Abstract
Coronavirus disease 2019 (COVID-19) is associated with fatal acute respiratory distress syndrome, which can be ameliorated by methylprednisolone pulse therapy, thereby reducing the risk of progression to respiratory failure and death. We aimed to determine the efficacy and safety of methylprednisolone pulse therapy for patients with COVID-19 pneumonia. In this retrospective, observational cohort study, seventy patients (age, 35–91 years) who were admitted to Saitama Medical University Hospital in Japan between March 2020 and January 2021 due to COVID-19 pneumonia were included. The difference in mortality between the methylprednisolone pulse therapy (n = 22) and dexamethasone therapy (n = 48) groups was the primary outcome. Between-group differences in the average length of intensive care unit stay, duration of invasive mechanical ventilation, and incidence of treatment-related adverse events were the secondary outcomes. The methylprednisolone pulse therapy group showed a significantly lower mortality rate (3.8% vs. 20.2%, p = 0.006) and increased survival rate compared with the dexamethasone therapy group (p = 0.044). Additionally, without statistical significance, the average length of intensive care unit stay tended to be shorter in the methylprednisolone pulse therapy group (11.5 ± 6.1 days) than in the dexamethasone therapy group (22.3 ± 23.1 days) (p = 0.793). The average duration of invasive mechanical ventilation also tended to be shorter in the methylprednisolone pulse therapy group (15.3 ± 10.1 vs. 28.8 ± 9.2 days, p = 0.120). There were no significant differences in the incidence of treatment-related serious adverse events between the two groups. In conclusion, methylprednisolone pulse therapy for patients with COVID-19 pneumonia significantly reduced mortality and increased the survival rate compared to conventional dexamethasone therapy.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is responsible for the coronavirus disease 2019 (COVID-19) pandemic, which originated in late 2019 in the Chinese city of Wuhan [1]. Its clinical manifestations range from asymptomatic to severe pneumonia, thrombosis, and multiple organ failure [2,3]. Elderly individuals and people with comorbidities are more likely to suffer from severe COVID-19, and the rate of death during hospitalization is 7.5% [2]. Cytokine storm has been reported to be associated with one of the causes of exacerbations and death from COVID-19 [3]. A cytokine storm comprises macrophages, neutrophils, and lymphocytes that infiltrate alveolar epithelial cells, which then release large amounts of inflammatory cytokines, chemokines, and leukocytosis factors, such as interleukin (IL)-6, tumor necrosis factor-α, IL-1β, interferon, IL-18, interferon gamma-induced protein 10, monocyte chemoattractant protein-1, monocyte colony-stimulating factor, and granulocyte colony-stimulating factor [3]. These inflammatory mediators induce a systemic inflammatory response, thereby causing fatal acute respiratory distress syndrome (ARDS) [3].
In clinical practice, corticosteroids [4], JAK inhibitors [5], and anti-IL-6 receptor antibodies [6] have been used to treat patients with COVID-19 pneumonia. In a randomized controlled clinical trial of moderate and severe COVID-19 pneumonia conducted in 2020 in the United Kingdom, 6 mg of dexamethasone was administered daily for 10 days to patients requiring oxygen therapy or mechanical ventilation. The mortality rate of the dexamethasone therapy group was significantly lower than that of the usual care group [4]. Additionally, in a randomized clinical trial of moderate and severe COVID-19 pneumonia conducted in Spain in 2021, a high dose of dexamethasone (20 mg once daily for 5 days, followed by 10 mg once daily for an additional 5 days) compared with a low dose of dexamethasone (6 mg once daily for 10 days) significantly decreased clinical worsening within 11 days [7]. A high dose of dexamethasone was not associated with an increased risk of treatment-related serious adverse events in this population with COVID-19 pneumonia. Furthermore, in a randomized clinical trial of severe COVID-19 pneumonia conducted in Denmark, India, Sweden, and Switzerland in 2020 and 2021, a higher dose of dexamethasone (12 mg once daily for maximum 10 days) compared with the currently recommended dose of dexamethasone (6 mg once daily for maximum 10 days) had significantly lower mortality and fewer serious treatment-related adverse events at day 28 [8].
