1. Introduction
The Omicron (B.1.1.529) variant was first reported in South Africa in November 2021 and showed a stronger immune escape capacity compared to the SARS-CoV-2 prototype and other variants [
1]. The major mutation sites were in the spike protein, with 15 mutation sites in the RBD domain, far exceeding those of previously identified mutant strains [
2]. A large number of mutation sites in the spike protein of the Omicron strain is strongly related to the speed of its transmissibility and immune escape ability [
3].
Vaccines are important tools to effectively fight against the epidemic. So far, many vaccines have been developed to fight against COVID-19, such as BNT162b2 (Pfizer BioNTech, Comirnaty
®) and ChAdOx1-S (Vaxzevria, AstraZeneca). However, due to the high mutation level of Omicron, the protective effect of the vaccine against the variant strain of Omicron was significantly reduced. The research showed that previous vaccination provided low protection against the Omicron virus, with vaccine effectiveness ranging from 17.0% to 29.5% [
4]. In response to the great threat of the Omicron variant of concern to the public, we have developed an inactivated candidate Omicron vaccine, which provides good protection against the Omicron virus [
5].
In addition to its vaccine effectiveness, another important focus of the vaccine is safety. Different evaluation methods of the toxicity of the vaccines were used in previous studies. For CoviVac (Chumakov), BALB/c mice and guinea pigs were used to test acute toxicity, chronic toxicity, and reproductive toxicity, and the results confirmed the safety [
6]. For BIV1-CovIran (Shifa Pharmed) inactivated vaccine, BALB/c mice, Guinea pigs, and Wistar Rats were used as animal models to evaluate the acute toxicity and repeated dose toxicity test [
7]. For the BBIBP-CorV (Sinopharm) inactivated vaccine, the acute toxicity was evaluated through Sprague-Dawley rats and the systemic allergic reactions of guinea pigs [
8].
A previous study evaluated the immunogenicity by injecting different doses of candidate Omicron vaccine into BALB/c mice and evaluated its safety by acute toxicity, systemic anaphylaxis, and muscle stimulation experiments [
5]. In this study, we further evaluate repeated-dose toxicity. During vaccine development, repeated-dose toxicity assessment in a single animal species is an important means of determining the nonclinical safety of a vaccine [
9]. Sprague-Dawley (SD) rats are proven to be effective animal models for repeat-dose toxicity studies [
10] and have been applied to the preclinical study of COVID-19 vaccines [
11]. Here, we evaluate the repeat-dose toxicity of an inactivated candidate Omicron vaccine (Vero cell) by repeating the intramuscular injection of Sprague-Dawley rats for 6 weeks and then allowing them to recover for 4 weeks. In the test, the preclinical safety of this vaccine was evaluated by analyzing the physiological condition of the rats, including ophthalmology, urine, body weight, food intake, body temperature, clinical pathology such as blood biochemistry, urine, neutralizing antibody, inflammation at the injection site, and organs weight.
2. Results
2.1. Clinical Observations
All the rats survived until the end of the experiment, and all were euthanized according to the protocol. No abnormal symptoms, such as erythema, hyperemia, swelling, ulcer, or induration, were observed at the injection sites in each group. No other relevant clinical signs were noted.
