Prevalence of Post COVID-19 Condition among Healthcare Workers: Self-Reported Online Survey in Four African Countries, December 2021–January 2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Clearance
2.2. Study Participants and Survey Methodology
2.3. Study Variables
2.4. Questionnaire Design
2.5. Data Analysis
3. Results
3.1. Healthcare Worker Demographics
3.2. COVID-19 Infection among Healthcare Workers
3.3. Work Performance
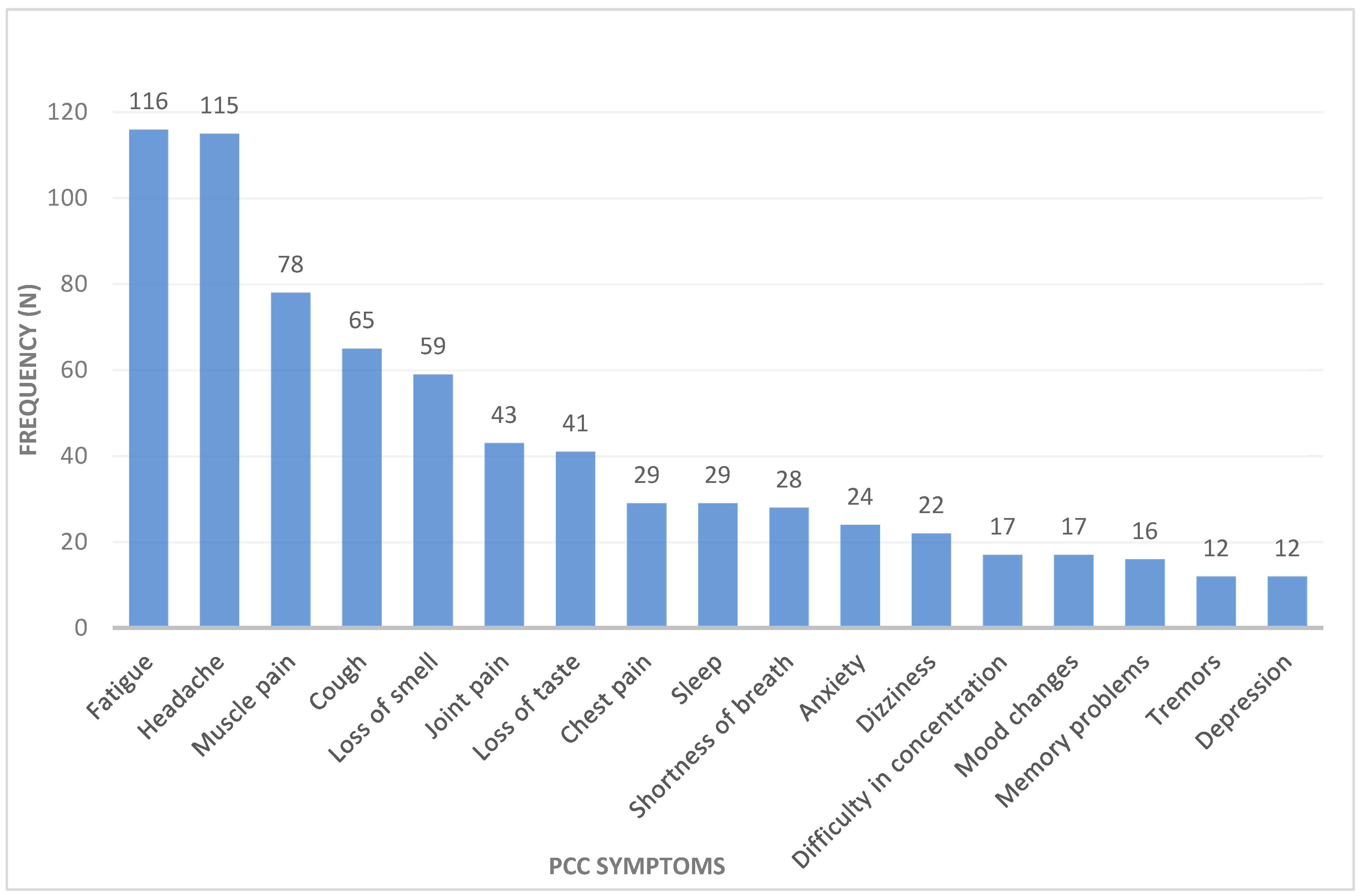
3.4. Post-COVID Condition among African Healthcare Workers
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022, 322, C1–C11. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 8 March 2022).
- Eroglu, B.; Nuwarda, R.F.; Ramzan, I.; Kayser, V. A Narrative Review of COVID-19 Vaccines. Vaccines 2021, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus Disease (COVID-19) Pandemic, World Health Organization. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 15 June 2023).
