Abstract
Introduction: Studies conducted in real-life scenarios on vaccine protection against COVID-19 constitute an important global priority, but one that is currently mostly neglected in low- and middle-income countries such as Angola. Here, we analyze for the first-time vaccine protection against COVID-19 in a real-life scenario after 6 months of implementing a multi-vaccination plan in Angola. Methods: 4232 vaccinated and unvaccinated individuals with the result of a rapid antigen diagnostic test against SARS-CoV-2 performed from 27 to 28 December 2021 were included in the study. The general and sex-adjusted and age-adjusted odds ratios were evaluated by comparing the chances of vaccination between cases and controls, and their associated 95% CI, which were calculated using the Mantel–Haenszel stratification method. Vaccine efficacy was calculated using the odds ratio applying the accepted statistical vaccine efficacy formula: (1 − odds ratio) × 100. For all estimates, a p-value < 0.05 was considered statistically significant. Results: The odds of SARS-CoV-2 infection were 0.85 (95% CI 0.70–1.03)-times lower in vaccinated compared to unvaccinated individuals, with p = 0.09. The overall vaccine efficacy (VE) was 15% (95% CI −3–30). Conclusion: There was no statistically significant decrease in the chances of SARS-CoV-2 infection in vaccinated versus unvaccinated individuals.
1. Introduction
On 2 March 2021, Angola began vaccinating the first people against COVID-19 upon receipt of the first batch of 624,000 doses of the AstraZeneca vaccine, recombinant ChAdOx1-S from the University of Oxford, an adenovirus vector vaccine, donated by COVID-19 Vaccines Global Access (COVAX) initiative [1]. The first published results on the efficacy of this vaccine against COVID-19, based on phase 3 trials, showed an efficacy of 72% (95% CI: 63–79%) against symptomatic SARS-CoV-2 infection, with its immediate use being strongly recommended by the World Health Organization (WHO, Geneva, Switzerland) with the aim of protecting against serious conditions and death, especially in low- and middle-income countries such as Angola, which may have a limited supply of vaccines [2]. The largest mass vaccination campaign in the country was then started on 6 March 2021 [3], almost a year after the notification of the first cases of COVID-19 in the country and at a time when the epidemic was in a phase of deceleration, and a cumulative total of 21,055 confirmed cases, 903 active cases and 512 deaths were recorded throughout the country, with a daily incidence rate of 0.05/100,000 inhabitants and a daily fatality rate of 6.25% [4]. The vaccination campaign has adopted two modalities, the first has to do with the construction of high-performance vaccination posts which are fixed vaccination posts in different locations and the constitution of mobile vaccination teams or brigades to reach locations with difficult access and where health resources are scarce or non-existent. At that time, Angola already had a cold chain made up of the National Vaccine Depot, located in the province of Luanda, in the capital of the country, with a temperature chamber +2 °C + 8 °C with 123 m3 of liquid capacity and a temperature chamber −15 °C at −25 °C of 70 m3 liquid. In addition, it also had capacity for ultra-cold storage at −70 °C with 10 chests of 780 L of storage capacity each and even production of dry ice for sending mRNA vaccines by private companies in Luanda. The dry storage capacity was about 900 m3. For the provincial level, 19 additional ultra-cold chests were strategically distributed in provincial capitals. At the provincial level, all 18 provinces have 7 m3 net cold storage chambers, except Benguela, which has 15 m3 net freezing and refrigeration chambers, and Cuando Cubango, which does not have chamber, due to its small population. At the national and provincial level, there is sufficient storage capacity for vaccines against COVID-19, and at the municipal level, it was planned to take advantage of the installed capacity of the Polio vaccination campaigns as well as the acquisition of additional refrigerators and freezers for all municipalities in accordance with the storage needs of each municipality. In addition, the well-established management strategy for the cold chain for vaccines against COVID-19 also aimed at the use of 20 L isothermal boxes and vaccine holders previously acquired for the Yellow Fever and Polio campaigns carried out in the country in addition to the acquisition of new equipment for vaccination against COVID-19. The IOTA Vaccine Logistics Management Digital Platform—provided by Logistimo—was also implemented, which allowed the management of cold chain temperatures, and real-time monitoring of the COVID-19 vaccine stock and vaccination material with the aim of guaranteeing the integrity of COVID-19 vaccines and vaccination material throughout the supply chain, as well as access to tools for real-time monitoring of the entire logistics and vaccination process. Likewise, health technicians—such as logistics staff and vaccinators who had already worked in previous vaccination campaigns carried out in the country, as well as new middle and senior technicians recruited voluntarily—were trained and trained with the support of senior professionals from international organizations such as The Global Alliance for Vaccines and Immunization (GAVI) and The United Nations Children’s Fund (UNICEF) in partnership with the Expanded National Vaccination Program, with programmatic materials on vaccination against COVID-19 developed by the WHO and adopted by the National Directorate of Public Health. The first phase of the National Vaccination Plan against COVID-19 provided for the vaccination of 95% of people ≥40 years of age based on priority groups. Almost a year after the notification of the first cases of COVID-19 in the country and at a time when the epidemic was in a deceleration phase with a cumulative total of 21,055 confirmed cases, 903 active cases, and 512 deaths with a daily incidence rate of 0.05/100,000 population and a daily fatality rate of 6.25% [5].
