Abstract
Direct optical detection methods such as surface plasmon resonance imaging (SPRi) and photonic-integrated-circuits (PIC)-based biosensors provide a fast label-free detection of COVID-19 antibodies in real-time. Each technology, i.e., SPRi and PIC, has advantages and disadvantages in terms of throughput, miniaturization, multiplexing, system integration, and cost-effective mass production. However, both technologies share similarities in terms of sensing mechanism and both can be used as high-content diagnostics at or near to point of care, where the analyte is not just quantified but comprehensively characterized. This is significant because recent results suggest that not only the antibody concentration of the three isotypes IgM, IgG, and IgA but also the strength of binding (affinity) gives an indication of potential COVID-19 severity. COVID-19 patients with high titers of low affinity antibodies are associated with disease severity. In this perspective, we provide some insights into how SPR and PIC technologies can be effectively combined and complementarily used for a comprehensive COVID-19 severity monitoring. This opens a route toward an immediate therapy decision to provide patients a treatment in an early stage of the infection, which could drastically lowers the risk of a severe disease course.
1. Introduction
High-content diagnostics at the point of care where the analyte is not just quantified but comprehensively characterized are urgently needed to monitor COVID-19 severity. Previous studies showed that when a person suffers with COVID-19, the quality of the generated antibodies is crucial to predict the severity of the infection [1]. This allows an immediate therapy decision to provide patients a treatment in an early stage of the infection, which drastically lowers the risk of a severe disease course. Currently, this extremely relevant information cannot be provided by any diagnostic device available on the market.
For now three decades, direct-optical biosensors such as surface plasmon resonance (SPR) spectroscopy are the gold standard in the pharmaceutical lead generation and qualification process because they are able to deliver high-content data within a short time. In diagnostics, there are numerous applications, where these features would offer enormous benefits. However, up to now, instrument complexity together with prohibitive cost impeded a widespread application in the medical field. Therefore, a dedicated SPR imaging instrument is required to bridge this gap and to bring this technology near to point of care applications, thus allowing a fast and qualified medical decision.
Besides that, portable and cost-effective point-of-care systems based on photonic integrated circuits (PIC) [2] are promising candidates for initial quantitative patient screening. In this way, PIC-based biosensors can prescreen the patients in terms of COVID-19 severity to decide for a booster vaccination or further in-depth analysis using SPR imaging with multiplex targets as well as for the purpose of treatment and therapy of COVID-19 patients close to the point of care. PIC-based biosensors are intensively studied [3,4] and their sensitivity and limit of detection was improved over the last decade, reaching similar performance as SPR devices [5,6]. However, a widespread use as point-of-care device requires novel fabrication approaches to realize disposable COVID-19 tests [7]. One promising approach is the local backside release of the photonic sensor in order to separate the microfluidic channel from the electrical and optical interconnects [8,9]. This novel approach opens a route toward a cost-effective mass-production of packaged PIC-based biosensors for point-of-care applications.
In contrast to current diagnostics, both technologies will allow not only to analyze the concentration but also the strength of binding to get a fast indication of potential severity within 5 min. The strength of binding is highly significant because it gives information about the severity of an infection by SARS-CoV-2 or a mutant, which is essential for vaccinated and nonvaccinated patients.
Currently, hospitals have no diagnostics to decide for advanced therapeutic monoclonal antibody cocktail treatments. In the following, we will discuss the possibility to combine SPR imaging instruments and PIC technologies to solve this problem, which is urgently needed to get the pandemic under control.
Clinical Background
While antibodies against nucleocapsid (NCP) and spike proteins (S) are detectable in sera of individuals who have been infected with SARS-CoV-2, only antibodies against spike-RBD are measured for individuals in whom immunity has been acquired by vaccination only. The vaccine will not generate anti-NCP antibodies. As such, serological antibody testing is essential to assess the fraction of the population that is immune to the virus.
In the longer term, the persistence of immunity to SARS-CoV-2 infections may need to be periodically assessed to ensure public health and prevent or monitor the resurgence of the virus, context of viral infection, and vaccine efficacy monitoring. As such, developing a multiplex test for antibodies against nucleocapsid and spike protein and subepitopes (e.g., EP9) are needed to gain knowledge on the immunity of populations and whether this immunity was acquired following an (asymptomatic) infection or through vaccination.