Methylprednisolone pulse therapy has been used for the purpose of lifesaving and rescue since 1969 [9]. This can be attributed to its high immediate effect when patients are at risk of death or irreversible organ damage, particularly in those with autoimmune diseases. The method involves the intravenous administration of a large amount of methylprednisolone (1000 mg daily) for 3 days. Thus, it exerts more immediate anti-inflammatory and immunosuppressive effects compared with the conventional daily administration of 6 mg oral dexamethasone. Thus, we hypothesized that methylprednisolone pulse therapy for patients with COVID-19 pneumonia might improve mortality by suppressing the progression to cytokine storm-mediated ARDS.
In this study, we investigated the mortality rate and incidence of adverse events of methylprednisolone pulse therapy compared with conventional dexamethasone therapy in patients with COVID-19 pneumonia using propensity score-based inverse probability weighting (IPW). Additionally, we analyzed the differences in laboratory parameters between the methylprednisolone pulse therapy and dexamethasone therapy groups before and on days 3 and 7 of treatment.
2. Materials and Methods
2.1. Design Overview and Setting
We conducted this retrospective cohort study at Saitama Medical University Hospital, a specialized facility in Japan that has accepted patients with severe COVID-19 pneumonia since the earliest stage of the pandemic. We analyzed patients aged 35–91 years who were admitted to the hospital between March 2020 and January 2021 and diagnosed with COVID-19 pneumonia requiring oxygen administration. This study was conducted (1) in accordance with the revised tenets of the Declaration of Helsinki and (2) after approval by the Institutional Review Board (No. 20188.01) of Saitama Medical University Hospital. Clinical information was obtained by reviewing electronic medical records. The primary outcome was the difference in mortality between the methylprednisolone pulse therapy and dexamethasone therapy groups analyzed using propensity score-based IPW. The secondary outcomes were differences in the average length of intensive care unit (ICU) stay, duration of invasive mechanical ventilation, and incidence of treatment-related adverse events between the two groups. We also analyzed the differences in laboratory parameters including the levels of serum C-reactive protein (CRP), lactate dehydrogenase (LDH), albumin, creatinine, plasma D-dimer, and the absolute neutrophil or lymphocyte count between the two groups, before and on days 3 and 7 of treatment.
2.2. Participants
For the diagnosis of COVID-19, SARS-CoV-2 was identified by a reverse transcription polymerase chain reaction using a nasopharyngeal swab or sputum sample. A radiological diagnosis of COVID-19 pneumonia was made based on the presence of abnormal shadows (bilateral, subpleural, and peripheral ground-glass opacities) on computed tomography scans, which was confirmed by a radiologist. Patients who required oxygen administration indicated by percutaneous arterial oxygen saturation (SpO2) ≤ 94% in room air were enrolled as participants. Exclusion criteria were pregnant or lactating females, active malignancies, or aggressive treatment undesired.
2.3. Treatment Protocol
The initial management included supportive care by oxygenation aimed at achieving SpO2 ≥ 95%. In the absence of contraindications for antiviral drugs, remdesivir (n = 38) or favipiravir (n = 10) was administered at the time of hospitalization. In detail, remdesivir was used in 15 of 22 patients (68.2%) in the methylprednisolone pulse therapy group and 23 of 48 patients (48.0%) in the dexamethasone therapy group, and the methylprednisolone pulse therapy group was used more frequently than the dexamethasone therapy group. Favipiravir was used in 3 of 22 patients (13.6%) in the methylprednisolone pulse therapy group and 7 of 48 patients (14.6%) in the dexamethasone therapy group, and the frequency of use was almost the same in both groups. The patients then received either methylprednisolone pulse therapy (methylprednisolone 1000 mg/day injected intravenously for 3 days, followed by intravenous or oral administration of dexamethasone 6 mg/day) or dexamethasone therapy (dexamethasone 6 mg/day administered intravenously or orally daily), according to physicians’ decisions. The dosage of dexamethasone was decreased gradually every week and eventually discontinued. JAK inhibitors were not used in this study, and only one patient was treated with anti-IL-6 receptor antibodies.