On 13 March 2021, the first 40,000 doses of the Russian Sputnik V vaccine [6] arrived in the country, a recombinant heterologous vaccine based on adenovirus vectors (rAd) developed by the Research Center for Epidemiology and Microbiology–Gamaleya [7]. The primary results reported based on phase 3 clinical trials on the efficacy of Sputnik V were 91.6% (95% CI 85.6–95.2) [8], much higher than Oxford/AstraZeneca with similar technology.
On 25 March 2021, with the country entering the ‘second wave’ and a massive vaccination campaign having been launched 19 days prior—with the AstraZeneca and Sputnik V vaccines being administered to the target population of the National Vaccination Program, with demonstrated efficacy above 70% and approved by the WHO emergency plan [9] another 200,000 doses were delivered of the People’s Republic of China vaccine, Sinopharm. Sinopharm is produced by the Beijing Bio-Institute of Biological Products Co Ltd. (Beijing, China), a subsidiary of the China National Biotec Group (CNBG, Beijing, China), being an inactivated virus vaccine [10]. This vaccine has been reported to be 79% effective in individuals with symptomatic disease and those who were hospitalized in all age groups combined according to the WHO [9].
On 1 July 2021, the country received 100,620 doses of the first vaccine produced with mRNA technology, BNT162b2 from Pfizer–BioNTech [11]. This vaccine reached 91% efficacy (95% CI 89–93%) in phase 3 studies 7 days after administration of the second dose against symptomatic SARS-CoV-2 infection with the ancestral strain in people aged 16 years or longer, based on an average follow-up of two months [12].
On 8 August 2021, in the phase that marked the deceleration of the ‘second wave’ and the beginning of the ‘third wave’ of the epidemic in Angola—with 1,690,719 doses of the four vaccines having already been administered by using Oxford/AstraZeneca, Sputnik V, Sinopharm, and Pfizer—165,000 doses of the first single-dose regimen vaccine were deployed from the pharmaceutical company Janssen [13]. Janssen’s Ad26.COV2.S vaccine against COVID-19 is a recombinant, incompetent adenovirus vector for replication, serotype 26 (Ad26) encoding a full-length, stabilized SARS-CoV-2 spike protein [14].
On 1 October 2021, 209 days (more than 7 months) had elapsed since the start of the mass vaccination campaign against COVID-19, and more than 3,000,000 doses of the Oxford/AstraZeneca and Sputnik V vaccines had been administered. Sinopharm, Pfizer, and Jansen had been administered to a total population of 15,765,837; with 874,719 doses corresponding to the group of subjects in the two-dose vaccine regimen who were considered immunized and 167,755 doses corresponding to the group of subjects in the single-dose vaccine regimen being considered immunized, this gave us an ‘effective’ vaccination coverage of 6.60%, very low compared to the world average and still far from the 50% needed to be able to provide general protection to the population and counter the evolution of the epidemic. This is remarkable because at this point—on 24 December 2021, with the country experiencing the ‘fourth wave’ of the epidemic which had become more aggressive since the beginning of the epidemic—since the end of the first half of December, the country’s health authorities confirmed community circulation of the most transmissible variant of the SARS-CoV-2 virus identified since the beginning of the epidemic, Omicron. It was identified primarily in South Africa and also attributed to the emergence of a respiratory virus that predominated in the country’s capital, characterized mainly by sore throat, headache, and nasal obstruction in individuals of all age groups [15,16]. Vaccination coverage at this time was 24.88% given by the Oxford/AstraZeneca, Sputnik V, Sinopharm, Pfizer, and Jansen vaccines.
The emergence of this new variant identified in the country and associated with the high transmissibility that triggered the ‘fourth wave’ led the country’s health authorities to carry out a massive testing campaign in various parts of the country’s capital in order to assess the epidemiological situation [17] in a period when vaccine coverage was above 29%, but still insufficient to counteract a rapid growth of the epidemic that was marked with one of the highest daily incidence rates achieved since the beginning of the epidemic in the country of 6.06/100,000 people.
Justification
A review study on the “Evaluation of Protection by COVID-19 Vaccines after Deployment in Low- and Middle-Income Countries” [18] published in late December last year identified 58 published studies that included 85 evaluations of the effectiveness of different COVID-19 vaccines worldwide. In this study, only three had been carried out in low- and middle-income countries, and no impact studies were identified in these settings [18]. Furthermore, in March 2021, The World Health Organization (WHO) issued guidelines for the design and implementation of non-randomized vaccine protection efficacy studies for vaccines used in low- and middle-income countries on the basis that there is a divergence in vaccine protection that can occur when vaccines are deployed in public health practice compared to measurement in individually randomized, designed, phase 3 efficacy trials for licensing; because such trials do not take into account all the pertinent vaccine protection issues encountered in a real-life public health setting [19]. Therefore, studies conducted in real-life scenarios on vaccine protection against COVID-19 constitute an important global priority, are mostly neglected in low- and middle-income countries such as Angola.
Here, we analyze for the first-time vaccine protection against COVID-19 in a real-life scenario after 6 months of implementing a multi-vaccination plan in Angola. We provide an estimation of odds ratios and vaccine efficacy in a period that coincided with the identification of the Omicron variant in the country, assuming that there is no decreased risk of SARS-CoV-2 infections in vaccinated individuals compared to unvaccinated individuals. The results of this study will provide the first scientific evidence on the effectiveness and impact of vaccination against COVID-19 in Angola and will allow for the adoption of measures aimed at our reality, which is quite different from the reality of countries where most studies address vaccine protection.