In our recent publications [10,11], we showed a highly diverse immune profile consisting of mainly three isotypes, IgM, IgG, and IgA antibodies. Figure 1 shows SPRi measured immune profile of IgM, IgG, and IgA for COVID-19 negative, lower than and higher than 10 days after symptoms onset. Please note that the total immune response (IgM + IgG + IgA) is high for COVID-19 patients after 10 days of symptoms onset.
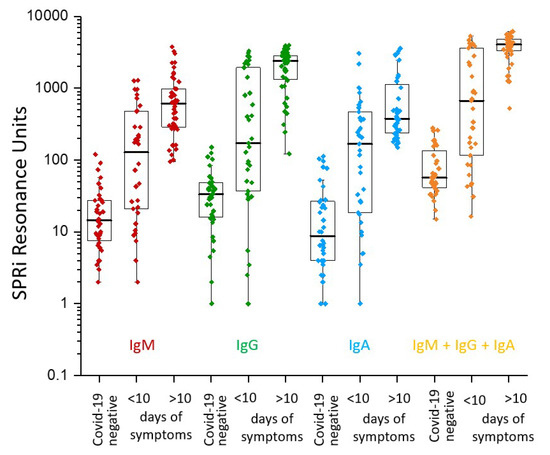
Figure 1.
SPRi measured immune profile of IgM, IgG, and IgA (Log SPRi resonance units) for COVID-19 negative, lower than and higher than 10 days after symptoms onset. The total immune response (IgM + IgG + IgA) is high for COVID-19 patients after 10 days of symptoms onset. Adopted from [10].
Affinity maturation of anti-SARS-CoV-2 antibodies was observed. Previous exposure to coronaviruses could lead to the production of non-neutralizing Abs and establish an overwhelming proinflammatory state through antibody-dependent enhancement (ADE) [12]. The immune complex against the nucleocapsid protein (NCP) [13] induces IL6 secretion by immune cells and activates a network of proinflammatory cytokines and profound systemic inflammatory response. NCP protein plays a critical role in viral pathogenesis, including aggravation of lung injury by (MASP)-2—mediated complement overactivation [14]. Generally, antibodies to the NCP protein will not neutralize the virus for infecting a cell but only antibodies against the spike protein. All current vaccines are generating antispike antibodies and not anti-NCP antibodies.
Effective methods for predicting COVID-19 disease trajectories are urgently needed. Additionally to our RBD/NCP ratio observation, recent evidence has been found [15] that non-neutralizing antibodies generated against a 21-residue epitope from nucleocapsid (termed Ep9) are associated with severe disease, including admission to the intensive care unit (ICU), requirement for ventilators, or even death. Importantly, anti-Ep9 antibodies can be detected within six days post-symptom-onset and sometimes within one day using an enzyme-linked immunosorbent assay (ELISA), which has similar sensitivity to SPRi [1]. Furthermore, anti-Ep9 antibodies correlate with various comorbidities and hallmarks of immune hyperactivity and sepsis. Thus, COVID-19 patients with high titers of low affinity antibodies against NCP associate with disease severity. The results will be the basis for a new type of COVID-19 prognostic biomarker in addition to the full NCP protein and S-RBD using label-free sensing technologies such as SPR and PIC to allow early identification and triage of high-risk patients. Such information will lead to effective therapeutic intervention. A patient with mild symptoms shows low titers, but the anti-RBD and anti-NCP can easily be detected. During our initial study, we could not measure vaccinated people, but many papers show that anti-NCP antibodies are not present in the sera of vaccinated people without SARS-CoV-2 infection. Although evidence with respect to the ratio of anti-RBD and anti-NCP should be confirmed in further studies for correlation with severity of the disease, it is clear that vaccinated versus nonvaccinated people (seropositive and seronegative) can clearly be identified. The anti-RBD value of vaccinated people (so negative for NCP) shows the status of immunity for COVID-19. Epidemiologic studies showing correlation with respect to vaccinated and seropositive patients are not necessary but only validation of patient data with traditional serology tests (e.g., ELISA).