- CDC. National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 22 February 2022).
- WHO. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021 (accessed on 8 December 2021).
- Pavli, A.; Theodoridou, M.; Maltezou, H.C. Post-COVID Syndrome: Incidence, Clinical Spectrum, and Challenges for Primary Healthcare Professionals. Arch. Med. Res. 2021, 52, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Lechner-Scott, J.; Levy, M.; Hawkes, C.; Yeh, A.; Giovannoni, G. Long COVID or post COVID-19 syndrome. Mult. Scler. Relat. Disord. 2021, 55, 103268. [Google Scholar] [CrossRef] [PubMed]
- Iheanacho, T.; Stefanovics, E.; Okoro, U.G.; Anyaehie, U.E.; Njoku, P.O.; Adimekwe, A.I. Assessing knowledge, attitude, practice and training related to COVID-19: A cross-sectional survey of frontline healthcare workers in Nigeria. BMJ Open 2021, 11, e050138. [Google Scholar] [CrossRef]
- Silva Andrade, B.; Siqueira, S.; de Assis Soares, W.R.; de Souza Rangel, F.; Santos, N.O.; dos Santos Freitas, A. Long-COVID and post-COVID health complications: An up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: A meta-analysis and systematic review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Das, K.M.; Lee, E.Y.; Singh, R.; Enani, M.A.; Al Dossari, K.; Van Gorkom, K.; Larsson, S.G.; Langer, R.D. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian. J. Radiol. Imaging 2017, 27, 342–349. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Liu, H.; Han, N.; Ju, J.; Kou, Y.; Chen, L.; Jiang, M.; Pan, F.; Zheng, Y.; et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: A 15-year follow-up from a prospective cohort study. Bone Res. 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Galanis, P.; Vraka, I.; Fragkou, D.; Bilali, A.; Kaitelidou, D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: A systematic review and meta-analysis. J. Hosp. Infect. 2021, 108, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ochoa, S.A.; Franco, O.H.; Rojas, L.Z.; Raguindin, P.F.; Roa-Díaz, Z.M.; Wyssmann, B.M. COVID-19 in Health-Care Workers: A Living Systematic Review and Meta-Analysis of Prevalence, Risk Factors, Clinical Characteristics, and Outcomes. Am. J. Epidemiol. 2021, 190, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Štěpánek, L.; Nakládalová, M.; Janošíková, M.; Štěpánek, L.; Kabrhelová, K.; Boriková, A. Predictors and characteristics of post-acute COVID-19 syndrome in healthcare workers. Infect. Diseases. 2023, 55, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.A.; Wood, R.R.; Hanefeld, J.; El-Bcheraoui, C. Seroprevalence and Risk Factors of COVID-19 in Healthcare Workers From Eleven African Countries: A Scoping Review and Appraisal of Existing Evidence. Health Policy Plan. 2022, 37, 505–513. [Google Scholar] [CrossRef]
- Sahu, A.K.; Amrithanand, V.T.; Mathew, R.; Aggarwal, P.; Nayer, J.; Bhoi, S. COVID-19 in health care workers—A systematic review and meta-analysis. Am. J. Emerg. Med. 2020, 38, 1727–1731. [Google Scholar] [CrossRef]
- Osikomaiya, B.; Erinoso, O.; Wright, K.O.; Odusola, A.O.; Thomas, B.; Adeyemi, O.; Bowale, A.; Adejumo, O.; Falana, A.; Abdus-Salam, I.; et al. “Long COVID”: Persistent COVID-19 symptoms in survivors managed in Lagos State, Nigeria. BMC Infect. Dis. 2021, 21, 304. [Google Scholar] [CrossRef]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-Term and Long-Term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Hyassat, D.; El-Khateeb, M.; Dahbour, A.; Shunnaq, S.; Naji, D.; Ata, E.B.; Abujbara, M.; Khawaja, N.; Batieha, A.; Ajlouni, K. Post-COVID-19 syndrome among healthcare workers in Jordan. East. Mediterr. Health J. 2023, 29, 247–253. [Google Scholar] [CrossRef]
