Timing of Initiation of Methylprednisolone Pulse Therapy in Patients with COVID-19
Abstract
:1. Introduction
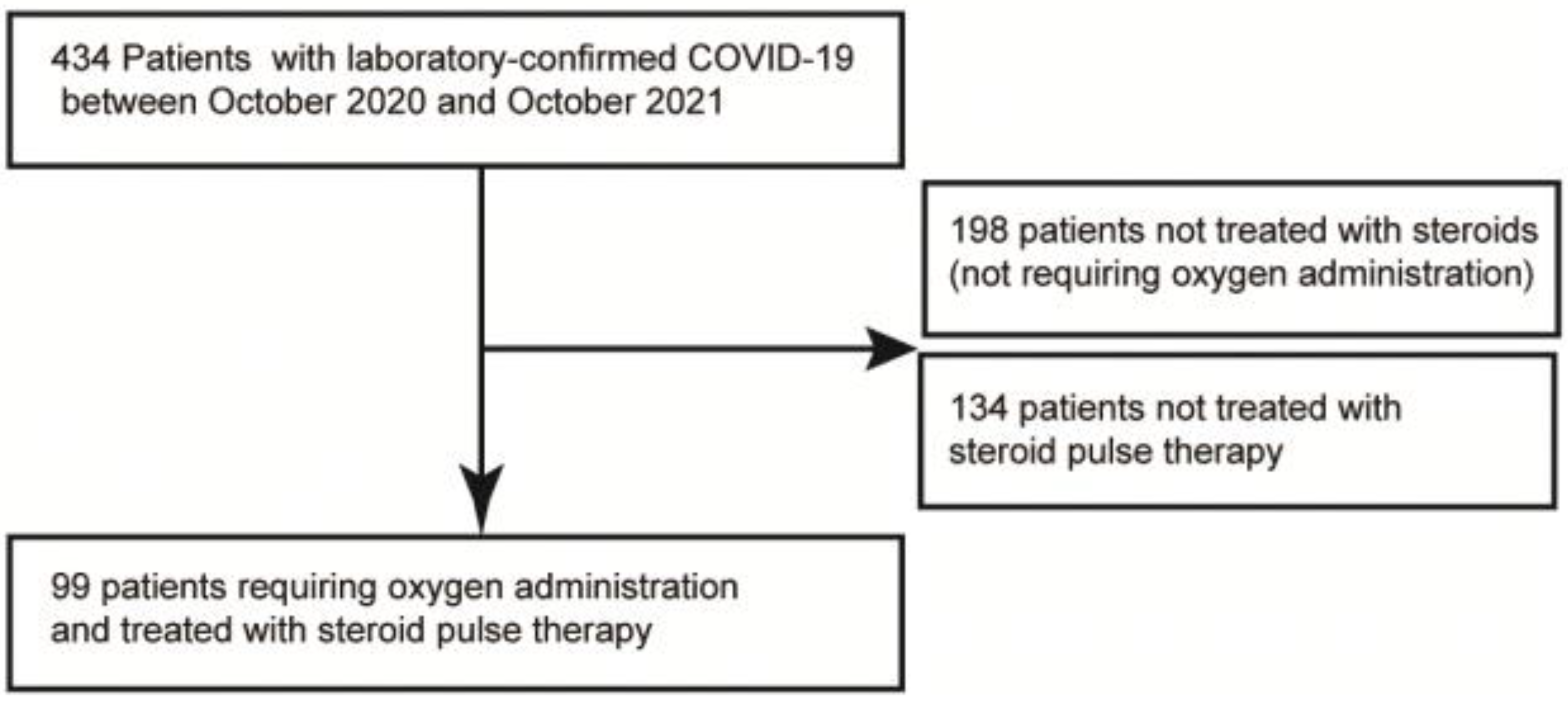
2. Materials and Methods
2.1. Design Overview
2.2. Definitions
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Primary Outcomes: Relationship between Disease Severity at the Initiation of MPT and Clinical Outcomes
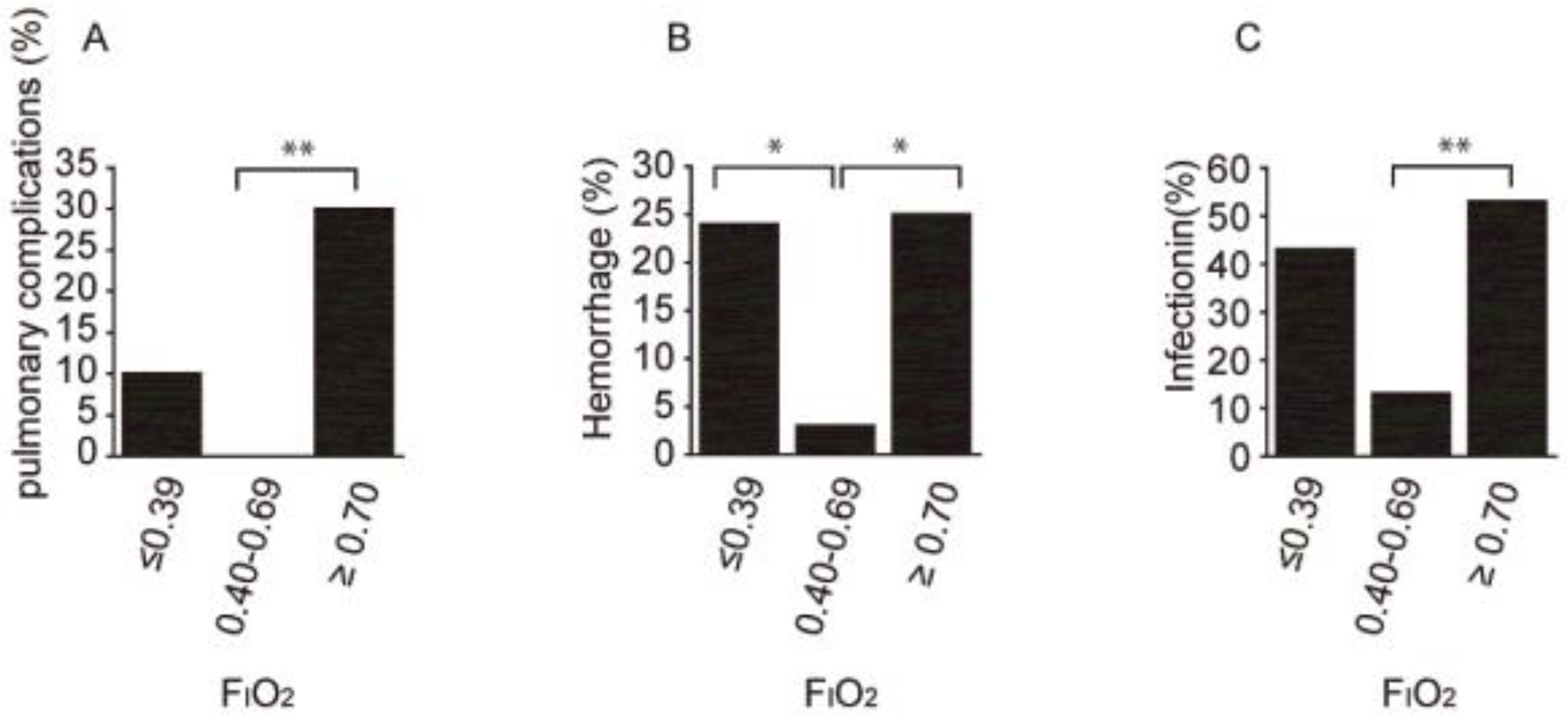
3.3. Secondary Outcomes: Relationship between Adverse Events and Disease Severity at the Initiation of MPT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COVID-19 | coronavirus disease 2019 |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| DIC | disseminated intravascular coagulation |
| ARDS | acute respiratory distress syndrome |
| RCT | randomized controlled trial |
| MPT | methylprednisolone pulse therapy |
| FIO2 | a fraction of inspired oxygen |
| IQR | interquartile range |
| HFNC | high-flow nasal cannula |
References
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, L.A.; Canna, S.W.; Schulert, G.S.; Volpi, S.; Lee, P.Y.; Kernan, K.F.; Caricchio, R.; Mahmud, S.; Hazen, M.M.; Halyabar, O.; et al. On the alert for cytokine storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020, 72, 1059–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, T.; Ayusawa, M.; Ujiie, M.; Omagari, T.; Oda, J.; Kato, Y.; Kamiya, H.; Kawana, A.; Kutsuna, S.; Kotani, T.; et al. Novel Coronavirus Infection COVID-19 Medical Practice Guidelines. Version 6.1. Available online: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000121431_00111.html (accessed on 10 January 2022).