2. Objectives
2.1. Study Main Objective
- To estimate the odds ratio of SARS-CoV-2 infection in vaccinated versus unvaccinated individuals.
2.2. Specifics Objectives
- Characterize the participants according to age group, sex, vaccination status, and rapid diagnostic test result;
- To estimate the odds ratio of SARS-CoV-2 infection in vaccinated versus unvaccinated individuals by sex and age group;
- To determine the overall vaccine efficacy (VE) by sex and age groups against SARS-CoV-2 infection.
3. Material and Methods
3.1. Study Design
We used a negative test case-control design to assess the effectiveness of vaccination against confirmed SARS-CoV-2 infection. This is a widely accepted design to determine vaccine efficacy in a population after a vaccine has been introduced [20].
3.2. Data Source, Population, and Period of Analysis
We extracted from the Ministry of Health’s Registo Digital Individual de Vacinação-Rediv (Individual Digital Vaccination Record) platform a total of 4232 records of individuals registered with rapid antigen diagnostic (RAD) test carried out on 27 and 28 December 2021. The Rediv platform is a dynamic and management platform for the digital pre-registration of users, the digital vaccination record, and the management of the logistics chain of vaccination against COVID-19. Rediv was designed based on an evolved and innovative architecture, and it foresees organic growth in order to meet other needs of the National Health System in the future. Based on this, it was also used to carry out the RAD test registration carried out in community testing campaigns.
3.3. Inclusion Criteria
All individuals who joined the mass testing campaign against COVID-19 in Luanda on 27 and 28 December 2021 were included. The mass testing campaign was carried out in points of high population concentration in the country’s capital from downtown, in Mutamba, an urban area where large multinational companies such as Sonangol (the largest state oil company in Angola, Luanda and several ministerial departments such as the Ministry of Finance and the provincial government of Luanda where a testing point was placed to serve the population of this locality which seems to be mostly middle class and high income. Other testing points were placed in informal markets, 30 markets located in the rural areas of the municipality of Viana, and Catinton market located in the district of Maianga. These two informal markets are large population clusters frequented mostly by low- and middle-income adult women who carry out their commercial activity, which is mainly focused on the sale of agricultural products. This strategy with the placement of several testing points from the urban area to the rural area of the city of Luanda made it possible to reduce the selection bias of specific population groups, such as individuals with higher educational and socioeconomic levels—and consequently with greater habitual demand for health care—residing predominantly in urban areas where there is also greater availability of health resources. Therefore, this reduced the asymmetries of the population studied that joined the mass testing campaign at random.
3.4. Exclusion Criteria
Individuals with more than one RAD test result performed in the period of analysis.
3.5. Ethical Aspects
The project was approved by the Ministry of Health of Angola (MINSA) Research Ethics Committee (opinion no. 26 of 4 August 2022).
3.6. Statistical Analysis
Descriptive statistics (frequency distributions and measures of central tendency) were used to characterize the universe of the study. The overall and adjusted odds ratios were calculated in a contingency table considering the vaccination exposure factor and the result of the RAD test to define cases and controls, adjusting for stratification by sex and age group using the Mantel–Haenszel method. The risk classification of Axel Kroeger, Piscoya, and Alarcon were used to interpret the odds ratio, considerable protection (OR 0–0.3), moderate protection (OR 0.4–0.5), negligible protection (OR 0.6–0.8), no effect (OR 0.9–1.1), negligible risk (OR 1.2–1.6), moderate risk (OR 1.7–2.5), and high risk (OR > 2.6) [21]. Vaccine efficacy was calculated using the odds ratio result by applying the statistical vaccine efficacy formula: (1 − odds ratio) × 100. This study interpreted the vaccine protective efficacy point and the respective 95% CI. We included negative estimates such as zero effectiveness. For all estimates, a value of p < 0.05 was considered statistically significant. To control for potential for repeat testing bias in RAD, positive individuals were tested in order to verify infection or repeat testing bias among controls (persons with a higher level of health care seeking behavior who are presumably at lower risk of infection). For each case, we considered the first positive RAD test during the analysis period from 27 December 2021 to 28 December 2021. We considered the first negative RAD test for each control during this period. This generated an independent universe of unique cases and controls. Statistical analyses were performed using version 7.5.2.0 of the CDC’s (Centers for Disease Control and Prevention, Atlanta, GA, USA) Epi Info.
3.7. Operational Definitions
Case: Every individual with confirmed SARS-CoV-2 infection through a positive rapid antigen diagnostic (RAD) test result.
Control: Any individual without SARS-CoV-2 infection confirmed by a negative rapid antigen diagnostic (RAD) test result.
Vaccination status: It was considered as an exposure factor, with individuals who received a vaccine against SARS-CoV-2 being classified as vaccinated (exposed) and those who did not receive any vaccine against SARS-CoV-2 as unvaccinated (unexposed).
Vaccine efficacy (VE): It represents the proportion of reduction in cases or infections by SARS-CoV-2 among the vaccinated group, compared to the unvaccinated group [22].
4. Results
Figure 1 shows the age pyramid of the population studied and Figure 2 shows the characteristics of the general Angolan population according to the latest data from the 2014 census. The studied population consisted of 1540 (63.63%) males and 2693 (36.37%) females. This distribution does not follow the distribution of the general population, which is mostly made up of women, as can be seen in Figure 2. The mean age of the population studied was 36 years with a standard deviation (SD) of 13. However, the age structure of the general population—distributed in Figure 2 in five-year intervals—is characterized by a young population, with the population of 0–14 years of 121,964.96 people representing 47% of the total resident population. The working age population (population aged 15 to 64) is 12,980,098 people, representing 50% of the country’s population. Meanwhile, the population aged 65 and over is only 612,430 people (2% of the country’s population).