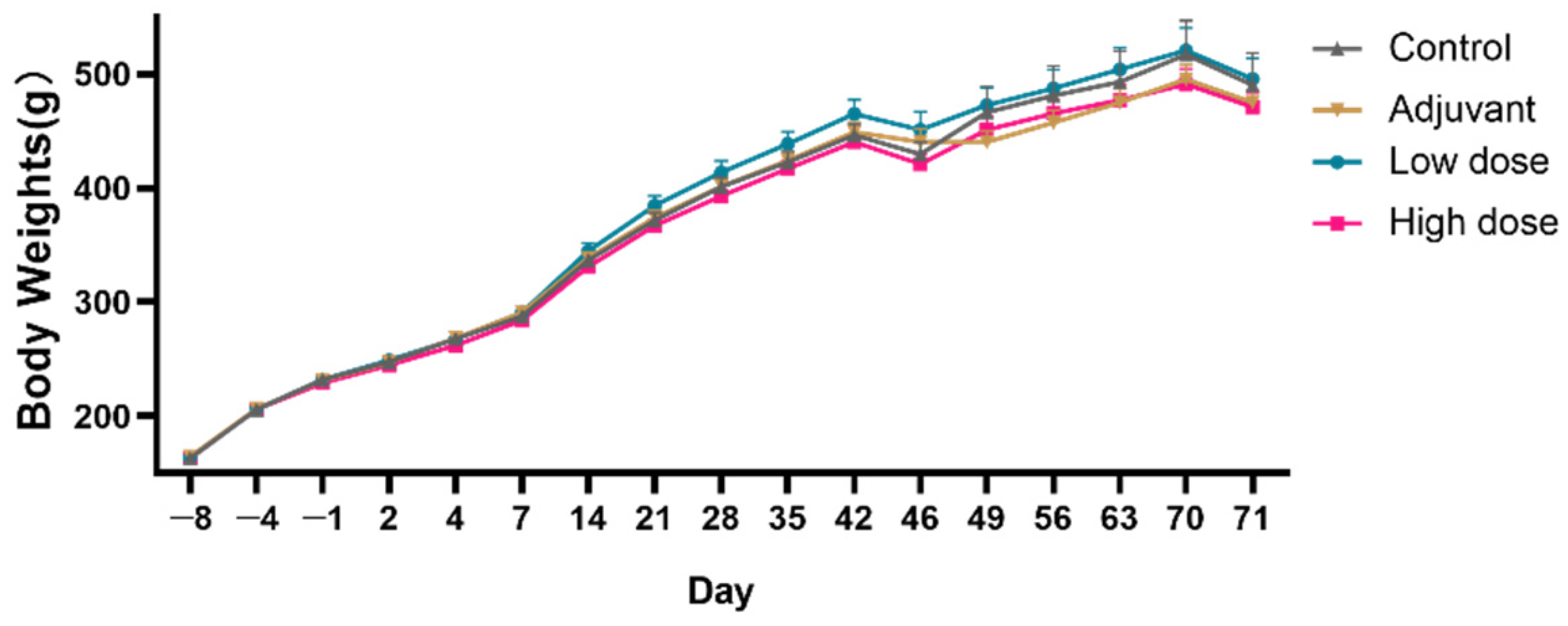
2.2. Body Weights
During the experiment, the body weights of the animals in each group showed a normal growth trend, and there was no obvious abnormal change related to vaccine administration, with no statistically significant differences between the four experimental groups (
Figure 1).
2.3. Food Intake
There was no obvious abnormal change in food intake related to the administration of the vaccines, and no statistical differences were observed between the four experimental groups (
Figure 2).
2.4. Body Temperature
There was no abnormal change in body temperature related to the vaccine administration in each group. The body temperature of the rats at day 1 in the adjuvant, low-dose, and high-dose groups were lower than in the control group, but this trend was also observed in pre-vaccination. Since a slight decrease in body temperature is generally not meaningful, it is not considered to have a toxicological significance (
Figure 3,
Table 1).
2.5. Ophthalmological Examination
No abnormal changes were found in the ophthalmological examination of the animals in each group, including the eyelids, conjunctiva, cornea, iris, pupil, lens, sclera, vitreous, and fundus.
2.6. Clinical Pathology
2.6.1. Complete Blood Count
Compared with the control group, the rats in the adjuvant group, low-dose, and high-dose groups showed that the number of neutrophils (Neut) and monocytes (Mono) cells (×10
9/L and/or %) increased, while the number of lymphocytes (Lymph) cells and Mean Corpuscular Hemoglobin cell (MCHC) cells (×10
9/L and %) was decreased at 3 days after the last dose (D46). The increase in the number of Neut and Mono cells may be associated with immune response and/or local irritation after administration of vaccine/adjuvant. The decrease in the MCHC of the rats in the high-dose group was not considered to be of toxicological significance because other major erythroid-related indicators (red blood cells (RBC), hemoglobin (HGB), hematocrit (HCT)) were not abnormally changed. However, the above indicators showed a complete recovery trend at the end of the 4-week recovery period (D71) (
Table 2). No differences were observed in the blood cells (
Table S1).