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [PubMed]
- Agarwal, A.; Rochwerg, B.; Siemieniuk, R.A.; Agoritsas, T.; Lamontagne, F.; Lisa, A.; Bausch, F.J.; Calfee, C.S.; Cao, B.; Cecconi, M.; et al. A Living WHO guideline on drugs for covid-19. BMJ 2020, 370, m3379. [Google Scholar] [CrossRef] [PubMed]
- Dolci, G.; Cassone, G.; Venturelli, F.; Besutti, G.; Revelli, M.; Corsini, R.; Sampaolesi, F.; Pavone, P.; Contardi, G.; Riva, N.; et al. High-dose glucocorticoids pulse-therapy for beta-coronaviridae pneumonia: A systematic literature review and case-series of Coronavirus disease-2019. Clin. Exp. Rheumatol. 2021, 39, 1119–1125. [Google Scholar] [PubMed]
- Edalatifard, M.; Akhtari, M.; Salehi, M.; Naderi, Z.; Jamshidi, A.; Mostafaei, S.; Najafizadeh, S.R.; Farhadi, E.; Jalili, N.; Esfahani, M.; et al. Intravenous methylprednisolone pulse as a treatment for hospitalized severe COVID-19 patients: Results from a randomised controlled clinical trial. Eur. Respir. J. 2020, 56, 2002808. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Ichinose, M. Guidelines for Oxygen Therapy, 2nd ed.; Medical Review: Tokyo, Japan, 2017. [Google Scholar]
- Otsuka, Y.; Omagari, T.; Sakamoto, F.; Sato, T.; Shimada, T.; Shirabe, K. Guidelines for Pathogen Testing for COVID-19. Version 4.1. Available online: https://www.mhlw.go.jp/content/000841541.pdf (accessed on 10 January 2022).
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shionoya, Y.; Taniguchi, T.; Kasai, H.; Sakuma, N.; Imai, S.; Shikano, K.; Takayanagi, S.; Yahaba, M.; Nakada, T.-A.; Igari, H.; et al. Possibility of deterioration of respiratory status when steroids precede antiviral drugs in patients with COVID-19 pneumonia: A retrospective study. PLoS ONE 2021, 16, e0256977. [Google Scholar] [CrossRef]
- Cruz, A.F.; Ruiz-Antorán, B.; Múñez Rubio, E.; López, A.S.; Callejas Díaz, A.; Avendaño-Solá, C.; Martínez, A.R. The Right Time for Steroids in COVID-19. Clin. Infect. Dis. 2021, 72, 1486–1487. [Google Scholar] [CrossRef]
- Fadel, R.; Morrison, A.R.; Vahia, A.; Smith, Z.R.; Chaudhry, Z.; Bhargava, P.; Miller, J.; Kenney, R.M.; Alangaden, G.; Ramesh, M.S.; et al. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin. Infect. Dis. 2020, 71, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, X.; Li, T.; Chan, S.; Yu, Y.; Ai, J.W.; Zhang, H.; Sun, F.; Zhang, Q.; Zhu, L.; et al. Corticosteroid Prevents COVID-19 Progression within Its Therapeutic Window: A Multicentre, Proof-of-Concept, Observational Study. Emerg. Microbes Infect. 2020, 9, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Nath, A.; Aggarwal, A.N.; Gupta, D. Do glucocorticoids decrease mortality in acute respiratory distress syndrome? A meta-analysis. Respirology 2007, 12, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.V.; John, P.; Graham, P.L.; Moran, J.L.; George, I.A.; Bersten, A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: Meta-analysis. BMJ 2008, 336, 1006–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantini, F.; Goletti, D.; Petrone, L.; Fard, S.N.; Niccoli, L.; Foti, R. Immune Therapy, or Antiviral Therapy, or Both for COVID-19: A Systematic Review. Drugs 2020, 80, 1929–1946. [Google Scholar] [CrossRef] [PubMed]
- Batirel, A.; Demirhan, R.; Eser, N.; Körlü, E.; Tezcan, M.E. Pulse steroid treatment for hospitalized adults with COVID-19. Turk. J. Med. Sci. 2021, 51, 2248–2255. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, H.; Greenberg, D.; Hwang, F.; Lee, C.; Vernik, D.; Manglani, R.; Wang, Z.; Murad, M.H.; Chandy, D.; Epelbaum, O. Comparison of pulse-dose and high-dose corticosteroids with no corticosteroid treatment for COVID-19 pneumonia in the intensive care unit. J. Med. Virol. 2022, 94, 349–356. [Google Scholar] [CrossRef] [PubMed]