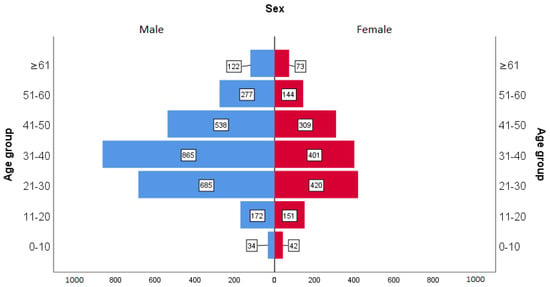
Figure 1.
Age pyramid of the population studied.
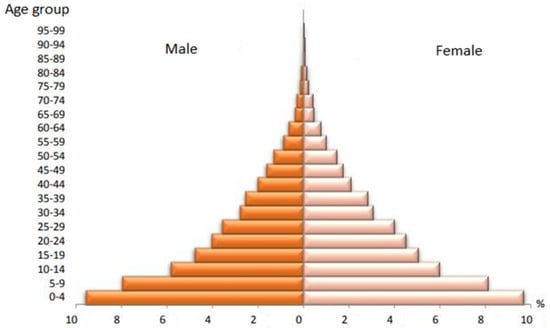
Figure 2.
Age pyramid of the general population. Source: https://www.ine.gov.ao/ (accessed on 16 December 2021).
Table 1 shows the distribution of individuals according to vaccine status against COVID-19 and the distribution of individuals according to the result of the rapid antigen diagnostic test against COVID-19. The sample consisted of 708 (16.73%) unvaccinated individuals and 3524 (83.27%) vaccinated individuals, of which 3309 (78.19%) individuals displayed a negative result and 923 (21.81%) individuals displayed a positive result.

Table 1.
Vaccination status and rapid antigen diagnostic (RAD) test result.
Table 2 shows the overall odds ratio (OR), by sex and age group, of SARS-CoV-2 infection in individuals vaccinated against COVID-19 versus unvaccinated and vaccine efficacy. The chances of SARS-CoV-2 infection is 0.85-times lower in vaccinated compared to unvaccinated individuals, with (95% CI 0.70–1.03) and p-value = 0.09. The overall vaccine efficacy (VE) was 15% (95% CI −3–30).

Table 2.
Estimate of the odds ratio of SARS-CoV-2 infection in vaccinated individuals and vaccine efficacy, overall, by sex and age group.
The chances of SARS-CoV-2 infection is 1.25-times higher in vaccinated females compared to unvaccinated females, with (95% CI 0.90–1.75) and p-value = 0.18. The resulting VE in this group was −25% (95% CI −75–10).
The chances of SARS-CoV-2 infection is 0.68-times lower in male vaccinated individuals compared to unvaccinated males of the same sex, with (95% CI 0.54–0.86) and p-value = 0.00. The resulting VE in this group was 32% (95% CI 14–46).
The chances of SARS-CoV-2 infection in the 0–10 year age group have not been determined because the number of exposed (vaccinated) individuals is zero.
The chances of infection by SARS-CoV-2 are 0.63-times lower in vaccinated individuals aged 11–20 years compared to unvaccinated individuals in the same age group, with (95% CI 0.38–1.05) and p-value = 0.08. The resulting VE in this age group was 37% (95% CI −5–62).
The chances of infection by SARS-CoV-2 are 1.11-times higher in vaccinated individuals aged 21–30 years compared to unvaccinated individuals in the same age group, with (95% CI 0.72–1.73) and p-value = 0.61. The resulting VE in this age group was −10% (95% CI −73–28). The chances of infection by SARS-CoV-2 are 1.71-times higher in vaccinated individuals aged 31–40 years compared to unvaccinated individuals in the same age group, with (95% CI 1.05 –2.78) and p-value = 0.02. The resulting VE in this age group was −71% (95% CI −178–−5). The chances of infection by SARS-CoV-2 are 0.75-times lower in vaccinated individuals aged 41–50 years compared to unvaccinated individuals in the same age group, with (95% CI 0.43–1.31) and p-value = 0.32. The resulting VE in this age group was 25% (95% CI −31–57). The chances of SARS-CoV-2 infection are 1.18-times higher in vaccinated individuals aged 51–60 years compared to unvaccinated individuals in the same age group, with (95% CI 0.54–2.55) and p-value = 0.67. The resulting VE in this age group was −18% (95% CI −155–46). The chances of SARS-CoV-2 infection is 0.41-times lower in vaccinated individuals in the age group of ≥61 years in relation to unvaccinated individuals in the same age group, with (95% CI 0.18–0.91) and p-value = 0.02. The resulting VE in this age group was 52% (95% CI 9–82).
Table 3 shows the determination of the adjusted odds ratio (OR) stratified by age group and sex with confidence intervals and p-value of the risk of infection by SARS-CoV-2 in individuals vaccinated against COVID-19. The chances of infection by SARS-CoV-2 are 0.34-times lower (95% CI 0.18–0.91) in male vaccinated individuals aged 41–50 years, with p = 0.00 and 8.63-times higher (95% CI 1.15–64.62) in vaccinated women of the same age group with p = 0.01.