2.6.2. Coagulation
Compared with the animals in the control group, the fibrinogen (FIB) was increased in the adjuvant group, low-dose, and high-dose groups on D2, D4, D44, and D46. FIB may be an indicator related to the immune response and/or local irritation caused by the administration of the vaccine/adjuvant, and significant recovery was observed at the end of the 4-week recovery period (
Table 3 and
Table S2). The FIB in D71 in the low-dose group was slightly increased, but it showed no dose-dependent adverse toxicological changes.
2.6.3. Biochemical Evaluation
During the experiment, no abnormal changes related to vaccine administration were found in the blood biochemical indexes. However, compared with the control group, the level of aspartate aminotransferase (AST) was increased in the high-dose group 3 days after the last administration (D46), and the alanine aminotransferase (ALT) was upregulated in the low-dose group at the end of the 4-week recovery period (D71). Since the AST showed a complete recovery trend at the end of the 4-week recovery period (D71), no dose-dependent adverse toxicological changes were observed in ALT, and a recovery trend was obvious, so it is not considered to have toxicological significance (
Table S3).
2.6.4. Urine
Compared with the rats in the control group in the same period, 2 days after the last dose (D45) and 3 days before the end of the recovery period (D68), no abnormal changes related to the vaccine administration were found in the examination of the urine of the animals in each dose group (
Table S4).
2.7. Anatomopathological Analysis
To investigate the toxicity of the omicron COVID-19 vaccine candidate, we carried out gross and histopathological examinations after euthanasia. There was no significant variation in the organs weights except for the testis, which demonstrated lower weights 3 days after the last dose (D46) in the high-dose group (0.6694%) compared with the control group (0.7553%) (
Table 4), although these weights recovered after 4 weeks (D71) (control: 0.6959% vs. high dose group: 0.7027%).
There were no modifications to organs by microscopy at the injection site only, in which we observed slight mononuclear infiltration and interstitial granulomatous inflammation. The first euthanasia group, the control group, and the Omicron COVID-19 vaccine candidate (low-dose/high-dose) group showed local interstitial granulomatous inflammation; the incidence of lesions was 20/20, and the degree of lesions was mild to moderate, mild to moderate, and slight to moderate in each group, respectively. The severity and incidence of acute inflammation in the adjuvant, low-dose, and high-dose groups were mild (3/20), slight to moderate (9/20), and slight to moderate (5/20), respectively. The lesion severity and incidence of bleeding were slight to mild (3/20), slight (3/20), and slight to moderate degree (4/20), respectively.
Interstitial granulomatous inflammation, acute inflammation, and hemorrhage were observed in the above adjuvant control group and test product group. Among them, the degree and incidence of local granulomatous inflammation and hemorrhage in the test-product group were not significantly different from those in the adjuvant control group, and there was no obvious dose dependence; therefore, the injection of local granulomatous inflammation, acute inflammation, and bleeding were all related to the adjuvant and not to the vaccines.
After the 4-week recovery period (D71), interstitial granulomatous inflammation at the injection site was still observed in the adjuvant control group and the test-product group, and the degree and incidence of lesions were slight to mild (10/10). At the end of the recovery period, the acute inflammation and hemorrhage at the injection site in each group were completely recovered; although the granulomatous inflammation did not completely recover, the recovery trend is obvious (
Table 5,
Figure 4).
2.8. Immunogenicity Analysis
In order to test the immunogenicity of the Omicron inactivated vaccine, the Omicron S1- and RBD-antigen-specific IgG antibody and neutralizing antibody to the Omicron virus were tested by ELISA and cytopathic effect (CPE). The S1 and RBD-antigen-specific IgG antibody and neutralizing antibody were up-regulated in the serum from the rats injected with the vaccine on day 14, D28, D42, D56, and D70 (
Figure 5A,C,E). The S1-antigen-specific IgG antibodies in the high dose of the vaccine were higher than the low doses on day D42, D56, and D70, but the RBD-antigen-specific IgG antibody and the neutralizing antibody titer showed no differences in those two groups. Furthermore, we also tested the RBD-antigen-specific IgG antibody of the HB02 inactivated vaccine as a control (
Figure 5B,D,F,G). These results indicated that the Omicron-inactivated vaccine induced high levels of S1- and RBD-antigen-specific IgG antibodies and neutralizing antibodies against the Omicron in the rats.