In our latest study [16], we compared the data between critical and moderate patients and we observe that the strength of binding toward all antigens is lower with increasing disease severity, as shown in Figure 2. We deduced that the strength of binding of anti-NCP antibodies is lower (dissociation rate higher) than the anti-RBD antibodies in moderate and critical patients.
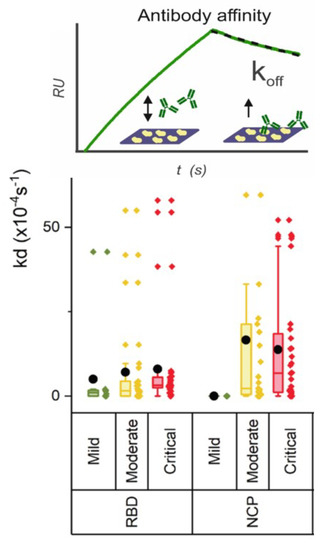
Figure 2.
The strength of binding of anti-NCP antibodies is lower (dissociation rate higher) than the anti-RBD antibodies in moderate and critical patients. Data obtained from Hendriks et al. [16].
Thus, although in critically ill patients, more antibodies to RBD, spike, and NCP are produced than in moderate patients, the binding strength is much less. As the affinity of the RBD domain for ACE2 receptor is high (≈10 nM [17]), this lower affinity is likely to have severe consequences for the effective neutralization. Additionally, to the correlation of the severity of COVID-19 using antibodies levels and strength of binding, strong correlation also exists with the expression of high levels of D-dimer, C-reactive protein (CRP), and Interleukin6, among others. These biomarkers can be implemented in the multiplex SPRi test for further in-depth analysis of COVID-19.
2. Advantages of SPR and PIC Technologies for COVID-19 Severity Monitoring
The SPR and PIC technologies are label-free and, therefore, the interaction of specific antibodies can directly be followed in real-time within three minutes, resulting in the fastest fully quantitative multiplex assay with respect to any other technology. In the following, we provide perspective advantages of both technologies for the specific scenario of COVID-19 severity monitoring:
- In our latest work [10,11], we address the importance to measure the levels and the anti-RBD/NCP ratios in concentration and strength of binding to get an indication of potential severity of COVID-19, which can be done with both technologies.
- The multiplex affinity parameter to varied antigens of SARS-CoV-2 can be measured, which is unique. We found a strong correlation between off-rate and the affinity equilibrium constant. We deduced an antibody affinity maturation effect after the beginning of symptoms onset.
- The ease with which photonic transducers can be duplicated as sensor building blocks makes them particularly suited for an important biosensing technique called multiplexing. Multiplex testing improves the diagnostic and prognostic outcome by simultaneously targeting more than one specific analyte in a sample.
- We found a strong correlation of antinucleocapsid antibodies with respect to anti-RBD and strength of binding with COVID-19 severity. Only label-free sensing technologies are able to measure this parameter in a 5 min time window. (3 min association and 2 min dissociation)
- The specific isotype responses (IgM, IgG, and IgA) can be implemented in the same run. IgG responses contribute to longer-lasting immunity, so this is important to track decreasing immunity.
- An important surrogate virus neutralization test (sVNT) using ACE2 antibody blocking can also be implemented in the same run. This new approach is important for testing not only general antibody concentration, but really, the virus neutralization capacity of the patient’s antibodies. Only anti-RBD antibodies contribute to the virus neutralization titer.
- SPR imaging in multiplex mode enables full profiling of a patient sample resulting in at least six antigen-specific antibody concentrations including three isotypes (18 values); six specific affinity parameters and sVNT values to the RBD specific ACE2 block in one run and fast.
As illustrated in Figure 3, a COVID-19 survivor or a COVID-19 deceased patient shows a different immune quality profile. It is still unclear why the human body generates such an enormous diverse polyclonal immune response to SARS-CoV-2 over the human population. Thus, the immune fingerprint should be tested for critical parameters in order to predict COVID-19 severity.
