The Impact of the COVID-19 Pandemic in The Netherlands on Primary Healthcare Use and Clinical Outcomes in Persons with Type 2 Diabetes
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Definitions
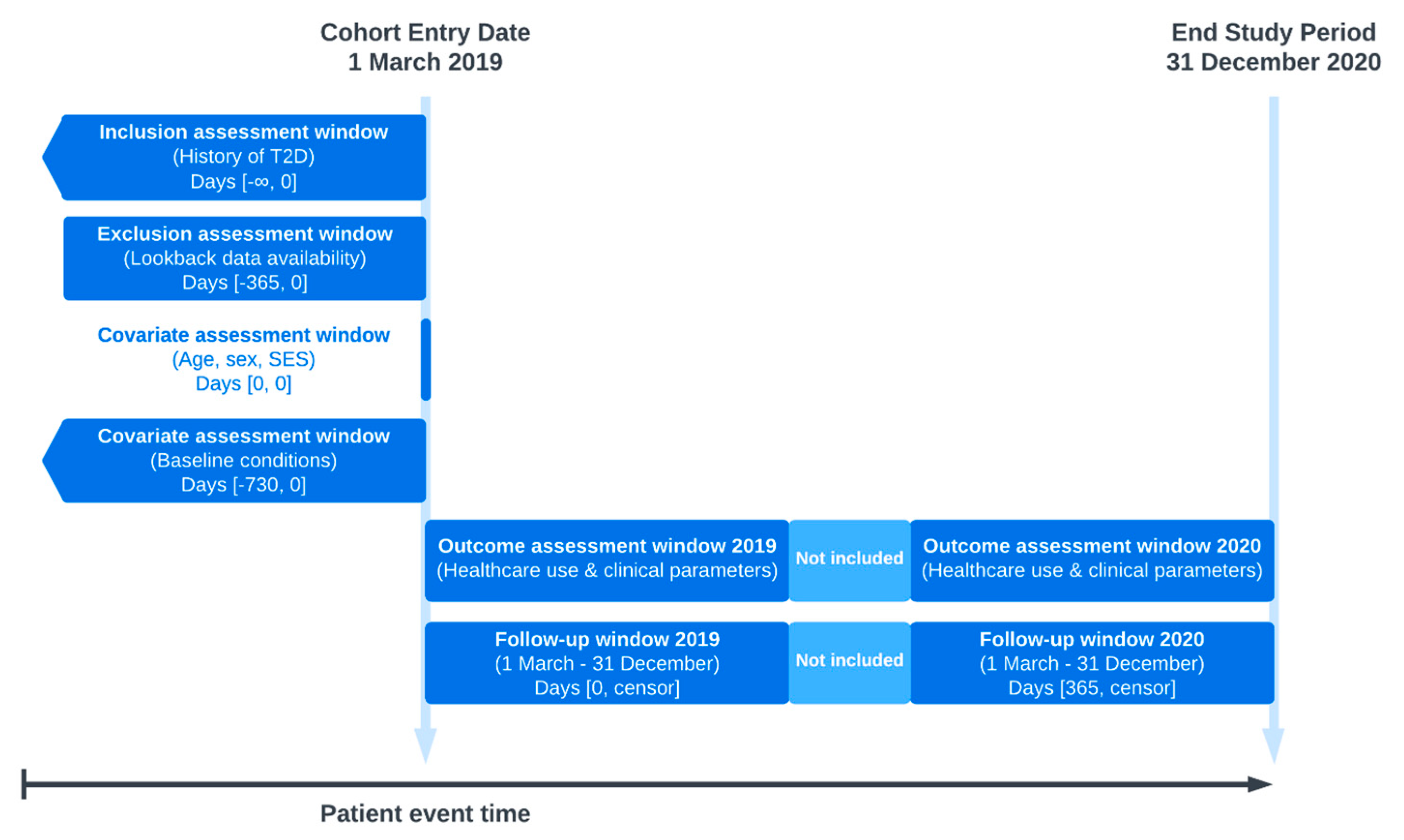
2.3.1. Study Period
2.3.2. Characteristics
2.3.3. Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Findings
4.2. Previous Studies
4.3. Interpretations and Implications for Clinic and Research
4.4. Strengths and Limitations
4.5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Findling, M.G.; Blendon, R.J.; Benson, J.M. Delayed Care with Harmful Health Consequences-Reported Experiences from National Surveys During Coronavirus Disease 2019. JAMA Health Forum 2020, 1, e201463. [Google Scholar] [CrossRef] [PubMed]
- Van Giessen, A.; De Wit, A.; Van den Brink, C.; Degeling, K.; Deuning, C.; Eeuwijk, J.; van den Ende, C.; van Gestel, I.; Gijsen, R.; van Gils, P. Impact van de Eerste COVID-19 Golf op de Reguliere Zorg en Gezondheid: Inventarisatie van de Omvang van het Probleem en Eerste Schatting van Gezondheidseffecten. 2020. Available online: https://rivm.openrepository.com/handle/10029/624583 (accessed on 4 April 2023).
