22, 209–221. [Google Scholar] [CrossRef]
- Garibaldi, B.T.; Wang, K.; Robinson, M.L.; Zeger, S.L.; Bandeen-Roche, K.; Wang, M.-C.; Alexander, G.C.; Gupta, A.; Bollinger, R.; Xu, Y. Comparison of Time to Clinical Improvement with vs. without Remdesivir Treatment in Hospitalized Patients with COVID-19. JAMA Netw. Open 2021, 4, e213071. [Google Scholar] [CrossRef]
- Garibaldi, B.T.; Wang, K.; Robinson, M.L.; Betz, J.; Alexander, G.C.; Andersen, K.M.; Joseph, C.S.; Mehta, H.B.; Korwek, K.; E Sands, K.; et al. Real-World Effectiveness of Remdesivir in Adults Hospitalized with Coronavirus Disease 2019 (COVID-19): A Retrospective, Multicenter Comparative Effectiveness Study. Clin. Infect. Dis. 2021, 75, e516–e524. [Google Scholar] [CrossRef]
- Garibaldi, B.T.; Wang, K.; Robinson, M.L.; Betz, J.; Alexander, G.C.; Andersen, K.M.; Joseph, C.S.; Mehta, H.B.; Korwek, K.; E Sands, K.; et al. Effectiveness of remdesivir with and without dexamethasone in hospitalized patients with COVID-19. medRxiv 2020. medRxiv:2020.11.19.20234153. [Google Scholar]
- Olender, S.A.; Perez, K.K.; Go, A.S.; Balani, B.; Price-Haywood, E.G.; Shah, N.S.; Wang, S.; Walunas, T.L.; Swaminathan, S.; Slim, J.; et al. Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care. Clin. Infect. Dis. 2020, 73, e4166–e4174. [Google Scholar] [CrossRef]
- Lapadula, G.; Bernasconi, D.P.; Bellani, G.; Soria, A.; Rona, R.; Bombino, M.; Avalli, L.; Rondelli, E.; Cortinovis, B.; Colombo, E.; et al. Remdesivir Use in Patients Requiring Mechanical Ventilation due to COVID-19. Open Forum Infect. Dis. 2020, 7, ofaa481. [Google Scholar] [CrossRef]
- Larson, D.T.; Ewers, E.C.; Gallagher, K.M.; Mahoney, A.M.; Paul, M.L.; Weina, P.J. Real World Impact of Remdesivir and Dexamethasone on Clinical Outcomes of Severe Coronavirus Disease 2019 in a Community Hospital. Mil. Med. 2022, usac052. [Google Scholar] [CrossRef]
- Ohl, M.E.; Miller, D.R.; Lund, B.C.; Kobayashi, T.; Miell, K.R.; Beck, B.F.; Alexander, B.; Crothers, K.; Sarrazin, M.S.V. Association of Remdesivir Treatment with Survival and Length of Hospital Stay Among US Veterans Hospitalized with COVID-19. JAMA Netw. Open 2021, 4, e2114741. [Google Scholar] [CrossRef]
- Lim, H.; Palaiodimos, L.; Berto, C.G.; Tedunjaiye, O.; Malik, P.; Nagraj, S.; Choi, H.; Seng, N.S.H.L.; Kladas, M.; Kharawala, A.; et al. Remdesivir in the Treatment of COVID-19: A Propensity Score-Matched Analysis from a Public Hospital in New York City Assessing Renal and Hepatic Safety. J. Clin. Med. 2022, 11, 3132. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.X.; et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Mozaffari, E.; Chandak, A.; Zhang, Z.; Liang, S.; Thrun, M.; Gottlieb, R.L.; Kuritzkes, D.R.; E Sax, P.; A Wohl, D.; Casciano, R.; et al. Remdesivir Treatment in Hospitalized Patients with Coronavirus Disease 2019 (COVID-19): A Comparative Analysis of In-hospital All-cause Mortality in a Large Multicenter Observational Cohort. Clin. Infect. Dis. 2021, 75, e450–e458. [Google Scholar] [CrossRef]
- Gressens, S.B.; Esnault, V.; De Castro, N.; Sellier, P.; Sene, D.; Chantelot, L.; Hervier, B.; Delaugerre, C.; Chevret, S.; Molina, J.-M.; et al. Remdesivir in combination with dexamethasone for patients hospitalized with COVID-19: A retrospective multicenter study. PLoS ONE 2022, 17, e0262564. [Google Scholar] [CrossRef]
- Lee, T.C.; Murthy, S.; Del Corpo, O.; Senécal, J.; Butler-Laporte, G.; Sohani, Z.N.; Brophy, J.M.; McDonald, E.G. Remdesivir for the treatment of COVID-19: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1203–1210. [Google Scholar] [CrossRef]
- China National Health Commission. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment. Available online: http://kjfy.meetingchina.org/msite/news/show/cn/3337.html (accessed on 8 August 2022).