Table 3.
Stratification by age group and sex of vaccine efficacy (VE) and estimate of the odds ratio (OR) of SARS-CoV-2 infection in vaccinated individuals.
5. Discussion
For the first time, we present results that allow the assessment of vaccine protection against COVID-19 in a real-life scenario by estimating the odds ratio and overall vaccine efficacy adjusted for sex and age groups.
The overall odds ratio of SARS-CoV-2 infection from among the 4,232 individuals who took part in this analysis was estimated at 0.85 (95% CI 0.70–1.03). According to the Axelodds ratio risk classification [21], this means that there is a protective effect of the vaccine, although this protection is negligible and therefore not statistically significant if we look at the confidence interval values for the odds ratio and for the value of p = 0.09. The estimated overall vaccine efficacy (VE) was 15%. Remember here that the minimum efficacy recommended by the WHO for an anti-SARS-CoV-2 vaccine is 50% in phase 3 clinical trials [23]. All vaccines used in the COVID-19 Vaccination Program in Angola had reported efficacy in phase 3 clinical trials superior to that recommended by the WHO [2,8,10,12,14]. The difference found in the general VE of our study with those published previously may be due to the fact that the phase 3 clinical trials in which the VE used in Angola was determined did not consider relevant factors present in the real world, such as the expansion of the range of recipients of vaccines beyond those eligible for testing initially, administration of vaccine with incomplete or mixed regimens, variation of dosing intervals, possibility of storage and incorrect administration of vaccines, concomitant administration with other drugs by vaccinated subjects that are not permitted for participants in phase 3 trial, the decline in vaccine immunity over time, and the emergence of new genetic variants of SARS-CoV-2 with protein targets not initially considered in the manufacture of currently available vaccines such as Omicron [18]. This last question seems to us to be the most relevant. The latest UK Health Safety Agency report on “SARS-CoV-2 Variants of Concern and Variants under Investigation in England” found an EV similar to that found in our study in vaccinated individuals of 13.0% (95% CI 11–15) where it evaluated the effectiveness of vaccination by comparing symptomatic individuals >18 years old infected with the Omicron and Delta variant in the period from 27 November 2021 to 6 January 2022 [24]. Furthermore, Jara et al. [25] in contrast reported that several studies [26,27,28,29] conducted in a real-life setting found a higher VE than that found in our study. It is also necessary here to emphasize the need for some consideration in the comparison of these results with ours, since in this analysis we did not consider several issues such as VE by type of vaccine, clinical status (asymptomatic vs. symptomatic), dominant variants, number of doses taken, and time elapsed since taking the first or second dose which were factors considered in some of the cited studies.
As for the stratified analysis of the odds ratio by sex, we found that there is a significant difference between the sexes. The odds of SARS-CoV-2 infection in male vaccinated individuals was 0.68 (95% CI 0.54–0.86)-times lower compared to unvaccinated male individuals. According to Axel’s odds ratio risk rating, this means that there is a ‘negligible’ protective effect of vaccination in men and this protection is statistically significant (p = 0.00). The same was not seen in women where the odds of SARS-CoV-2 infection in vaccinated were 1.25 (95% CI 0.90–1.75)-times higher than in unvaccinated. According to Axel’s odds ratio risk rating, this means that there is a ‘negligible’ risk of vaccination in women, although this is not statistically significant (p = 0.18). Thus, the VE in men was 32% (95% CI 14–46) and −25% in women (95% CI −75–10). In the published and unpublished literature, we did not encounter findings similar to ours and no reason to explain the difference in VE between the sexes. We postulate that, other non-biological factors—such as poor adherence to individual protection measures such as mask use, and greater exposure to the virus in places of large population areas such as informal markets frequented mostly by women—may explain the greater risk found in vaccinated women compared to men whose work activity is more focused on institutional environments where compliance with individual protection measures is mandatory, which is a synergistic measure for higher levels of vaccine protection seen in men. However, other host-related biological factors—such as the post-vaccination antibody production response, duration and type of vaccine, and vaccine type; as well as factors related to the viral agent such as the presence of genetic variants more transmissible in one sex—should be considered in further studies that are justified to clarify this difference found.