- Overbeek, J.A.; Swart, K.M.A.; van der Pal, E.Y.M.; Blom, M.T.; Beulens, J.W.J.; Nijpels, G.; Elders, P.J.M.; Herings, R.M.C. The DIAbetes MANagement and Treatment (DIAMANT) Cohort. Clin. Epidemiol. 2022, 14, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Volksgezondheidenzorg.info. Diabetes Mellitus. Available online: https://www.vzinfo.nl/diabetes-mellitus (accessed on 6 April 2023).
- Barents, E.S.E.; Bilo, H.J.G.; Bouma, M.; Dankers, M.; De Rooij, A.; Hart, H.E.; Houweling, S.T.; IJzerman, R.G.; Janssen, P.G.H.; Kerssen, A.; et al. NHG-Standaard Diabetes Mellitus Type 2; Dutch College of General Practitioners (NHG): Utrecht, The Netherlands, 2018. [Google Scholar]
- Khare, J.; Jindal, S. Observational study on effect of lock down due to COVID 19 on HBA1c levels in patients with diabetes: Experience from Central India. Prim. Care Diabetes 2022, 16, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Heins, M.; Hek, K.; Hooiveld, M.; Hendriksen, J.; Korevaar, J. Impact Coronapandemie op Zorgvraag bij Huisartsen (Factsheet A). 2020. Available online: https://www.nivel.nl/sites/default/files/bestanden/1003787.pdf (accessed on 4 April 2023).
- Kuiper, J.G.; Bakker, M.; Penning-van Beest, F.J.A.; Herings, R.M.C. Existing Data Sources for Clinical Epidemiology: The PHARMO Database Network. Clin. Epidemiol. 2020, 12, 415–422. [Google Scholar] [CrossRef] [PubMed]
- RIVM. Tijdlijn van Coronamaatregelen. 2020. Available online: https://www.rivm.nl/gedragsonderzoek/tijdlijn-van-coronamaatregelen-2020 (accessed on 7 April 2023).
- Amsah, N.; Md Isa, Z.; Ahmad, N.; Abdul Manaf, M.R. Impact of COVID-19 Pandemic on Healthcare Utilization among Patients with Type 2 Diabetes Mellitus: A Systematic Review. Int. J. Env. Res. Public Health 2023, 20, 4577. [Google Scholar] [CrossRef] [PubMed]
- Van Grondelle, S.E.; Van Bruggen, S.; Rauh, S.P.; Van der Zwan, M.; Cebrian, A.; Seidu, S.; Rutten, G.; Vos, H.M.M.; Numans, M.E.; Vos, R.C. The impact of the COVID-19 pandemic on diabetes care: The perspective of healthcare providers across Europe. Prim. Care Diabetes 2023, 17, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.J.; Wright, A.K.; Leelarathna, L.; Thabit, H.; Milne, N.; Kanumilli, N.; Ashcroft, D.M.; Rutter, M.K. Impact of COVID-19 restrictions on diabetes health checks and prescribing for people with type 2 diabetes: A UK-wide cohort study involving 618 161 people in primary care. BMJ Qual. Saf. 2022, 31, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Krupp, G.R.; Lo, J.Y. The COVID-19 Pandemic and Changes in Health Care Utilization among Patients with Type 2 Diabetes. Diabetes Care 2022, 45, e74–e76. [Google Scholar] [CrossRef] [PubMed]