2.4. Statistical Analyses
To adjust for potential confounding factors, the two groups were balanced by propensity score-based IPW. The propensity score was calculated using a logistic regression model that included the baseline characteristics (age, sex, body mass index, number of severe cases, total number of comorbidities, number of diabetes cases, number of hypertension cases, number of chronic kidney disease cases, serum CRP levels, LDH levels, albumin levels, creatinine levels, plasma D-dimer levels, and neutrophil or lymphocyte counts in peripheral blood). Covariate balance after the IPW was assessed by computing their standardized differences, and groups were considered balanced when the standardized differences (STDs) of all covariates were <0.1 [10]. Moreover, IPW-adjusted Kaplan–Meier survival curve analysis and the log-rank test were used to analyze the survival rates between the two groups. The average length of ICU stay and duration of invasive mechanical ventilation in the two groups were calculated as the average length of days and standard deviation. Furthermore, we subsequently performed Friedman’s nonparametric test to detect differences in the levels of serum CRP, LDH, and plasma D-dimer, before and on days 3 and 7 in both groups. The threshold for statistical significance was set at p < 0.05. All analyses were performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC, USA).
3. Results
3.1. Baseline Characteristics of the Participants
We enrolled 70 patients with COVID-19 pneumonia who started treatment with methylprednisolone pulse therapy or conventional dexamethasone therapy between March 2020 and January 2021. Twenty-two of them were assigned to methylprednisolone pulse therapy and the remaining 48 were assigned to dexamethasone therapy. They were followed-up from admission to discharge. While 62 out of 70 patients (88.6%) were discharged, 8 out of 70 (11.4%) patients died despite undergoing treatment. Table 1 summarizes the clinical data of all patients.

Table 1.
Aggregation of patient backgrounds before and after propensity score-based inverse probability weighting-adjustment.
Before performing an adjustment based on the propensity score-based IPW, the STD values of the body mass index, severe case, hypertension, CRP, LDH, and neutrophil count were all >0.1. However, following the adjustment, the STD values decreased below 0.1 for most of these variables, and the distribution of variables was relatively well balanced. The average age of all patients was 67.0 ± 14.5 years; 22 (31.4%) females and 48 (68.6%) males were included. The average number of days from the onset of symptoms to hospitalization was 7.5 ± 5.5 days. The average body weight was 62.7 ± 17.1 kg. Moreover, remdesivir, favipiravir, invasive ventilator machine, and extracorporeal membrane oxygenation were used in 38 (54.3%), 10 (14.2%), 11 (15.7%), and 5 (7.1%) patients, respectively.
3.2. Primary Outcome
The mortality rate in the methylprednisolone pulse therapy group was 4.5% (1/22), which was lower than that in the dexamethasone therapy group at 14.6% (7/48), before the propensity score-based IPW adjustment. The propensity score-based IPW-adjusted mortality rates for the methylprednisolone pulse therapy and dexamethasone therapy groups were 3.8% (95% confidence interval [CI], 0.8–8.5%) and 20.2% (95% CI, 8.4–32.1%), respectively (p = 0.006). Calculation using the Kaplan–Meier estimator of survival rate revealed a better survival rate in the methylprednisolone pulse therapy group compared to the dexamethasone therapy group (log-rank test; p = 0.044) (Figure 1).
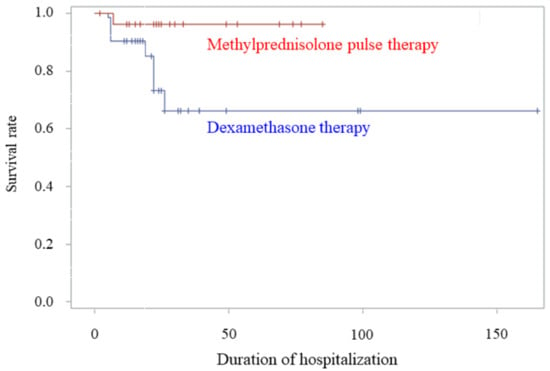
Figure 1.
Comparison of survival rates between the methylprednisolone pulse therapy and dexamethasone therapy groups. Kaplan–Meier estimate of the survival rate between the methylprednisolone pulse therapy and dexamethasone therapy groups after adjustment by propensity score-based inverse probability weighting (p = 0.044, log-rank test).