- Menges, D.; Ballouz, T.; Anagnostopoulos, A.; Aschmann, H.E.; Domenghino, A.; Fehr, J.S.; Puhan, M.A. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PLoS ONE. 2021, 16, e0254523. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.G.; Klepac, P.; Liu, Y.; Prem, K.; Jit, M.; Eggo, R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020, 26, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, P.; Moral, I.; Puy, A.; Cordero, E.; Chantada, N.; Cuixart, L.; Brotons, C. Prevalence of Post COVID-19 Condition in Primary Care: A Cross Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 1836. [Google Scholar] [CrossRef] [PubMed]
- Iwu, C.J.; Iwu, C.D.; Wiysonge, C.S. The occurrence of long COVID: A rapid review. Pan Afr. Med. J. 2021, 38, 65. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021, 5, 947–953. [Google Scholar] [CrossRef]
- Anjorin, A.A.; Odetokun, I.A.; Abioye, A.I.; Elnadi, H.; Umoren, M.V.; Damaris, B.F. Will Africans take COVID-19 vaccination? PLoS ONE 2021, 16, e0260575. [Google Scholar] [CrossRef]
- Venkatesan, P. Do vaccines protect from long COVID? Lancet Respir. Med. 2022, 10, e30. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Spoorthy, M.S.; Pratapa, S.K.; Mahant, S. Mental health problems faced by healthcare workers due to the COVID-19 pandemic–A review. Asian J. Psychiatry. 2020, 51, 102119. [Google Scholar] [CrossRef]
- Tan, B.Y.; Chew, N.W.; Lee, G.K.; Jing, M.; Goh, Y.; Yeo, L.L.; Zhang, K.; Chin, H.K.; Ahmad, A.; Khan, F.A.; et al. Psychological impact of the COVID-19 pandemic on health care workers in Singapore. Ann. Intern. Med. 2020, 173, 317–320. [Google Scholar] [CrossRef]
- Temsah, M.H.; Al-Sohime, F.; Alamro, N.; Al-Eyadhy, A.; Al-Hasan, K.; Jamal, A.; Al-Maglouth, I.; Aljamaan, F.; Al Amri, M.; Barry, M.; et al. The psychological impact of COVID-19 pandemic on health care workers in a MERS-CoV endemic country. J. Infect. Public Health 2020, 13, 877–882. [Google Scholar] [CrossRef]
- Vizheh, M.; Qorbani, M.; Arzaghi, S.M.; Muhidin, S.; Javanmard, Z.; Esmaeili, M. The mental health of healthcare workers in the COVID-19 pandemic: A systematic review. J. Diabetes Metab. Disord. 2020, 19, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Sun, P.; Wang, M.; Song, T.; Wu, Y.; Luo, J.; Chen, L. The psychological impact of COVID-19 pandemic on health care workers: A systematic review and meta-analysis. Front. Psychol. 2021, 12, 2382. [Google Scholar]
- Chersich, M.F.; Gray, G.; Fairlie, L.; Eichbaum, Q.; Mayhew, S.; Allwood, B.; English, R.; Scorgie, F.; Luchters, S.; Simpson, G.; et al. COVID-19 in Africa: Care and protection for frontline healthcare workers. Glob. Health 2020, 16, 46. [Google Scholar] [CrossRef]
- Jalili, M.; Niroomand, M.; Hadavand, F.; Zeinali, K.; Fotouhi, A. Burnout among healthcare professionals during COVID-19 pandemic: A cross-sectional study. Int. Arch. Occup. Environ. Health 2021, 94, 1345–1352. [Google Scholar] [CrossRef]
- Torrente, M.; Sousa, P.A.; Sánchez-Ramos, A.; Pimentao, J.; Royuela, A.; Franco, F.; Collazo-Lorduy, A.; Menasalvas, E.; Provencio, M. To burn-out or not to burn-out: A cross-sectional study in healthcare professionals in Spain during COVID-19 pandemic. BMJ Open 2021, 11, e044945. [Google Scholar] [CrossRef]