- Eberle, C.; Stichling, S. Impact of COVID-19 lockdown on glycemic control in patients with type 1 and type 2 diabetes mellitus: A systematic review. Diabetol. Metab. Syndr. 2021, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, K.; Sawada, M.; Aihara, M.; Doi, S.; Sekine, R.; Usami, S.; Ohe, K.; Kubota, N.; Yamauchi, T. Impact of the COVID-19 pandemic on the glycemic control in people with diabetes mellitus: A retrospective cohort study. J. Diabetes Investig. 2023, 14, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Jacob, L.; Rickwood, S.; Rathmann, W.; Kostev, K. Change in glucose-lowering medication regimens in individuals with type 2 diabetes mellitus during the COVID-19 pandemic in Germany. Diabetes Obes. Metab. 2021, 23, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Arora, B.; Gupta, R.; Anoop, S.; Misra, A. Effects of nationwide lockdown during COVID-19 epidemic on lifestyle and other medical issues of patients with type 2 diabetes in north India. Diabetes Metab. Syndr. 2020, 14, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roso, M.B.; Knott-Torcal, C.; Matilla-Escalante, D.C.; Garcimartin, A.; Sampedro-Nunez, M.A.; Davalos, A.; Marazuela, M. COVID-19 Lockdown and Changes of the Dietary Pattern and Physical Activity Habits in a Cohort of Patients with Type 2 Diabetes Mellitus. Nutrients 2020, 12, 2327. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Total Population | Age < 70 | Age ≥ 70 | HbA1c on Target | HbA1c off Target | Non-High-Risk T2D | High-Risk T2D |
|---|---|---|---|---|---|---|---|
| N = 182,048 | N = 86,443 | N = 95,605 | N = 106,495 | N = 54,654 | N = 158,750 | N = 23,298 | |
| Age, mean (SD) | 69.0 (13.1) | 58.2 (9.6) | 78.8 (6.4) | 70.9 (11.8) | 67.7 (12.4) | 67.5 (12.8) | 78.9 (9.9) |
| Female, n (%) | 85,204 (46.8) | 37,741 (43.7) | 47,463 (49.6) | 51,592 (48.4) | 24,246 (44.4) | 73,945 (46.6) | 11,259 (48.3) |
| T2D duration, n (%) | |||||||
| <2 years | 16,674 (9.2) | 11,265 (13.0) | 5409 (5.7) | 10,784 (10.1) | 4298 (7.8) | 15,705 (9.9) | 969 (4.2) |
| 2–4 years | 28,347 (15.6) | 18,190 (21.1) | 10,157 (10.6) | 16,787 (15.7) | 7143 (13.1) | 26,290 (16.6) | 2057 (8.8) |
| 5–9 years | 52,310 (28.7) | 27,137 (31.4) | 25,173 (26.3) | 30,732 (28.9) | 16,375 (30.0) | 46,998 (29.6) | 5312 (22.8) |
| 10+ years | 84,717 (46.5) | 29,851 (34.5) | 54,866 (57.4) | 48,192 (45.3) | 26,837 (49.1) | 69,757 (43.9) | 14,960 (64.2) |
| SES, n (%) | |||||||
| Low | 64,102 (35.2) | 24,742 (28.6) | 26,273 (27.5) | 36,215 (34.0) | 20,504 (37.5) | 55,206 (34.8) | 8896 (38.2) |
| Middle | 65,136 (35.8) | 31,126 (36.0) | 32,976 (34.5) | 38,706 (36.4) | 18,918 (34.6) | 56,794 (35.8) | 8342 (35.8) |
| High | 51,015 (28.0) | 29,622 (34.3) | 35,514 (37.1) | 30,607 (28.7) | 14,634 (26.8) | 45,163 (28.4) | 5852 (25.1) |