Stratification by age group also revealed differences in the odds ratio in the different groups. The odds of SARS-CoV-2 infection were 0.63 (95% CI 0.38–1.05)-times lower in vaccinated individuals aged 11–20 years compared to unvaccinated individuals in the same age group, although the value of p = 0.08 indicates that there is no statistical significance for this ‘negligible’ protection according to the Axel risk classification [21]. The VE in this age group was 37% (CI 95% −5–62). On the other hand, the chances of infection by SARS-CoV-2 in the following age group of 21–30 years old showed no difference between vaccinated and unvaccinated with OR = 1.11 (95% CI 0.72–1.73) and p-value = 0.61 confirming that there is no effect of vaccination in this group according to Axel’s risk classification. The VE in this age group was −10% (95% CI −73–28). The risk of infection by SARS-CoV-2 becomes moderate according to the Axel risk classification [21] in individuals aged 31–40, being 1.71 (95% CI 1.05–2.78)-times higher in vaccinated individuals compared to unvaccinated individuals and statistically significant for p = 0.02. The VE in this age group was −71% (95% CI −178–−5). In the 41–50 age group, the chances of SARS-CoV-2 infection were 0.75 (95% CI 0.43–1.31)-times lower in vaccinated individuals compared to unvaccinated individuals of the same age group, however this ‘negligible’ protection according to Axel’s risk classification, it was not statistically significant for p = 0.32. The VE in this age group was 25% (CI 95% −31–57). The odds of SARS-CoV-2 infection were 1.18 (95% CI 0.54–2.55)-times higher in vaccinated individuals aged 51–60 years compared to unvaccinated individuals in the same age group, however this negligible risk was not statistically significant with p = 0.67. The VE in this age group was −18% (95% CI −155–46). The last age group showed that there is 0.41 (95% CI 0.18–0.91)-times lower chance of SARS-CoV-2 infection in vaccinated elderly compared to unvaccinated elderly in the same age group, this difference being statistically significant with p-value = 0.02. According to the Axel risk score [21], there was moderate protection conferred by vaccination in this age group with a VE of 59% (95% CI 9–46). There is a possible reason for better VE seen among the elderly. According to the Axel risk score [21], there was moderate protection conferred by vaccination in this age group with an VE of 59% (95% CI 9–46). A possible reason for better VE seen in the age group of ≥61 years and not seen in the lower age groups may be because this group has developed lasting immunity as this is a risk group that was prioritized at the beginning of the vaccination campaign, supporting the fact that the protective effect of vaccine-induced immunity is also supported by long-term components of the humoral response, including memory B cells, as is the response to infection.
Adjusting the odds ratio and VE in each age group by sex, we found that men had a lower risk and, consequently, a higher VE compared to women in all age groups. A protective effect was seen in all age groups for men except the 31–40 age group where the odds of SARS-CoV-2 infection was 1.49 (95% CI 0.84–2.65)-times higher in vaccinated men compared to unvaccinated men. Protective effect was only seen in women aged 11–20 and in women ≥61 years of age, although the negligible protective effect was not statistically significant in both age groups for women. The resulting VE in these age groups was 29% (95% CI −50–66) and 34% (95% CI −141–82) respectively. On the other hand, in men in the range ages 41–50 and ≥61 years old, there was a statistically significant protective effect with VE of 66% (95% CI 37–82) and 73% (95% CI 25–90) respectively. Protection in these age groups was moderate, different from the ‘negligible’ efficacy seen in men of other strata. Surprisingly, we cannot fail to point out here that vaccinated women aged 41–50 years had an odds ratio of SARS-CoV-2 infection of 8.63 (95% CI 1.15–64.62)-times greater than unvaccinated women of the same age group, this risk according to the Axel classification [21] considered highwas statistically significant for p = 0.01. Why did vaccination have a better effect on men than on women in virtually all age groups? For now, the best explanation we can find for such differences is limited to non-biological or environmental factors or also socio-cultural factors with possible antagonistic effects on the effectiveness of vaccination seen in women who most frequent places in population clusters such as informal markets where there is greater viral exposure. However, there may be a combination of factors also linked to the viral agent, because 81.94% (1262) of the women of our sample were vaccinated and—despite this—increased risk was verified in practically all age groups except the age groups from 11–20 and from ≥61. This leads us to think that this risk in women may be an epidemiological translation of the presence of viral genetic variants with vaccine evasion capacity, as supported by a study recently published by researchers from the University of New South Wales, Australia, who mapped an infection rate of the Omicron variant compared to the old Delta variant—Omicron was 10-times higher in overcoming protections of vaccines against COVID-19 and acquired resistance [30].
There were no vaccinated individuals in the 0–10 age group, which is why the odds ratio in this age group was not estimated because Angola—as of the date that this analysis was made—had not yet included this age group in its vaccination program against COVID-19.
6. Conclusions
There was no statistically significant decrease in the odds of SARS-CoV-2 infection in vaccinated versus unvaccinated individuals. The sex-adjusted and VE-adjusted risk decreases were statistically significant only in men. The age-adjusted risk revealed a statistically significant decrease in the risk of SARS-CoV-2 infection only in vaccinated individuals aged 61 years and over and who had a VE greater than 50%. In the adjusted risk for age group and sex, there was a statistically significant decrease in the risk of infection by SARS-CoV-2 only in men aged 41–50 and older than or equal to 61 years of age and VE greater than 60%. Vaccinated women aged 41–50 years had a risk of infection with SARS-CoV-2 that is considered high and VE demonstrated no protective effect.
7. Limitations
This study has some limitations: first, as an observational study, it is subject to confounding. We do not provide estimates of effect (odds ratio) or vaccine efficacy (VE) in vaccinated individuals taking into account the different types of vaccine, number of doses given, or the time elapsed since the administration of the vaccine who may also be exerting a residual confounding effect. However, all of these aspects will be considered in a complementary analysis
Second, as the selection test for cases and controls used was a rapid antigen diagnostic test and there was no laboratory confirmation of the results by gold standard testing—in this case the RT-PCR—we consider that there may have been a risk of classification bias cases and controls due to the possibility of false positives (considered in the case group) and false negatives (considered in the control group). As the accuracy of the RAD test is also highly dependent on the execution according to the manufacturer’s guidelines, another possible source of bias may be related to the level of training of the technicians who performed and interpreted the RAD test results following or not following the manufacturer’s instructions.