There were eight hospital deaths (one in the methylprednisolone pulse therapy group and seven in the dexamethasone therapy group). The causes of death were intraperitoneal bleeding (one patient in the methylprednisolone pulse therapy group), respiratory failure (five patients in the dexamethasone therapy group), and acute myocardial infarction and gastrointestinal bleeding (one patient each in the dexamethasone therapy group). All of the deaths occurred within 1 month of hospitalization. In addition, in the methylprednisolone pulse therapy group, 1/15 patients (6.7%) died with the use of remdesivir and 0/3 patients (0.0%) died with favipiravir. On the other hand, in the dexamethasone therapy group, 3/23 patients (13.0%) died with the use of remdesivir, and 3/7 patients (42.9%) died with favipiravir.
3.3. Secondary Outcomes
Excluding the abovementioned fatal cases, 15 patients in the methylprednisolone pulse therapy group and 4 in the dexamethasone therapy group were admitted to the ICU for intensive care. Although not statistically significant, the average length of ICU stay in the methylprednisolone pulse therapy group was 11.5 ± 6.1 days, which tended to be 10.8 days shorter than that in the dexamethasone therapy group (22.3 ± 23.1 days) (p = 0.793). A total of 11 patients (5 in the methylprednisolone pulse therapy group and 6 in the dexamethasone therapy group) underwent invasive mechanical ventilation. The average duration of invasive mechanical ventilation in the methylprednisolone pulse therapy group was 15.3 ± 10.1 days, which tended to be 13.5 days shorter than that in the dexamethasone therapy group (28.8 ± 9.2 days) (p = 0.120). Regarding the incidence of treatment-related adverse events, no significant difference was observed between the two groups. The most common treatment-related adverse event requiring treatment was the exacerbation of impaired glucose tolerance, with insulin therapy being initiated or increased in 21 (95.4%) and 34 (70.8%) patients in the methylprednisolone pulse therapy and dexamethasone therapy groups, respectively. Fungal infection was the next most common adverse event, occurring in three (13.6%) and two (4.2%) patients in the methylprednisolone pulse therapy and dexamethasone therapy groups, respectively. Gastrointestinal bleeding followed in one case in the dexamethasone therapy group. All events were relieved and cured by treatments, and did not affect the length of hospitalization.
3.4. Laboratory Parameters
Blood biochemical tests revealed a significant reduction in serum CRP levels from the pretreatment values on day 3 and 7 of treatment in both groups (p < 0.01 for all) (Figure 2a,b).
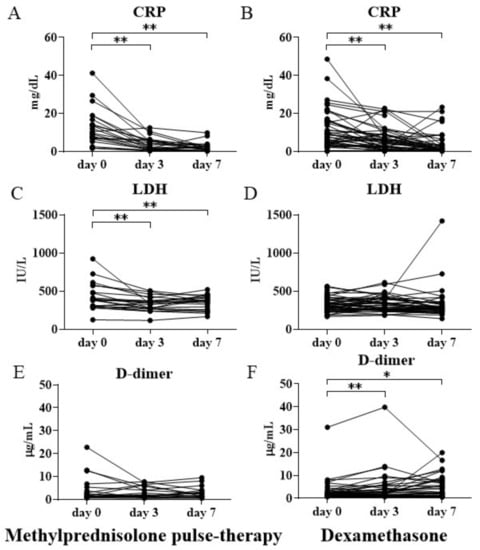
Figure 2.
Serum CRP, LDH, and plasma D-dimer levels before and after initiating methylprednisolone pulse therapy or dexamethasone therapy. The changes in CRP (A,B), LDH (C,D), and D-dimer (E,F) levels before treatment and on days 3 and 7 after treatment initiation in the methylprednisolone pulse therapy and dexamethasone therapy groups. Values are presented as mean ± SEM. * p < 0.05, ** p < 0.01. CRP = C-reactive protein; LDH = lactate dehydrogenase; SEM = standard error of the mean.
Notably, the decrease in CRP levels in the methylprednisolone pulse therapy group was more noticeable than that in the dexamethasone therapy group. The methylprednisolone pulse therapy group had a 70% reduction in the levels of CRP on day 3 compared to the pretreatment level, whereas the dexamethasone therapy group had a 40% reduction in CRP levels on day 3. The serum LDH levels significantly decreased on days 3 and 7 of therapy compared to the pretreatment level in the methylprednisolone pulse therapy group (p < 0.01 for all) (Figure 2c). The methylprednisolone pulse therapy group had a 20% reduction in LDH levels on days 3 and 7 when compared to the pretreatment level. However, the LDH levels were almost unchanged in the dexamethasone therapy group (Figure 2d). Conversely, the levels of plasma D-dimer significantly increased both on days 3 and 7 of treatment in the dexamethasone therapy group (Figure 2f), whereas the corresponding levels in the methylprednisolone pulse therapy group showed little change (Figure 2e).