3. Discussion
During the COVID-19 pandemic, vaccines developed against SARS-CoV-2 have greatly contributed to reducing infection and mortality rates. However, the continuous emergence of new genetic variants of SARS-CoV-2 challenges the clinical effectiveness of current vaccines. The large number of mutations found in the Omicron variant enables it to have a higher infection rate and immune-evasion ability [
12,
13,
14]. The development of an effective vaccine against Omicron remains the most effective intervention to limit the COVID-19 pandemic.
We have developed a candidate Omicron-inactivated vaccine against SARS-CoV-2 Omicron with a promising effect of efficacy [
5]. This study evaluated the repeated-dose toxicity of SD rats after repeated intramuscular administration of the SARS-CoV-2 Omicron-inactivated vaccine (Vero cell) at the local and systemic levels [
11,
15].
The efficacy and immunotoxicity of the vaccine can be assessed by analyzing changes in clinical, physiological, and pathological parameters over short and long periods of time after repeated administration. The possible toxicity of a product can be classified into local and systemic. Depending on the different occurrence times, these can be divided into acute toxicity and delayed toxicity, which is manifested as transient local inflammation, acute phase response, local delayed-type hypersensitivity, and rarely, autoimmune reaction [
16]. After repeated administration, the animals in each group showed no significant signs of toxicity when evaluated for clinical conditions. Common clinical signs of toxicity, such as erythema, congestion, swelling at the injection sites, changes in body weight, food and water consumption, and ocular lesions, were not present in the study [
17]. The animals did not develop a fever during the evaluation period, and the slight decrease in body temperature after initial administration was non-dose-dependent and within the normal physiological range.
Delayed reactions after administration can be observed at the injection sites or systemically. An inflammatory reaction at the injection site causes local-tissue lesions, such as foreign-body granulomas, which consist of the foreign object and its surrounding distribution of macrophages, multi-nucleated giant cells, lymphocytes, and varying numbers of plasma cells and eosinophils [
16,
18]. After the repeated-dose administration of the vaccines, some local inflammatory lesions were observed by histopathological sections at the injection sites. Three days after the last dose (D46), interstitial granulomatous inflammation, acute inflammation, and hemorrhage were observed in the control group and the Omicron COVID-19 vaccine candidate (low-dose/high-dose) group. Studies have shown that the aluminum adjuvant can also lead to local inflammatory reactions, such as granulomatosis [
19,
20]. Gender difference is not observed. The toxicity of all animals showed no dose-dependent behavior, and at the end of the 4-week recovery period (D71), the above changes showed a clear recovery trend. Therefore, it is believed that the abnormal changes in the injection site in this trial may be related to the aluminum adjuvant contained in it and are not to be considered a security issue.
The acute-phase response is a transient syndrome that summarizes different endocrine, metabolic, and neurological changes caused by the inflammatory response [
21]. The possible systemic toxicity of repeated administration was investigated according to the clinicopathological indicators. Hematological and blood chemistry parameters can be used to assess changes in rat immunotoxicology and animal health over time [
22]. After the repeated-dose administration of vaccines, blood-cell counts (except for Neut, Lymph, Mono, and MCHC), the coagulation function (except for FIB), blood biochemistry, and urine examination indicators showed no regular changes compared with the control group. In particular, the increase in the number of Neut, Mono, and FIB may be associated with the immune response and/or local irritation after the administration of vaccine/adjuvant, as well as with the fluctuation in the remaining indicators. These indicators showed a complete/significant recovery trend at the end of the 4-week recovery period (D71). In addition, we carried out gross examinations after euthanasia. There was no significant variation in the organs weights except for the testis, which demonstrated a lower weight 3 days after the last dose (D46) in the high-dose group, but recovered after 4 weeks (D71).