Third, at the time of writing this paper, a preprint published by researchers at the University of New South Wales, Australia found that the Omicron variant had a 10-times greater ability to evade COVID-19 vaccines compared to Delta. Although the national genomic surveillance for SARS-CoV-2 in Angola has reported the circulation of the Alpha, Gamma, Delta, and Omicron variants, we do not have data to estimate their effect on vaccine efficacy because these data are not obtained from the positive results during the performance of routine RT-PCR testing in Angola as there are no resources to perform genotyping in-house, and antigen tests do not provide this information.
Fourth, estimates of vaccine efficacy (VE) were obtained under specific epidemiological and vaccine conditions, and therefore we assume here that they can clearly vary over time and across these conditions.
Author Contributions
Conceptualization, S.S.A.d.L.; Data curation, S.S.A.d.L.; Formal analysis, C.A.P.d.S.; Investigation, S.S.A.d.L.; Methodology, S.S.A.d.L.; Project administration, S.S.A.d.L.; Supervision, B.d.S. and C.A.P.d.S.; Validation, B.d.S. and C.A.P.d.S.; Visualization, C.A.P.d.S.; Writing—original draft, B.d.S.; Writing—review & editing, S.S.A.d.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ministry of Health of Angola (MINSA) Research Ethics Committee (opinion no. 26 of 4 August 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank all health professionals and entities involved in carrying out this work, especially Helga Reis Freitas from the National Directorate of Public Health, Luanda, Angola, and Engineers José Moura and Fabio Lima from Equilibrium SA, Luanda, Angola who provided access to data available on the Rediv platform of the Ministry of Health of Angola and to all Digital Health staff at Equilibrium SA, Luanda, Angola who assisted in the processing of data; the Rectory and Board of the Faculdade de Medicina da Universidade Agostinho Neto, Luanda, Angola, and the Private University of Angola—UPRA, Luanda, Angola, for the additional financial support for the Article Processing Charges.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| % | Percentage |
| VE | Vaccine efficacy |
| CI | Confidence interval |
| COVAX | COVID-19 Vaccines Global Access |
| GAVI | The Global Alliance for Vaccines and Immunization |
| OR | Odds ratio |
| WHO | World Health Organization |
| p | Probability |
| RT-PCR | Reverse transcription polymerase chain reaction |
| RAD | Rapid antigen diagnostic |
| Rediv | Registo Digital Individual de Vacinação (Individual Digital Vaccination Record) |
| UNICEF | The United Nations Children’s Fund |
References
- Radio and Television of Portugal—RTP Angola Opens Vaccine Warehouse and Starts Vaccination Campaign. Angola Opens Vaccine Warehouse and Starts Vaccination Campaign. 2021. Available online: https://www.rtp.pt/noticias/mundo/angola-inaugura-vaccine-deposit-and-start-up-with-vaccination-campaign_n1301332 (accessed on 25 January 2022).
- World Health Organization. Interim Recommendations for Use of the ChAdOx1-S [Recombinant] Vaccine against COVID-19 (AstraZeneca COVID-19 Vaccine AZD1222 VaxzevriaTM, SII COVISHIELDTM): Interim Guidance, First Issued 10 February 2021, Updated 21 April 2021, Last Updated 30 July 2021 (WHO/2019-nCoV/Vaccines/SAGE_recommendation/AZD1222/2021.3); World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/handle/10665/343388 (accessed on 26 January 2022).
- News from Angola. COVID-19: Mass Vaccination Campaign Begins in This Saturday. News in Angola. Available online: https://noticiasdeangola.co.ao/covid-19-campanha-vacinacao-massa-comeca-neste-sabado/ (accessed on 26 January 2022).
- World Health Organization. Weekly Epidemiological Update—9 March 2021. 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update10-march-2021 (accessed on 26 January 2022).
- World Health Organization. Weekly Epidemiological Update—30 March 2021. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update---31-march-2021 (accessed on 26 January 2022).
- Angola Newspaper. Jornal de Angola—News—Sputnik Vaccine Arrives to Angola; Angola Newspaper: Luanda, Angola, 2021; Available online: https://www.jornaldeangola.ao (accessed on 26 January 2022).
- Gamaleya. НИЦЭМ им. Н. Ф. Гамалеи. 2021. Available online: https://www.gamaleya.org/ (accessed on 26 January 2022).
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomized controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- World Health Organization. WHO Lists Additional COVID-19 Vaccine for Emergency Use and Issues Interim Policy Recommendations. 2021. Available online: https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations (accessed on 26 January 2022).
- World Health Organization. Background Document on the Inactivated COVID-19 Vaccine BIBP Developed by China National Biotec Group (CNBG), Sinopharm: Background Document to the WHO Interim Recommendations for Use of the Inactivated COVID-19 Vaccine BIBP Developed by China National Biotec Group (CNBG), Sinopharm, 7 May 2021 (WHO/2019-nCoV/Vaccines/SAGE_recommendation/BIBP/Background/2021.1); World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/handle/10665/341252 (accessed on 26 January 2022).
- Embassy of the Republic of Angola in Portugal. Embassy of the Republic of Angola in Portugal—Angola Receives More Than 100,000 Doses of Vaccines from Pfizer. Embassy of the Republic of Angola in Portugal. Available online: http://www.embaixadadeangola.pt/angola-recebe-mais-de-100-mil-vaccine-dose-from-pfizer/ (accessed on 26 January 2022).