- de Pablo, G.S.; Vaquerizo-Serrano, J.; Catalan, A.; Arango, C.; Moreno, C.; Ferre, F.; Shin, J.I.; Sullivan, S.; Brondino, N.; Solmi, M.; et al. Impact of coronavirus syndromes on physical and mental health of health care workers: Systematic review and meta-analysis. J. Affect. Disord. 2020, 275, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.M.; McCann-Pineo, M.; Bellehsen, M.; Singh, V.; Malhotra, P.; Rasul, R.; Corley, S.S.; Jan, S.; Parashar, N.; George, S.; et al. The Impact of Physicians’ COVID-19 Pandemic Occupational Experiences on Mental Health. J. Occup. Environ. Med. 2022, 64, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.W.S.; Lee, G.K.H.; Tan, B.Y.Q.; Jing, M.; Goh, Y.; Ngiam, N.J.H.; Yeo, L.L.L.; Ahmad, A.; Ahmed Khan, F.; Napolean Shanmugam, G.N.; et al. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav. Immun. 2020, 88, 559–565. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. Eclinicalmedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Twycross, A. Living with long COVID: Some reflections 14 months down the line. Evid. Based Nurs. 2021, 24, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Loewenson, R. COVID-19 in East and Southern Africa: Rebuilding Differently and Better Must Start Now. MEDICC Rev. 2020, 22, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, M.; Nel, J.; Blumberg, L.; Madhi, S.A.; Dryden, M.; Stevens, W.; Venter, F.W.D. Long-COVID: An evolving problem with an extensive impact. S. Afr. Med. J. 2020, 111, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Randremanana, R.; Lazoumar, R.H.; Tejiokem, M.C.; Manirakiza, A.; Bicaba, B.W.; Rajatonirina, S.; Battaglia, S.; Pons, G.; Richard, V. Institut Pasteur International Network’s efforts to guide control measures against the coronavirus disease 2019 (COVID-19) epidemic among healthcare workers in Africa. Int. J. Infect. Dis. 2021, 103, 525–526. [Google Scholar] [CrossRef]
- van Kessel, S.A.M.; Hartman, T.C.O.; Lucassen, P.L.B.J.; van Jaarsveld, C.H.M. Post-acute and long-COVID-19 symptoms in patients with mild diseases: A systematic review. Fam. Pract. 2022, 39, 159–167. [Google Scholar] [CrossRef]
- Hopman, J.; Allegranzi, B.; Mehtar, S. Managing COVID-19 in Low- and Middle-Income Countries. JAMA 2020, 323, 1549–1550. [Google Scholar] [CrossRef]
- Lam, M.H. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: Long-term follow-up. Arch. Intern. Med. 2009, 169, 2142–2147. [Google Scholar] [CrossRef]
- Ngai, J.C.; Ko, F.W.; Ng, S.S.; To, K.-W.; Tong, M.; Hui, D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology 2010, 15, 543–550. [Google Scholar] [CrossRef]
- Karacic, J.; Bursztajn, H.J.; Arvanitakis, M. Who Cares What the Doctor Feels: The Responsibility of Health Politics for Burnout in the Pandemic. InHealthcare 2021, 9, 1550. [Google Scholar] [CrossRef]
- Aiash, H.; Khodor, M.; Shah, J.; Ghozy, S.; Sheble, A.; Hassan, A.; Abbadi, S.; Sabry, K.; Zeid, A.A.; Abdelbary, A. Integrated multidisciplinary post-COVID-19 care in Egypt [published correction appears in Lancet Glob Health. Lancet Glob. Health 2021, 9, e908–e909. [Google Scholar] [CrossRef] [PubMed]
| Variables | Number (%) |
|---|---|
| Nationality | |
| Cameroon | 104 (14.7) |
| Egypt | 281 (39.8) |
| Nigeria | 210 (29.8) |
| Somalia | 111 (15.7) |
| Occupation | |
| Dentist | 38 (5.4) |
| Nurse | 256 (36.3) |
| Physician | 143 (20.3) |
| Pharmacist | 42 (6.1) |
| Laboratory technician | 74 (6.7) |
| Veterinarian | 79 (11.2) |
| Others * | 74 (6.7) |
| Age | |
| 18–24 | 283 (40.1) |
| 25–34 | 252 (35.7) |
| 35–44 | 125 (17.7) |
| 45–54 | 36 (5.1) |
| >55 | 10 (1.4) |
| Gender | |
| Female | 394 (55.8) |
| Male | 301 (42.6) |
| Prefer not to say | 11 (1.6) |
| Do you have a known underlying disease? | |
| No | 646 (91.5) |
| Yes | 60 (8.5) |
| Variables | Number (%) |
|---|---|
| |
| No | 568 (80.5) |
| Yes | 138 (19.5) |
| |
| No | 509 (72) |
| Yes | 59 (8.4) |
| |
| <1 month ago | 38 (27.5) |
| 2–3 months | 13 (9.4) |
| 3–6 Months | 20 (14.5) |
| 6–12 months | 30 (21.7) |