Previous studies on candidate inactivated vaccines for 2019-nCoV had evaluated their respective humoral immunogenicities in different animal models, which can induce strong anti-SARS-CoV-2 antibodies in SD rats [
8,
10]. We found that after immunization with the SARS-CoV-2 Omicron-inactivated vaccine (Vero cell), the SD rats could also induce high levels of specific IgG antibodies and neutralizing antibodies against the S1-antigen-specific antibody and Omicron virus, respectively. After the 4-week recovery period (D71), the immunization antibody titers and neutralizing antibody titers in the vaccinated group remained at high levels, providing protection against SARS-CoV-2 Omicron. This suggests that, in addition to being safe, the SARS-CoV-2 Omicron-inactivated vaccine (Vero cell) can induce a long-lasting immune response.
Based on the above indicators and previous reports on safety related to acute toxicity, systemic anaphylaxis, and muscle stimulation experiments [
5], it is believed that the SARS-CoV-2 Omicron-inactivated vaccine (Vero cell) offers good safety, providing an experimental basis for further clinical trials and a reference frame for related follow-up studies.
Limitations
This study featured three limitations, which could be improved in future studies. Firstly, more time is required to detect the recovery from lesions. In D71, a recovery trend was observed, but the animals did not completely recover from the granulomatous inflammation. Therefore, it is necessary to further monitor the lesions until complete recovery. Secondly, as indicated in a paper cited above [
16], clearer indications of vaccine toxicity need to be considered, such as the cellular-response factors induced by the vaccine. Thirdly, for the vaccine to be finally applied to humans, a primate model should be considered to confirm its safety in future studies.
4. Materials and Methods
4.1. Animal Ethics Statement
Animal feeding and management were carried out in accordance with the Standard Operating Procedures (SOP) of Beijing Zhaoyan New Drug Research Center Co., Ltd. and the 8th edition of the Guide for the Feeding and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; National Academy Press; Washington, DC; 2010) and the United States Department of Agriculture (USDA) Animal Welfare Regulations (Public Law 99–198). The institution has been accredited by the international AAALAC organization. All animal experiments were approved by the Institutional Animal Ethics Committee (IACUC) prior to manipulation (IACUC approval code: B-ACU22-0010).
4.2. Vaccine Preparation
Omicron strain HK-OM-P0 was isolated from a patient’s pharyngeal swab. The strain was used for vaccine development, as described previously [
5].
4.3. Animals
SD rats used in the experiment were purchased from Beijing Weitonglihua Experimental Animal Technology Co., Ltd. A total of 75 male and 75 female SD rats were housed in polycarbonate boxes, 5 animals per cage, and the environment was controlled as an SPF animal room with good ventilation (temperature 20 °C~26 °C; humidity 40~70%; the light was alternated between light and dark for approximately 12 h) except for some adjustments required for the experimental operation. Male rats were selected as representatives to check the physiological condition of the rats, including their ophthalmological condition, body weight, food intake, body temperature, blood biochemistry, urine, neutralizing antibody, inflammation at the injection site, and organs weight. Female rats were checked for histopathological changes at the sites of injection.