| Unknown | 1795 (1.0) | 953 (1.1) | 842 (0.9) | 967 (0.9) | 597 (1.1) | 1587 (1.0) | 208 (0.9) |
| Smoking, n (%) | |||||||
| Current | 23,812 (13.1) | 15,096 (17.5) | 8716 (9.1) | 13,691 (12.8) | 8619 (15.8) | 21,173 (13.4) | 2639 (11.3) |
| Former | 55,181 (30.3) | 22,704 (26.3) | 32,477 (34.0) | 35,553 (33.4) | 17,288 (31.6) | 47,497 (29.9) | 7684 (33.0) |
| Never | 75,013 (41.2) | 32,339 (37.4) | 42,674 (44.6) | 46,494 (43.7) | 25,116 (46.0) | 65,229 (41.1) | 9714 (41.7) |
| Unknown | 28,042 (15.4) | 16,304 (18.8) | 11,738 (12.3) | 10,757 (10.1) | 3640 (6.6) | 24,781 (15.6) | 3261 (14.0) |
| Clinical parameters, mean (SD) | |||||||
| HbA1c (mmol/mol) | 54.6 (13.8) | 55.7 (15.6) | 53.7 (12.0) | 47.4 (6.3) | 67.3 (13.4) | 54.4 (13.8) | 56.4 (14.0) |
| BMI (kg/m2) | 29.8 (5.4) | 30.0 (5.7) | 28.9 (5.0) | 29.5 (5.3) | 30.3 (5.5) | 29.8 (5.4) | 29.4 (5.4) |
| LDL (mmol/L) | 2.44 (0.92) | 2.55 (0.95) | 2.36 (0.90) | 2.45 (0.91) | 2.42 (0.93) | 2.47 (0.92) | 2.31 (0.93) |
| SBP (mmHg) | 137.4 (17.0) | 135.1 (16.4) | 139.5 (17.2) | 137.4 (16.9) | 137.5 (16.8) | 137.1 (16.6) | 139.9 (19.2) |
| eGFR (mL/min/1.73 m2) | 76.0 (21.0) | 87.7 (17.5) | 66.0 (18.3) | 74.1 (20.1) | 79.4 (21.2) | 81.0 (16.7) | 44.2 (16.8) |
| 2019 (N = 182,048) | 2020 (N = 168,097) | ||
|---|---|---|---|
| GP visit counts, % | |||
| Office visits | 0 | 20.9% | 19.9% |
| 1 | 7.2% | 8.2% | |
| >1 | 71.9% | 71.9% | |
| Home visits | 0 | 85.3% | 85.2% |
| 1 | 6.3% | 6.8% | |
| >1 | 8.4% | 8.0% | |
| Phone calls | 0 | 72.3% | 68.3% |
| 1 | 12.1% | 12.8% | |
| >1 | 15.6% | 18.9% | |
| E-mails | 0 | 98.0% | 98.4% |
| 1 | 1.6% | 1.2% | |
| >1 | 0.4% | 0.4% | |
| Clinical measurement counts, % | |||
| HbA1c | 0 | 27.3% | 32.4% |
| 1 | 23.6% | 25.3% | |
| >1 | 49.1% | 42.3% | |
| BMI | 0 | 28.7% | 38.4% |
| 1 | 18.9% | 24.9% | |
| >1 | 52.4% | 36.7% | |
| LDL | 0 | 37.5% | 44.9% |
| 1 | 51.6% | 43.7% | |
| >1 | 10.9% | 11.4% | |
| SBP | 0 | 23.3% | 34.0% |
| 1 | 16.4% | 24.1% | |
| >1 | 60.3% | 41.9% | |
| eGFR | 0 | 30.4% | 36.7% |
| 1 | 47.2% | 42.6% | |
| >1 | 22.4% | 20.7% | |
| Clinical measurement values, mean (SD) | |||
| HbA1c (mmol/mol) | 54.2 (11.8) | 55.4 (12.3) | |
| BMI (kg/m2) | 29.6 (5.3) | 29.5 (5.2) | |
| LDL (mmol/L) | 2.45 (0.89) | 2.41 (0.88) | |
| SBP (mmHg) | 136.6 (14.5) | 137.9 (15.0) | |
| eGFR (mL/min/1.73m2) | 74.5 (20.7) | 74.1 (20.9) | |
| Total GP Visits | GP Office Visits | GP Home Visits | GP Phone Calls | |
|---|---|---|---|---|
| 2020 vs. 2019, rate ratios (95% CI) | ||||
| Univariate | 1.09 (1.08; 1.10) ** | 1.07 (1.06; 1.08) ** | 0.93 (0.91; 0.96) ** | 1.33 (1.31; 1.35) ** |
| Multivariate | 1.09 (1.08; 1.09) ** | 1.06 (1.06; 1.07) ** | 1.02 (0.99; 1.04) NS | 1.33 (1.31; 1.35) ** |
| Stratified models | ||||
| Age < 70 | 1.07 (1.06; 1.08) ** | 1.04 (1.03; 1.06) ** | N.A. | N.A. |