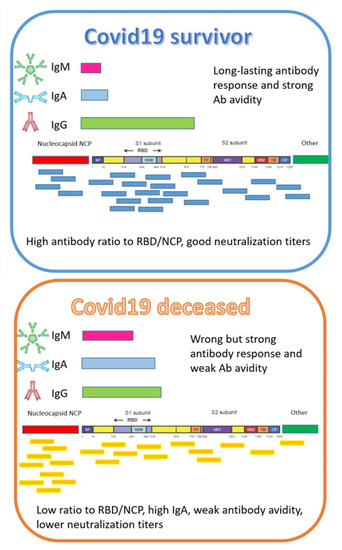
Figure 3.
A COVID-19 survivor or a COVID-19 deceased patient shows a different immune quality profile, as illustrated in this sketch, which is unique for each patient. The figure is a schematic presentation of antibody epitope binding profiles of antibodies to SARS-CoV-2. The fingerprint of antibodies to these epitopes are different for each patient. Vaccinated persons will not develop anti-NCP antibodies. The balance of neutralizing anti-RBD antibodies to non-neutralizing NCP antibodies reveals the status of recovering patients. Strength of binding of antibodies against RBD and NCP correlates with the severity of COVID-19. Critical and fatal COVID-19 patients developed high IgA titers. There are indications that patients with long COVID have an imbalance in these RBD/NCP antibodies.
3. Why and How to Combine SPR Imaging and PIC Technologies
Combining SPR imaging and PIC biosensors takes advantage of technological synergy effects because both technologies are based on the same physical principle (evanescent field sensing) and, hence, essentially the same surface functionalization and fluidics injection procedures can be used for both types of sensors. Furthermore, the software algorithm to analyze the sensor signal (sensorgram) can be the same for both technologies.
Furthermore, SPR imaging and PIC technologies are complementary because they can solve two different challenges: PIC biosensors allow a fast and portable point-of-care testing and SPR imaging enables a fast, in-depth analysis, important for a therapy or medical decision of patients with COVID-19. The combination of both technologies could enable a comprehensive monitoring of the severity of an infection by SARS-CoV-2 or a mutant in two stages and at the point of care, enabling an immediate therapy and medical decision.
In the following section, we describe a prospective approach for SARS-CoV-2 serology testing to identify the severity of COVID-19 in two stages:
- Early warning: Point-of-care testing by means of PIC sensors for prescreening of potential severityPIC technology has the potential for a miniaturized point-of-care system with sensor chips that can be mass-fabricated for cost-effective and, hence, disposable tests. An early detection of COVID-19 severity based on the concentration and off-rates of RBD/NCP could provide an ease of decision for hospitals. At this stage, the patient will be prescreened in terms of COVID-19 severity to decide for a booster vaccination or further in-depth analysis.
- Confirmation: Laboratory test by means of SPRi for in-depth COVID-19 severity screeningQuantitative measurements of IgG, IgM, and IgA neutralization antibodies (Nab’s) specific for SARS-CoV-2 epitopes and mutants and their apparent affinity in polyclonal sera of COVID-19 patients can be performed with SPR imaging. At this stage, an in-depth severity monitoring of COVID-19 is possible. This has the potency to screen vaccinated patients, e.g., patients with immune suppressive medication or high age, for sufficient Nab’s. If the Nab’s are too low in concentration, it suggests a booster vaccination. Furthermore, a selection of COVID-19 patients for advanced therapeutic monoclonal antibody cocktail treatments can be based on the in-depth analysis of the immune response of these patients.
Following the aforementioned two-stage COVID-19 severity monitoring allows to predict COVID-19 disease trajectories, which is urgently needed because when a patient is exposed to SARS-CoV-2 virus or the patient is vaccinated against SARS-CoV-2, the immune system responds differently. The immune systems express antibodies at levels that can be easily quantitatively detected and monitored by SPRi and PIC. In order to identify the fraction of the population that either (i) was vaccinated partly (single injection), (ii) has a second injection, (iii) was vaccinated additionally to exposure to SARS-CoV-2 (e.g., also asymptomatically), or (iv) was never exposed to SARS-CoV-2 or vaccinated is important for getting the pandemic under control. Because the immune system reacts differently from patient to patient, from the young to the elderly, from the healthy to the vulnerable, etc., a physician can assess the level of COVID-19 protection after multiplex tests with SPR imaging and PIC biosensors. In the longer term, the persistence of immunity to SARS-CoV-2 infections may need to be periodically assessed to ensure the public health and prevent or monitor the resurgence of the virus, context of viral infection, and vaccine efficacy monitoring.