4. Discussion
In this study, statistical adjustment to minimize the confounding bias using propensity score-based IPW demonstrated a significantly lower mortality rate in the methylprednisolone pulse therapy group than in the dexamethasone therapy group. Comparison of survival curves in the two groups revealed a significantly higher survival rate in the methylprednisolone pulse therapy group. The average length of ICU stay and the number of days of mechanical ventilation were shorter in the methylprednisolone pulse therapy group than in the dexamethasone therapy group. Contrastingly, we observed no serious treatment-related adverse events in the methylprednisolone pulse therapy group. Our findings strongly suggest that methylprednisolone pulse therapy is safe and efficacious for patients with COVID-19 pneumonia.
Previous studies have reported no benefit of glucocorticoids for severe preceding coronavirus infections including SARS and Middle East respiratory syndrome (MERS) [11,12]. This is considered plausible because of the slower clearance of viral RNA observed in patients with SARS and MERS who were treated with systemic glucocorticoids [4]. In fact, patients with MERS-CoV and SARS-CoV excrete the peak amount of viral RNA at 14 and 10 days after the onset of the symptoms, respectively [13]. Contrastingly, the peak amount of viral RNA excreted from individuals infected with SARS-CoV-2 was observed immediately before and after the onset of symptoms [13]. Moreover, we initiated methylprednisolone pulse therapy and dexamethasone therapy 6.9 and 7.0 days on average after the onset of symptoms, respectively. Therefore, at the beginning of methylprednisolone pulse therapy or dexamethasone therapy administration, the amount of SARS-CoV-2 RNA had already peaked and subsequently decreased during the treatment period in all cases. Thus, methylprednisolone pulse therapy or dexamethasone therapy is considered to exert little effect on the delay in SARS-CoV-2 RNA elimination.
No randomized controlled trial is available yet for methylprednisolone pulse therapy defined as ≥500 mg/day methylprednisolone in patients with COVID-19 pneumonia. Edalatifard et al. reported a single-blind, randomized controlled trial with respect to the presence or absence of 250 mg methylprednisolone for 3 days in addition to standard treatment in 68 patients with COVID-19 [14]. In this study, the standard care group did not receive glucocorticoids. The methylprednisolone group showed a significantly higher improvement rate (94.1% vs. 57.7%, p = 0.001) and lower mortality rate (5.9% vs. 42.9%, p < 0.001) compared to the standard treatment group. Ruiz-Irastorza et al. performed a comparative observational study of 242 patients with COVID-19 [15]. Sixty-one patients (25%) received methylprednisolone 125–250 mg/day for 3 days within the second week after the onset of symptoms. The methylprednisolone group had fewer deaths than the non-methylprednisolone group (hazard ratio, 0.35; 95% CI, 11–106%; p = 0.064). Furthermore, Dolci et al. included 14 patients with moderate to severe respiratory failure caused by COVID-19 pneumonia requiring oxygen therapy. The patients received methylprednisolone (1000 mg/day) for 3 days; out of the 14 patients, 10 survived and 4 died [16].
This study is the first to demonstrate that patients with COVID-19 pneumonia who received methylprednisolone pulse therapy had a lower mortality rate and better survival rate than patients who received conventional dexamethasone therapy according to the propensity score-based IPW. Our findings are expected to not only help suppress the outbreak of SARS-CoV-2 nosocomial infections but also to prevent medical collapse because of COVID-19 infection overload.