4.4. Experimental Design
In the present study, we selected 75 male and 75 female SD rats (6–7 weeks old) with qualified and similar body weights, randomly divided into seven groups by using the Provantis 9.4.3.0 system animal-management module. The main test groups (groups 1–4) and satellite groups (groups 5–7) contained 15 animals and 5 animals, respectively. Group 1 was the control group (receiving 0.9% physiological saline solution, 4B21021606, Qidu Pharma Co., Ltd.), group 2 was the adjuvant group (receiving adjuvant with PBS), and groups 3 and 4 were the low-dose and high-dose group, respectively (receiving Omicron COVID-19 Vaccine (Vero Cell)). Animals received 12 µg of the vaccine antigen in a volume of 0.5 mL per dose. The specific animal grouping and vaccination protocol are listed in
Table 6:
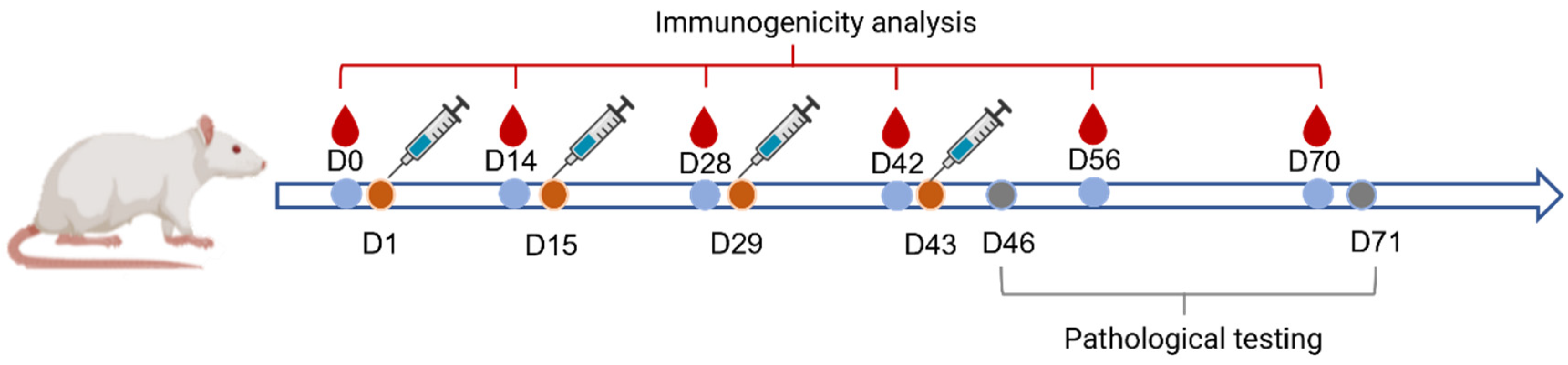
4.5. Frequency and Route of Administration
The vaccine was administered once every 2 weeks for 6 continuous weeks, i.e., at D1, D15, D29, and D43, respectively (
Figure 6). The injection sites were divided at multiple points in the gastrocnemius muscle of the animal’s bilateral hind limbs with a volume of 0.25 mL each. The first 10 rats and the remaining 5 rats in groups 1–4 were dissected at D46 and D71, respectively.
4.6. Clinical Observations
Male animals in groups 1–7 were subjected to general clinical observation during the quarantine adaptation period and during the trial period twice daily. Observations included mortality, mental status, behavioral activity, morbidity, respiration, secretions, feces, and diet and water intake. Animals in groups 1 to 4 were observed clinically in detail before the first dose (D-1) and then once a week after removal from the rearing cages for detailed clinical observation. Observations included mental status, behavioral activity, skin, hair, eyes, ears, nose, mouth, abdomen, external genitalia, anus, extremities, feet, and respiration. The animals in groups 1 to 4 were observed locally for erythema, congestion, swelling, ulceration, and sclerosis at the injection site once on the day before and after each dose administration and once a week for the rest of the remainder of the experiment.
4.7. Weight and Body Temperature
Male animals were weighed after receiving injections and before grouping (D-4). The animals in groups 1–4 were weighed at D-1, D2, D4, and D7, and for the remainder of the experiment, they were weighed once a week. Body temperature was measured in 5 animals in groups 1 to 4 before the first vaccine administration (D-1), 2–5 h after the first dose (D1), the day after the first dose (D2), 2–5 h after the last dose (D43), the day after the last dose (D44) and before the end of the recovery period (D70).
4.8. Ophthalmic Examinations
Five male rats in groups 1 to 4 underwent ophthalmologic examination at D44 and D68. The animals were observed for abnormalities of the eyelids, conjunctiva, sclera, cornea, iris, pupil, lens, vitreous, and fundus by visual and ophthalmoscopic examination, and pupil dilators were used before the examination.
4.9. Blood-Cell Count
Three days after the first dose (D4), approximately 0.5 mL blood was collected from the jugular veins of 5 male animals in groups 1 to 4; three days after the last dose (D46) and at the end of the recovery period (D71), approximately 0.5 mL blood was collected from the abdominal aorta after anesthesia from the same group. Blood samples were anticoagulated with ethylenediaminetetraacetic acid and later counted by a fully automated blood-cell analyzer.