4. Route for Commercialization of SPRi and PIC Devices
The COVID-19 study using SPR imaging was performed with two commercial research instruments. These general research instruments are less suitable to transfer to a clinical setting or to the point of care. The size of the instruments is large and it weighs >250 kg (LSA) and >100 kg (IBIS MX96 with spotter). The investment of the multipurpose equipment is in the order of 500 k€ for the LSA of Carterra and 250 k€ for the IBIS MX96 and this will be too high for a widespread acceptation. Definitely, the prize of the research-based SPR imagers will be reduced further at a higher volume, but because of complicated manufacturing, it will be less feasible that the prize drops below 50 k€. The software for analyzing the data of the COVID-19 patients should be streamlined for getting instant results (within 10–15 min). Therefore, it is needed to develop a new clinical-based cost-effective SPR imaging instrument with dedicated sensors for fingerprinting the antibodies of an infected patient for current or future pandemics. For COVID-19 antibody monitoring, we suggested to measure antibody binding/avidity against, e.g., six SARS-CoV-2 antigens (e.g., RBD, spike (S1 + S2), S1, S2, nucleocapsid, and EP9) and serotyping IgM, IgG, and IgA. Such an instrument does not need an autosampler, but the fluidics should be designed in such a way that it can perform a kinetic titration including automatic off-rate determination for all the analytes simultaneously. In this way, the SPR imager will be a dedicated plug-and-play instrument and can be used by uneducated operators at the point of care (e.g., a nurse without understanding of SPR). When prespotted sensors are applied, a nurse can then inject a sample of the patient (serum or whole blood) and the SPR imager can be considered as a clinical-data-generating black box. The instrument will make dilutions automatically and injects the patient sample to measure the biomolecular interactions and off-rates at the various spots in real time. Automatically including internal controls, the data will be processed and presented to the operator. It enables the operator to make important decisions within, e.g., 10 min to treat the patient further. The in-depth data profiles can be embedded in artificial intelligence (AI) software.
Recently, we found that the antibody concentration and off-rate gives us an indication to predict COVID19 severity. Only label-free technologies—in particular, photonic devices including SPR imaging—may fulfill this gap. While SPR imaging can be applied as an in-depth antibody profiling device for infectious disease monitoring, the photonic integrated circuit (PIC) device can be applied as an early warning device.
The costs of the PIC device should be in the order of a lateral flow device. Although concentration of the specific antibodies can be qualitatively measured with an LFA, the kinetics and off-rates cannot be measured. Since we know that the quality/avidity of the antibodies as an additional parameter is important to predict COVID-19 severity (and perhaps this counts for other infectious diseases as well), a mass-fabricated PIC sensor should be applied in future pandemics and/or local infectious disease outbreaks. In order to enter in this field, such a device should be available for performing clinical studies after an infectious disease outbreak.
Currently, there are a few companies using PIC-based sensors, namely Siphox, Genalyte, Bioherent, Lionix, and Lumensia. However, up to now, there is no point-of-care device on the market due to some technical challenges. For example, the light source is typically a tunable laser, which is expensive and not suitable for miniaturized systems. To overcome this issue, Leuermann et al. have developed a coherent light detection approach using a single wavelength laser source [18]. Besides that, the realization of a pluggable sensor solution, which needs to be simple and cost-effective, is challenging due to the required alignment tolerances between optical fiber and chip. Relying on standard single mode optical fibers for fiber-to-chip light coupling hinders the development of pluggable sensor solutions because an alignment precision below 1 m is required. An appropriated solution to tackle this problem is the use of GRIN-lensed fibers, which was demonstrated as cheap fiber coupling technique with improved alignment tolerance, enabling a low-cost and pluggable sensor solution [19]. In this way, PIC sensors can be implemented in point-of-care devices with low weight and keeps the production costs low because it avoids expensive and huge fiber alignment stages (about 3000 EUR).