| 12–18 months | 24 (17.4) |
| >18 months | 2 (2.2) |
| |
| No | 153 (77.7) |
| Yes | 44 (22.3) |
| |
| No | 237 (33.6) |
| Yes | 469 (66.4) |
| |
| No | 91 (19.4) |
| Yes | 378 (80.6) |
| Variable | Number (%) |
|---|---|
| |
| No | 592 (83.9) |
| Yes | 114 (16.1) |
| |
| No | 127 (64.5) |
| Yes | 70 (35.5) |
| |
| Mild/moderate | 181 (94.3) |
| Severe | 10 (5.2) |
| Very severe | 1 (0.5) |
| |
| No | 183 (93.1) |
| Yes | 14 (6.9) |
| |
| No | 125 (63.4) |
| Yes | 72 (36.6) |
| Variable | Number (%) |
|---|---|
| |
| Less stressful | 87 (44) |
| The same environmental stress level | 52 (26.3) |
| More stressful | 58 (29.7) |
| |
| No | 114 (58) |
| Yes | 83 (42) |
| |
| 1 | 76 (38.8) |
| 2 | 43 (22) |
| 3 | 47 (24) |
| 4 | 23 (11.7) |
| 5 | 7 (3.5) |
| |
| I get tired faster now | 97 (49.3) |
| I feel less motivated | 44 (22.3) |
| I feel more stressed | 49 (24.9) |
| I have less tolerability | 39 (19.8) |
| I get more forgetful | 32 (16.2) |
| |
| No | 63 (43.5) |
| Yes | 82 (56.5) |
| Outcome Variable | Variable | Baseline Category | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | ||||||
| Prevalence of PCC among HCWs | Age (years) | 18–24 | 25–34 | 3.50 (2.17, 5.65) | 0.027 | 0.47 (0.08, 2.71) | <0.001 |
| 35–44 | 2.27 (1.26, 4.09) | 0.86 (0.11, 6.52) | |||||
| 45–54 | 4.00 (1.78, 9.00) | 1.70 (1.01, 10.59) | |||||
| >55 | 6.1 (1.61, 22.81) | 0.34 (0.01, 6.01) | |||||
| Admission due to COVID-19 | No | Yes | 1.14 (0.53, 2.47) | 0.738 | - | - | |
| Gender | Male | Female | 0.89 (0.42, 1.86) | 0.322 | - | - | |
| Nationality | Cameroon | Egypt | 10.14 (3.00, 34.27) | <0.01 | 14.57 (2.62, 26.76) | 0.002 | |
| Nigeria | 3.28 (0.92,11.78) | 3.18 (0.51, 19.78) | |||||
| Somalia | 0.78 (0.47, 1.31) | 0.82 (0.47, 1.48) | |||||
| Occupation | Physician | Nurse | 2.47 (0.87,6.95) | 0.051 | - | - | |
| Pharmacist | 3.57 (0.94, 13.44) | ||||||
| Technician | 1.91 (0.50, 7.23) | ||||||
| Veterinarian | 0.35 (0.06, 1.83) | ||||||
| Others | 0.59 (0.14, 2.56) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elnadi, H.; Al-Mustapha, A.I.; Odetokun, I.A.; Anjorin, A.A.; Mosbah, R.; Fasina, F.O.; Razouqi, Y.; Awiagah, K.S.; Nyandwi, J.B.; Mhgoob, Z.E.; et al. Prevalence of Post COVID-19 Condition among Healthcare Workers: Self-Reported Online Survey in Four African Countries, December 2021–January 2022. COVID 2023, 3, 1663-1676. https://doi.org/10.3390/covid3110114
Elnadi H, Al-Mustapha AI, Odetokun IA, Anjorin AA, Mosbah R, Fasina FO, Razouqi Y, Awiagah KS, Nyandwi JB, Mhgoob ZE, et al. Prevalence of Post COVID-19 Condition among Healthcare Workers: Self-Reported Online Survey in Four African Countries, December 2021–January 2022. COVID. 2023; 3(11):1663-1676. https://doi.org/10.3390/covid3110114
Chicago/Turabian StyleElnadi, Hager, Ahmad I. Al-Mustapha, Ismail A. Odetokun, AbdulAzeez Adeyemi Anjorin, Rasha Mosbah, Folorunso O. Fasina, Youssef Razouqi, Kwame Sherrif Awiagah, Jean Baptiste Nyandwi, Zuhal E. Mhgoob, and et al. 2023. "Prevalence of Post COVID-19 Condition among Healthcare Workers: Self-Reported Online Survey in Four African Countries, December 2021–January 2022" COVID 3, no. 11: 1663-1676. https://doi.org/10.3390/covid3110114
APA StyleElnadi, H., Al-Mustapha, A. I., Odetokun, I. A., Anjorin, A. A., Mosbah, R., Fasina, F. O., Razouqi, Y., Awiagah, K. S., Nyandwi, J. B., Mhgoob, Z. E., Gachara, G., Yusuf Mohamud, M. F., Damaris, B. F., Maisara, A. M. O., & Radwan, M. (2023). Prevalence of Post COVID-19 Condition among Healthcare Workers: Self-Reported Online Survey in Four African Countries, December 2021–January 2022. COVID, 3(11), 1663-1676. https://doi.org/10.3390/covid3110114