| Oxygen Devices | Oxygen (L) | FIO2 (%) |
|---|---|---|
| Nasal cannula | 1 | 24 |
| 2 | 28 | |
| 3 | 32 | |
| Face mask | 4 | 30 |
| 5 | 40 | |
| 6 | 50 | |
| Reservoir face mask | 7 | 70 |
| 8 | 80 | |
| 9 | 90 | |
| 10 | 99 |
| Baseline Clinical Characteristics of 99 Patients at the Start of MPT | |||||
|---|---|---|---|---|---|
| Total, n = 99 | Early Treatment Group, n = 21 (FIO2 ≤ 0.39) | Middle Treatment Group, n = 18 (FIO2 0.40–0.69) | Late Treatment Group, n = 40 (FIO2 ≥ 0.70) | p-Value | |
| Demographic characteristics | |||||
| Age (years) | 64 (21–94) | 55 (40–76) | 56 (21–94) | 74 (35–91) | <0.001 ‡ |
| Male sex (%) | 79 (79.9) | 17 (81.0) | 30 (78.9) | 32 (80.0) | 1 † |
| Body mass index | 25 (17–41) | 24 (17–30) | 26 (17–41) | 24 (17–30) | 0.168 ‡ |
| Comorbidities | |||||
| Malignant tumor (%) | 10 (10.1) | 3 (14.3) | 2 (5.3) | 5 (12.5) | 0.468 † |
| Asthma or COPD (%) | 11 (11.1) | 4 (19.0) | 3 (7.9) | 4 (10.0) | 0.417 † |
| Chronic kidney disease (%) | 12 (12.1) | 0 (0.0) | 7 (18.4) | 5 (13) | 0.096 † |
| Diabetes mellitus (%) | 33 (33.3) | 5 (23.8) | 18 (47.4) | 10 (12.5) | 0.072 † |
| Hypertension (%) | 39 (39.4) | 4 (19.0) | 20 (52.6) | 15 (37.5) | 0.04 † |
| Hyperlipidemia (%) | 18 (18.1) | 4 (19.0) | 9 (23.7) | 5 (12.5) | 0.445 † |
| Obesity (%) | 47 (47.5) | 7 (33.3) | 23 (60.5) | 17 (42.5) | 0.095 † |
| History | |||||
| COVID-19 vaccination (%) | 6 (6.0) | 0 (0.0) | 4 (10.5) | 2 (5.0) | 0.258 † |
| Smoker (%) | 29 (29.3) | 5 (23.8) | 11 (28.9) | 13 (32.5) | 0.779 † |
| Treatment | |||||
| Days from onset to hospitalization | 7 (0–19) | 3 (0–11) | 6 (0–11) | 6 (0–19) | 0.150 ‡ |
| Days from onset to start of oxygen therapy | 7 (0–20) | 7 (0–20) | 7 (0–19) | 7 (0–19) | 0.240 ‡ |
| Days from onset to start of corticosteroid therapy | 7 (0–19) | 6 (0–11) | 8 (0–11) | 7 (0–19) | 0.365 ‡ |
| Anticoagulation (%) | 40 (40.4) | 8 (38.1) | 14 (36.8) | 18 (45.0) | 0.778 † |
| Remdesivir (%) | 79 (80.8) | 18 (85.7) | 30 (78.9) | 31 (77.5) | 0.768 † |
| Favipiravir (%) | 8 (8.0) | 0 (0.0) | 4 (10.5) | 4 (10.0) | 0.354 † |
| Baricitinib (%) | 47 (47.4) | 5 (23.8) | 23 (60.5) | 19 (47.5) | 0.025 † |
| Tocilizumab (%) | 24 (24.2) | 3 (14.3) | 8 (21.1) | 13 (32.5) | 0.290 † |
| Dexamethasone (%) | 53 (53.5) | 11 (52.4) | 25 (65.8) | 17 (42.5) | 0.060 † |
| Oxygen devices | |||||
| HFNC (%) | 29 (29.3) | 1 (4.8) | 13 (34.2) | 15 (37.5) | 0.012 † |
| Ventilator (%) | 12 (12.1) | 0 (0.0) | 1 (2.6) | 11 (27.5) | <0.001 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokano, M.; Tarumoto, N.; Imai, K.; Sakai, J.; Ishibashi, N.; Yokota, K.; Nakayama, H.; Haga, Y.; Maesaki, S. Timing of Initiation of Methylprednisolone Pulse Therapy in Patients with COVID-19. COVID 2022, 2, 532-539. https://doi.org/10.3390/covid2050039
Tokano M, Tarumoto N, Imai K, Sakai J, Ishibashi N, Yokota K, Nakayama H, Haga Y, Maesaki S. Timing of Initiation of Methylprednisolone Pulse Therapy in Patients with COVID-19. COVID. 2022; 2(5):532-539. https://doi.org/10.3390/covid2050039
Chicago/Turabian StyleTokano, Mieko, Norihito Tarumoto, Kazuo Imai, Jun Sakai, Noriomi Ishibashi, Kazuhiro Yokota, Hideto Nakayama, Yoshiyuki Haga, and Shigefumi Maesaki. 2022. "Timing of Initiation of Methylprednisolone Pulse Therapy in Patients with COVID-19" COVID 2, no. 5: 532-539. https://doi.org/10.3390/covid2050039
APA StyleTokano, M., Tarumoto, N., Imai, K., Sakai, J., Ishibashi, N., Yokota, K., Nakayama, H., Haga, Y., & Maesaki, S. (2022). Timing of Initiation of Methylprednisolone Pulse Therapy in Patients with COVID-19. COVID, 2(5), 532-539. https://doi.org/10.3390/covid2050039