One further challenge to realize the point-of-care approach as described in this work is the surface functionalization, which is optimized for flat gold surfaces of SPR sensors. This, however, needs to be transferred to structured silicon surfaces for the PIC sensors. Indeed, every chip technology has this challenge, but the back-side integration approach as reported in [8] mitigates this problem drastically. Besides that, the back-side integration approach allows a simplified and cost-effective packaging of the sensor chip. It opens a way for a fully wafer-scale production of sensor chips, microfluidic, and electronic packaging. This is essential in order to provide low-cost and disposable tests as it is required for the envisioned COVID-19 severity monitoring in two stages.
5. Expected Impact
If COVID-19 at risk can be monitored regarding their immunity status including sepsis detection, it will be a great impact on society and well-being. Both systems can be easily adapted to screen other infectious diseases by functionalizing the sensor surface with other specific ligand molecules. SPR imaging and PIC biosensors are complementary because they can be used either as point-of-care devices (PIC biosensors) or for a fast, in-depth analysis with high throughput (SPR imaging). In this way, a two stage diagnostic scenario is possible, as described in the previous section. Conclusively, both systems can help to manage the present but also future pandemics and, therefore, it is expected not only to reduce costs for hospitalization and treatment of COVID-19 patients but also to improve well-being and quality of life.
Author Contributions
P.S.: conceptualization, writing—review and editing. R.B.M.S.: conceptualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding in part by the ATTRACT Project funded by European Council (EC) under Grant 777222 and in part by the European Regional Development Fund under Grant 10.13039/501100008530.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Vega, G.; Soler, M.; Lechuga, L.M. Nanophotonic biosensors for point-of-care COVID-19 diagnostics and coronavirus surveillance. J. Phys. 2021, 3, 011002. [Google Scholar] [CrossRef]
- Steglich, P.; Hülsemann, M.; Dietzel, B.; Mai, A. Optical Biosensors Based on Silicon-On-Insulator Ring Resonators: A Review. Molecules 2019, 24, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steglich, P.; Rabus, D.G.; Sada, C.; Paul, M.; Weller, M.G.; Mai, C.; Mai, A. Silicon Photonic Micro-Ring Resonators for Chemical and Biological Sensing: A Tutorial. IEEE Sens. J. 2021. [Google Scholar] [CrossRef]
- Leuermann, J.; Fernandez-Gavela, A.; Torres-Cubillo, A.; Postigo, S.; Sanchez-Postigo, A.; Lechuga, L.M.; Halir, R.; Molina-Fernandez, I. Optimizing the Limit of Detection of Waveguide-Based Interferometric Biosensor Devices. Sensors 2019, 19, 3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steglich, P.; Villringer, C.; Pulwer, S.; Heinrich, F.; Bauer, J.; Dietzel, B.; Mai, C.; Mai, A.; Casalboni, M.; Schrader, S. Hybrid-Waveguide Ring Resonator for Biochemical Sensing. IEEE Sens. J. 2017, 17, 4781–4790. [Google Scholar] [CrossRef]
- Cognetti, J.S.; Steiner, D.J.; Abedin, M.; Bryan, M.R.; Shanahan, C.; Tokranova, N.; Young, E.; Klose, A.M.; Zavriyev, A.; Judy, N.; et al. Disposable photonics for cost-effective clinical bioassays: Application to COVID-19 antibody testing. Lab Chip 2021, 21, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Steglich, P.; Bondarenko, S.; Mai, C.; Paul, M.; Weller, M.G.; Mai, A. CMOS-Compatible Silicon Photonic Sensor for Refractive Index Sensing Using Local Back-Side Release. IEEE Photonics Technol. Lett. 2020, 32, 1241–1244. [Google Scholar] [CrossRef]