| Age ≥ 70 | 1.10 (1.09; 1.11) ** | 1.08 (1.07; 1.09) ** | N.A. | N.A. |
| HbA1c on target | N.A. | N.A. | N.A. | 1.36 (1.33; 1.39) ** |
| HbA1c not on target | N.A. | N.A. | N.A. | 1.28 (1.24; 1.31) ** |
| Non-high-risk T2D | N.A. | N.A. | 1.06 (1.05; 1.07) ** | N.A. |
| High-risk T2D | N.A. | N.A. | 0.94 (0.90; 0.99) * | N.A. |
| HbA1c (mmol/mol) | BMI (kg/m2) | LDL (mmol/L) | SBP (mmHg) | |
|---|---|---|---|---|
| 2020 vs. 2019, estimate (95% CI) | ||||
| Univariate | 1.66 (1.60; 1.72) ** | −0.07 (−0.08; −0.06) ** | −0.03 (−0.03; −0.02) ** | 1.25 (1.16; 1.34) ** |
| Multivariate | 1.65 (1.59; 1.70) ** | −0.07 (−0.08; −0.06) ** | −0.03 (−0.03; −0.03) ** | 1.27 (1.18; 1.36) ** |
| Stratified models | ||||
| Age < 70 | 1.84 (1.75; 1.93) ** | −0.01 (−0.03; −0.00) * | −0.03 (−0.04; −0.02) ** | 1.58 (1.46; 1.71) ** |
| Age ≥ 70 | 1.48 (1.42; 1.55) ** | −0.12 (−0.13; −0.11) ** | −0.03 (−0.04; −0.02) ** | 1.01 (0.88; 1.13) ** |
| HbA1c on target | 2.30 (2.24; 2.36) ** | −0.04 (−0.05; −0.03) ** | N.A. | 1.37 (1.26; 1.48) ** |
| HbA1c not on target | 0.37 (0.27; 0.49) ** | −0.14 (−0.15; −0.12) ** | N.A. | 1.06 (0.91; 1.21) ** |
| Non-high-risk T2D | 1.68 (1.62; 1.74) ** | −0.06 (−0.07; −0.05) ** | −0.03 (−0.03; −0.02) ** | 1.37 (1.28; 1.47) ** |
| High-risk T2D | 1.38 (1.21; 1.55) ** | −0.15 (−0.18; −0.12) ** | −0.04 (−0.06; −0.03) ** | 0.48 (0.19; 0.77) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Berg, J.M.; Blom, M.T.; Swart, K.M.A.; Overbeek, J.A.; Remmelzwaal, S.; Elders, P.J.M.; Herings, R.M.C. The Impact of the COVID-19 Pandemic in The Netherlands on Primary Healthcare Use and Clinical Outcomes in Persons with Type 2 Diabetes. COVID 2023, 3, 1677-1687. https://doi.org/10.3390/covid3110115
van den Berg JM, Blom MT, Swart KMA, Overbeek JA, Remmelzwaal S, Elders PJM, Herings RMC. The Impact of the COVID-19 Pandemic in The Netherlands on Primary Healthcare Use and Clinical Outcomes in Persons with Type 2 Diabetes. COVID. 2023; 3(11):1677-1687. https://doi.org/10.3390/covid3110115
Chicago/Turabian Stylevan den Berg, Jesse M., Marieke T. Blom, Karin M. A. Swart, Jetty A. Overbeek, S. Remmelzwaal, Petra J. M. Elders, and Ron M. C. Herings. 2023. "The Impact of the COVID-19 Pandemic in The Netherlands on Primary Healthcare Use and Clinical Outcomes in Persons with Type 2 Diabetes" COVID 3, no. 11: 1677-1687. https://doi.org/10.3390/covid3110115
APA Stylevan den Berg, J. M., Blom, M. T., Swart, K. M. A., Overbeek, J. A., Remmelzwaal, S., Elders, P. J. M., & Herings, R. M. C. (2023). The Impact of the COVID-19 Pandemic in The Netherlands on Primary Healthcare Use and Clinical Outcomes in Persons with Type 2 Diabetes. COVID, 3(11), 1677-1687. https://doi.org/10.3390/covid3110115