- World Health Organization. Interim Recommendations for Use of the Pfizer–BioNTech COVID-19 Vaccine, BNT162b2, under Emergency Use Listing: Interim Guidance, First Issued 8 January 2021, Updated 15 June 2021, Updated 19 November 2021, Updated 21 January 2022 (WHO/2019-nCoV/Vaccines/SAGE_recommendation/BNT162b2/2022.1); World Health Organization: Geneva, Switzerland, 2022; Available online: https://apps.who.int/iris/handle/10665/351139 (accessed on 26 January 2022).
- Lusa, A. COVID-19. Angola Received 165,000 Doses of Janssen Vaccines. Observer. 2021. Available online: https://observador.pt/2021/08/08/covid-19-angola-recebeu-165-thousand-dose-of-vaccines-janssen/ (accessed on 26 January 2022).
- World Health Organization. Interim Recommendations for the Use of the Janssen Ad26.COV2.S (COVID-19) Vaccine: Interim Guidance, 17 March 2021 (WHO/2019-nCoV/Vaccines/SAGE_recommendation/Ad26.COV2.S/2021.1); World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/handle/10665/340203 (accessed on 26 January 2022).
- AngoRussia. National Director of Public Health Warns That Outbreak in the Flu He Can to Be Signal in COVID-19. Available online: https://angorussia.com/noticias/directora-nacional-de-saude-publica-adverte-que-surto-de-gripe-pode-ser-sinal-de-covid-19/ (accessed on 27 January 2022).
- Angola Newspaper. Jornal de Angola—Notícias—Angola Already Has Community Circulation of the Omicron Variant; Angola Newspaper: Luanda, Angola, 2021. [Google Scholar]
- RFI. Omicron Forces Mass Testing of the Angolan Population. RFI. Available online: https://www.rfi.fr/pt/angola/20211227-%C3%B3micron-obriga-the-mass-testing-%C3%A0-population%C3%A7%C3%A3o-Angolan (accessed on 27 December 2021).
- Clemens, J.; Aziz, A.B.; Tadesse, B.T.; Kang, S.; Marks, F.; Kim, J. Evaluation of protection by COVID-19 vaccines after deployment in low and lower-middle income countries. EClinicalMedicine 2022, 43, 101253. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (Regional Office for Europe). Estimating COVID-19 Vaccine Effectiveness against Severe Acute Respiratory Infections (SARI) Hospitalisations Associated with Laboratory-Confirmed SARS-CoV-2: An Evaluation Using the Test-Negative Design: Guidance Document; Report No.: WHO/EURO: 2021-2481-42237-58308; World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/handle/10665/341111 (accessed on 26 January 2022).
- Vandenbroucke, J.P.; Brickley, E.B.; Vandenbroucke-Grauls, C.M.J.E.; Pearce, N. A Test-Negative Design with Additional Population Controls Can Be Used to Rapidly Study Causes of the SARS-CoV-2 Epidemic. Epidemiology 2020, 31, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Silva, B. Estatística para Ciências Humanas, 3rd ed; Gráfica Lito-Tipo: Luanda, Angola, 2006; 179p, ISBN 97897729202445. [Google Scholar]
- Tavares, C. Understand How the Effectiveness of a Vaccine Is Measured—Fiotec. 2021. Available online: https://www.fiotec.fiocruz.br/noticias/outros/7353-entenda-como-e-medida-a-effectiveness-of-a-vaccine (accessed on 15 March 2022).
- UOL. Vaccine with 50% Effectiveness Could Be Approved, Admits Chief Scientist Gives WHO. 2021. Available online: https://www.uol.com.br/vivabem/noticias/redacao/2020/09/21/vacina-com-50-de-efficacy-can-be-approved-admits-who-chief-scientist.htm (accessed on 27 January 2022).
- UKHSA. SARS-CoV-2 Variants of Concern and Variants under Investigation in England: Technical Briefing 34. 2022. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050236/technical-briefing-34-14-january-2022.pdf (accessed on 18 February 2022).
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, B.A.; Gerlovin, H.; Madenci, A.L.; Kurgansky, K.E.; Ferolito, B.R.; Figueroa Muñiz, M.J.; Gagnon, D.R.; Gaziano, J.M.; Cho, K.; Casas, J.P.; et al. Comparative Effectiveness of BNT162b2 and mRNA-1273 Vaccines in US Veterans. N. Engl. J. Med. 2022, 386, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Taylor, D.; Purver, M.; Chapman, D.; Fowler, T.; Pouwels, K.B.; Walker, A.S.; Peto, T.E.A. Effect of Covid-19 Vaccination on Transmission of Alpha and Delta Variants. N. Engl. J. Med. 2022, 386, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Young-Xu, Y.; Korves, C.; Roberts, J.; Powell, E.I.; Zwain, G.M.; Smith, J.; Izurieta, H.S. Coverage and Estimated Effectiveness of mRNA COVID-19 Vaccines among US Veterans. JAMA Netw. Open 2021, 4, e2128391. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Stella, A.O.; Walker, G.; Akerman, A.; Milogiannakis, V.; Brilot, F.; Amatayakul-Chantler, S.; Roth, N.; Coppola, G.; Schofield, P.; et al. SARS-CoV-2 Omicron: Evasion of Potent Humoral Responses and Resistance to Clinical Immunotherapeutics Relative to Viral Variants of Concern. 2022, 16. Available online: https://kirby.unsw.edu.au/sites/default/files/kirby/news/Pre-Print_Omicronevasion%20of%20potent%20humoral%20responses%20%26%20resistance%20to%20clinical%20immunotherapeutics%20relative%20to%20viral%20variants%20of%20concern.pdf/ (accessed on 21 February 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).