- Steglich, P.; Paul, M.; Mai, C.; Böhme, A.; Bondarenko, S.; Weller, M.G.; Mai, A. A monolithically integrated micro fluidic channel in a silicon-based photonic-integrated-circuit technology for biochemical sensing. In Optical Sensors 2021; Baldini, F., Homola, J., Lieberman, R.A., Eds.; International Society for Optics and Photonics, SPIE: Bellingham, WA, USA, 2021; Volume 11772, pp. 1–5. [Google Scholar] [CrossRef]
- Schasfoort, R.; van Weperen, J.; van Amsterdam, M.; Parisot, J.; Hendriks, J.; Koerselman, M.; Karperien, M.; Mentink, A.; Bennink, M.; Krabbe, H.; et al. Presence and strength of binding of IgM, IgG and IgA antibodies against SARS-CoV-2 during CoViD-19 infection. Biosens. Bioelectron. 2021, 183, 113165. [Google Scholar] [CrossRef]
- Schasfoort, R.; van Weperen, J.; van Amsterdam, M.; Parisot, J.; Hendriks, J.; Koerselman, M.; Karperien, M.; Mentink, A.; Bennink, M.; Krabbe, H.; et al. High throughput surface plasmon resonance imaging method for clinical detection of presence and strength of binding of IgM, IgG and IgA antibodies against SARS-CoV-2 during CoViD-19 infection. MethodsX 2021, 8, 101432. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Foo, S.S.; Bruzzone, R.; Vu Dinh, L.; King, N.J.C.; Mahalingam, S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015, 268, 340–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batra, M.; Tian, R.; Zhang, C.; Clarence, E.; Sacher, C.S.; Miranda, J.N.; De La Fuente, J.R.O.; Mathew, M.; Green, D.; Patel, S.; et al. Role of IgG against N-protein of SARS-CoV2 in COVID19 clinical outcomes. Sci. Rep. 2021, 11, 3455. [Google Scholar] [CrossRef] [PubMed]
- Flude, B.M.; Nannetti, G.; Mitchell, P.; Compton, N.; Richards, C.; Heurich, M.; Brancale, A.; Ferla, S.; Bassetto, M. Targeting the Complement Serine Protease MASP-2 as a Therapeutic Strategy for Coronavirus Infections. Viruses 2021, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.R.; Sanders, E.C.; Gabriel, K.N.; Miller, B.M.; Isoda, H.M.; Salcedo, G.S.; Garrido, J.E.; Dyer, R.P.; Nakajima, R.; Jain, A.; et al. Predicting COVID-19 Severity with a Specific Nucleocapsid Antibody plus Disease Risk Factor Score. mSphere 2021, 6, e00203-21. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, J.; Schasfoort, R.; Koerselman, M.; Dannenberg, M.; Cornet, A.; Beishuizen, A.; Krabbe, H.; Mulder, L.; Karperien, M. High titers of low affinity antibodies in Covid-19 patients are associated with disease severity. Front. Immunol. 2022. accepted. [Google Scholar]
- Fiedler, S.; Piziorska, M.A.; Denninger, V.; Morgunov, A.S.; Ilsley, A.; Malik, A.Y.; Schneider, M.M.; Devenish, S.R.A.; Meisl, G.; Kosmoliaptsis, V.; et al. Antibody Affinity Governs the Inhibition of SARS-CoV-2 Spike/ACE2 Binding in Patient Serum. ACS Infect. Dis. 2021, 7, 2362–2369. [Google Scholar] [CrossRef] [PubMed]
- Leuermann, J.; Stamenkovic, V.; Ramirez-Priego, P.; Sánchez-Postigo, A.; Fernández-Gavela, A.; Chapman, C.A.; Bailey, R.C.; Lechuga, L.M.; Perez-Inestrosa, E.; Collado, D.; et al. Coherent silicon photonic interferometric biosensor with an inexpensive laser source for sensitive label-free immunoassays. Opt. Lett. 2020, 45, 6595–6598. [Google Scholar] [CrossRef] [PubMed]
- Bondarenko, S.; Hülsemann, M.; Mai, A.; Steglich, P. Fiber-to-chip light coupling using a graded-index lensed fiber collimator. Opt. Eng. 2021, 60, 014105. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).