CRP, an acute phase protein, is induced by IL-6 in the liver. It correlates with the severity of COVID-19 infection, and is an index for predicting life prognosis [15]. IL-6 is closely involved in the induction of cytokine storm [3,17]. The methylprednisolone pulse therapy group demonstrated a marked decrease in serum CRP levels on days 3 and 7 after initiating the treatment compared to the dexamethasone therapy group. In other words, methylprednisolone pulse therapy strongly suppressed the production of pro-inflammatory cytokines centered on IL-6. Furthermore, the serum LDH levels are a marker for the severity of COVID-19 infection as well as an index for the assessment of severity of multiple organ failure and ARDS-associated acute lung injury [18]. Serum LDH levels were significantly reduced in the methylprednisolone pulse therapy group on days 3 and 7 compared to those before therapy. Methylprednisolone pulse therapy possibly suppressed the pathophysiological condition of multiple organ failure and acute lung injury caused by ARDS. Therefore, it led to an improvement in life prognosis. Additionally, plasma D-dimer levels are considered markers of COVID-19 aggravation, besides being an index of coagulation activation and thrombus formation [19]. The D-dimer levels decreased in the methylprednisolone pulse therapy group on days 3 and 7 after commencing the therapy. Contrastingly, they significantly increased in the dexamethasone therapy group compared to the pretreatment level. Thus, methylprednisolone pulse therapy controlled the cytokine storm and helped combat the disease, thereby reducing the D-dimer levels. In contrast, the disease did not subside in the dexamethasone therapy group; hence, the D-dimer levels remained relatively high.
The limitations of this study include a small sample size, having an open-label, non-randomized retrospective design, and potentially biased evaluators. In addition, due to sample size constraints, some variables may not be well balanced even after IPW and are one of the limitations of this study. Additionally, the methylprednisolone pulse therapy group used remdesivir more frequently than the dexamethasone therapy group, which may have correlated with the study outcomes. Further investigation is needed in this regard. Because some diabetics may develop severe COVID-19 pneumonia, attention should be paid for exacerbation of impaired glucose tolerance when initiating methylprednisolone pulse therapy. Moreover, we did not evaluate long-term adverse events.
5. Conclusions
Methylprednisolone pulse therapy had comparable safety to conventional dexamethasone therapy in real clinical practice and has the potential to decrease the total number of COVID-19 deaths worldwide. Anti-IL-6 receptor antibodies and JAK inhibitors have been applied to help combat cytokine storm. However, they are very expensive, and few countries can afford these agents. Contrastingly, glucocorticoids, such as methylprednisolone used in pulse therapy, are inexpensive. Despite the presence of randomized controlled trials on the efficacy of corticosteroids against ARDS [20], there are no reports on the efficacy of anti-IL-6 receptor antibodies and JAK inhibitors. Methylprednisolone pulse therapy exerts strong anti-inflammatory and anti-immunosuppressive actions by controlling numerous types of intracellular inflammatory pathways. Therefore, dexamethasone (6 mg daily) is considered inadequate to suppress cytokine storm, and methylprednisolone pulse therapy should be considered as one of the treatment options when stronger immunosuppression is required. To confirm this, a large, randomized, double-blind comparative study on methylprednisolone pulse therapy and dexamethasone therapy is required.
Author Contributions
Conceptualization, K.Y. and T.M.; methodology, K.Y., H.N. (Hisashi Noma), and T.M.; software, K.Y. and H.N. (Hisashi Noma); validation, K.Y., H.N. (Hisashi Noma), and T.M.; formal analysis, K.Y. and H.N. (Hisashi Noma); investigation, K.Y., N.T., N.I., J.S., S.M., S.I., Y.U., T.U., H.N. (Hideto Nakayama), and Y.H.; resources, T.M.; data curation, K.Y., H.N. (Hisashi Noma), and T.M.; writing—original draft preparation, K.Y. and T.M.; writing—review and editing, H.N. (Hisashi Noma), S.I., H.N. (Hideto Nakayama), and Y.H.; visualization, K.Y., H.N. (Hisashi Noma), and T.M.; supervision, T.M.; project administration, T.M.; funding acquisition, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted after approval by the Institutional Review Board (No. 20188.01) of Saitama Medical University Hospital.
Informed Consent Statement
Although we have not received informed consent from the subjects because of the retrospective study, we have disclosed the information of the study and guaranteed the opportunity for the study subjects to refuse. The information disclosure is on the Saitama Medical University Hospital Institutional Review Board website (URL: http://www.saitama-med.ac.jp/hospital/outline/irb.html).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, T.M., upon reasonable request.
Acknowledgments
We would like to thank the patients and their families for their patience and courage, and all the doctors and medical staff for their enormous efforts in providing medical care. We would also like to express our sincerest gratitude to Takashi Miyazaki and Midori Kamei (Social Medicine, Saitama Medical University, Saitama, Japan) for their contributions to the variable discussion.