- Cano, E.J.; Fuentes, X.F.; Campioli, C.C.; O’Horo, J.C.; Abu Saleh, O.; Odeyemi, Y.; Yadav, H.; Temesgen, Z. Impact of Corticosteroids in Coronavirus Disease 2019 Outcomes: Systematic Review and Meta-analysis. Chest 2020, 159, 1019–1040. [Google Scholar] [CrossRef]
- Wang, J.; Yang, W.; Chen, P.; Guo, J.; Liu, R.; Wen, P.; Li, K.; Lu, Y.; Ma, T.; Li, X.; et al. The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0249481. [Google Scholar] [CrossRef]
- Bahl, A.; Johnson, S.; Chen, N.-W. Timing of corticosteroids impacts mortality in hospitalized COVID-19 patients. Intern. Emerg. Med. 2021, 16, 1593–1603. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Johnson, M.L.; Crown, W.; Martin, B.C.; Dormuth, C.R.; Siebert, U. Good Research Practices for Comparative Effectiveness Research: Analytic Methods to Improve Causal Inference from Nonrandomized Studies of Treatment Effects Using Secondary Data Sources: The ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report—Part III. Value Health 2009, 12, 1062–1073. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]
- Rubin, D.B. Estimating causal effects from large data sets using propensity scores. Ann. Intern. Med. 1997, 127 (8 Pt 2), 757–763. [Google Scholar] [CrossRef]
- Schneeweiss, S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol. Drug Saf. 2006, 15, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B. The design versus the analysis of observational studies for causal effects: Parallels with the design of randomized trials. Stat. Med. 2007, 26, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.B. Using propensity scores to help design observational studies: Application to the tobacco litigation. Health Serv. Outcomes Res. Methodol. 2001, 2, 169–188. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Survival Analysis: Regression Modeling of Time to Event Data; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Hernán, M.A.; Robins, J.M. Causal Inference; Chapman & Hall/CRC, Forthcoming: Boca Raton, FL, USA, 2016. [Google Scholar]
- Austin, P.C. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat. Med. 2016, 35, 5642–5655. [Google Scholar] [CrossRef]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef]
- Gilead. Veklury MOA, December 2022. Available online: https://www.vekluryhcp.com/about/about-moa.php (accessed on 30 January 2023).
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines–Immunomodulators-Orticosteroids. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/ (accessed on 30 January 2023).
- Divyansh, B.; Ankit, A.; Manasvi, G.; Gaurav, M.; Kurt, H.; Umesha, B. Dexamethasone versus Methylprednisolone in Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis. Int. J. Crit. Care Emerg. Med. 2021, 7, 128. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Wang, X.Q.; Iwashyna, T.J.; Prescott, H.C. Readmission and Death After Initial Hospital Discharge Among Patients with COVID-19 in a Large Multihospital System. JAMA 2021, 325, 304–306. [Google Scholar] [CrossRef]
- Finn, A.; Jindal, A.; Andrea, S.B.; Selvaraj, V.; Dapaah-Afriyie, K. Association of Treatment with Remdesivir and 30-day Hospital Readmissions in Patients Hospitalized with COVID-19. Am. J. Med. Sci. 2022, 363, 403–410. [Google Scholar] [CrossRef]
- Lucijanic, M.; Cikara, T.; Bistrovic, P.; Papic, I.; Hadziabdic, M.O.; Busic, N.; Lackovic, M.; Cesar, N.; Koscak, V.; Mitrovic, J.; et al. Remdesivir use in COVID-19 patients might predispose bacteremia, matched case-control analysis. J. Infect. 2022, 85, 174–211. [Google Scholar] [CrossRef]
- Alam, M.S.; Costales, M.G.; Cavanaugh, C.; Williams, K. Extracellular Adenosine Generation in the Regulation of Pro-Inflammatory Responses and Pathogen Colonization. Biomolecules 2015, 5, 775–792. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef]
- Markovskaya, Y.; Gavioli, E.M.; Cusumano, J.A.; Glatt, A.E. Coronavirus disease 2019 (COVID-19): Secondary bacterial infections and the impact on antimicrobial resistance during the COVID-19 pandemic. Antimicrob. Steward. Heal. Epidemiol. 2022, 2, e114. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Lopez, A.A.; Diez-Remesal, Y.; Castro, N.M.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Dupper, A.C.M.; Malik, Y.; Cusumano, J.A.P.; Nadkarni, D.B.; Banga, J.; Caban, A.B.; Twyman, K.; Obla, A.; Patel, D.M.; Mazo, D.M.; et al. Longer Steroid Treatment Increases Secondary Bloodstream Infection Risk among Patients with COVID-19 Requiring Intensive Care. Infect. Dis. Clin. Pr. 2022, 30, 1188. [Google Scholar] [CrossRef]
- Ackley, T.W.; McManus, D.; Topal, J.E.; Cicali, B.; Shah, S. A Valid Warning or Clinical Lore: An Evaluation of Safety Outcomes of Remdesivir in Patients with Impaired Renal Function from a Multicenter Matched Cohort. Antimicrob. Agents Chemother. 2021, 65, e02290-20. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).