4.10. Coagulation Function
Approximately 0.2 mL of blood was obtained from the jugular veins of 5 male animals in groups 1 to 4 at D2, D4, and D44; approximately 2 mL of blood was obtained from the abdominal aorta in the same group at D46 and D71. Blood samples were anticoagulated by mixing sodium citrate and plasma at a ratio of 1:9. The fibrinogen content (FIB, g/L) was measured at D2, D4, and D46 by an SYSMEX fully automated blood coagulation analyzer, and the prothrombin time (PT, s), activated partial thromboplastin time (APTT, s) and fibrinogen content (FIB, g/L) were measured at the remaining time points.
4.11. Blood Biochemistry
About 2.5 mL of blood was collected from the abdominal aorta at D46 and D71 in groups 1 to 4 (male rats). The serum was separated within 2 h after blood collection, and the blood biochemical indexes were measured using a TBA-120FR automatic biochemical analyzer.
4.12. Urine Analysis
Approximately 2 mL of fresh urine was collected from animals in groups 1 to 4 (male rats) at D45 and D68 using the metabolic-cage method for urine-index analysis. Urine samples were analyzed using the Cobas 6500 urinalysis analyzer.
4.13. Anatomopathological Examination
Animals in groups 1 to 4 were subjected to systematic gross anatomical observation, and tissues/organs were collected and weighed according to the protocol requirements. Routine histological processing, paraffin embedding, sectioning, mounting, HE staining, and microscopic observation were performed on all tissues/organs collected from animals in groups 1 and 4 and upon injection localization in animals from groups 2 and 3. The injuries were graded using standard terminology for diagnosis and classification, using a 5-level method (slight mild, moderate, severe, and serious).
4.14. Anti-S1 IgG and Anti-RBD ELISA
The anti-S1 IgG antibody was measured based on the ELISA method. Briefly, the S1 protein was precoated on a plate, followed by washing 3 times. Next, then the plate was sealed with DPBS-T solution (Joinn Laboratories (China) Co., Ltd.) containing 3% BSA (SHANGHAI BIO SCIENCE&TECHNOLOGY.CO., LTD.) and incubated at 37 °C for about 1 h. The plate was washed with a DPBS solution containing 0.05% Tween-20, and the serum samples were added, followed by incubation at 37 °C for 1 h. Next, Anti-Rat IgG-Peroxidase antibody (SIGMA) in goat and TMB (Surmodics) chromogenic solution was added to react for 10 min at room temperature before being stopped by termination solution (Joinn Laboratories (China) Co., Ltd.). The absorbance was measured at 450 nm by a microplate reader.
The anti-RBD IgG antibody was measured based on the ELISA method. The prototype and Omicron RBD protein (Sino biological, 40592-V08H88, 40592-V08H121) were precoated on a plate and washed 3 times using a washing buffer, PBS solution containing 0.05% Tween-20. Next, the plate was sealed with PBS solution containing 1% BSA (Sinopharm Chemical Reagent Co., Ltd.) and incubated at 37 °C for about 2 h. Next, the contents were poured and the serum samples diluted with a PBS solution containing 1% BSA and 0.05% Tween-20 were added, followed by incubation at 37 °C for 1.5 h. After washing 3 times using washing buffer, the Goat Anti-Rat IgG-Peroxidase antibody (SIGMA) and TMB (SeraCare Life Sciences, lnc.) chromogenic solution were added to react for 15 min at room temperature before stopped by termination solution. The absorbance was measured at 450 nm and 630 nm by a microplate reader.
4.15. Neutralization Assay
Blood was collected from the satellite group at D14, D28, D42, D56, and D70, and the serum was isolated. The neutralization assay was based on the micro-plate CPE method. Briefly, the serum to be tested was diluted by a certain ratio. Next, the omicron virus was added to initiate neutralization. The cell suspension was then added and incubated for another 4 days, followed by observing the CPE. The detailed protocol was as described previously [
8].
4.16. Statistical Analysis
Statistical analysis was performed using Provantis SAS 9.4. If the variance was not significant (p > 0.05), Levene’s test was performed; if the variance was significantly different (p ≤ 0.05), Dunnett’s multiple comparisons (parametric method) were performed.