Conflicts of Interest
The authors have no conflicts of interest (COI).
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Hayakawa, K.; Terada, M.; Ohtsu, H.; Asai, Y.; Tsuzuki, S.; Suzuki, S.; Toyoda, A.; Suzuki, K.; Endo, M.; et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: Report of the COVID-19 registry Japan. Clin. Infect. Dis. 2020, 73, e3677–e3689. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Canna, S.W.; Schulert, G.S.; Volpi, S.; Lee, P.Y.; Kernan, K.F.; Caricchio, R.; Mahmud, S.; Hazen, M.M.; Halyabar, O.; et al. On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020, 72, 1059–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef] [PubMed]
- REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N. Engl. J. Med 2021, 384, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Taboada, M.; Rodríguez, N.; Varela, P.M.; Rodríguez, M.T.; Abelleira, R.; González, A.; Casal, A.; Peromingo, J.A.D.; Lama, A.; Domínguez, M.J.; et al. Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 Pneumonia: An open-label, randomised clinical trial. Eur. Respir. J. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Granholm, A.; Munch, M.W.; Myatra, S.N.; Vijayaraghavan, B.K.T. Dexamethasone 12 mg versus 6 mg for patients with COVID-19 and severe hypoxaemia: A pre-planned, secondary Bayesian analysis of the COVID STEROID 2 trial. Intensive Care Med. 2022, 48, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kountz, S.L.; Cohn, R. Initial treatment of renal allografts with large intrarenal doses of immunosuppressive drugs. Lancet 1969, 1, 338–340. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockman, L.J.; Bellamy, R.; Garner, P. SARS: Systematic Review of Treatment Effects. PLoS Med. 2006, 3, e343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabi, Y.M.; Mandourah, Y.; Al-Hameed, F.; Sindi, A.A.; Almekhlafi, G.A.; Hussein, M.A.; Jose, J.; Pinto, R.; Kharaba, A.; Kharaba, A.; et al. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Ejima, K.; Iwanami, S.; Fujita, Y.; Ohashi, H.; Koizumi, Y.; Asai, Y.; Nakaoka, S.; Watashi, K.; Aihara, K.; et al. A quantitative model used to compare within-host SARS-CoV-2, MERS-CoV, and SARS-CoV dynamics provides insights into the pathogenesis and treatment of SARS-CoV-2. PLoS Biol. 2021, 19, e3001128. [Google Scholar] [CrossRef] [PubMed]
- Edalatifard, M.; Akhtari, M.; Salehi, M.; Naderi, Z.; Jamshidi, A.; Mostafaei, S.; Najafizadeh, S.R.; Farhadi, E.; Jalili, N.; Esfahani, M.; et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: Results from a randomised controlled clinical trial. Eur. Respir. J. 2020, 56, 2002808. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Irastorza, G.; Pijoan, J.-I.; Bereciartua, E.; Dunder, S.; Dominguez, J.; Garcia-Escudero, P.; Rodrigo, A.; Gomez-Carballo, C.; Varona, J.; Guio, L.; et al. Second week methyl-prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: An observational comparative study using routine care data. PLoS ONE 2020, 15, e0239401. [Google Scholar] [CrossRef] [PubMed]
- Dolci, G.; Cassone, G.; Venturelli, F.; Besutti, G.; Revelli, M.; Corsini, R.; Sampaolesi, F.; Pavone, P.; Contardi, G.; Riva, N.; et al. High-dose glucocorticoids pulse-therapy for beta-coronaviridae pneumonia: A systematic literature review and case-series of Coronavirus disease-2019. Clin. Exp. Rheumatol. 2021, 39, 1119–1125. [Google Scholar] [PubMed]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Sun, N.-N.; Gao, H.-N.; Chen, Z.-Y.; Yang, Y.; Ju, B.; Tang, L.-L. Risk factors analysis of COVID-19 patients with ARDS and prediction based on machine learning. Sci. Rep. 2021, 11, 2933. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Villar, J.; Ferrando, C.; Martínez, D.; Ambrós, A.; Muñoz, T.; Soler, J.A.; Aguilar, G.; Alba, F.; González-Higueras, E.; Conesa, L.A.; et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020, 8, 267–276. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).