Comparison of SARS-CoV-2 Antigen Tests in Asymptomatic Testing of Passengers at German Airports under Time Constraints: Application of Three Different Antigen Test Formats
Abstract
:1. Introduction
- (I)
- Define the performance test characteristics of three different antigen testing formats: (1) point-of-care colorimetric read out, (2) point-of-care fluorometric, and (3) instrument-based chemiluminescent in asymptomatic testing of customers at German airports.
- (II)
- Assess the robustness of antigen testing performance over time by comparing positive and negative outcomes with RT-PCR results from samples collected and processed in parallel.
2. Materials and Methods
3. Results
3.1. Validation Results in Asymptomatic Travelers
3.2. Surveillance Results in Asymptomatic Travelers
3.3. Normalized Distribution of Positively Confirmed Antigen Tests
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathuria, J.P.; Yadav, R.; Rajkumar. Laboratory diagnosis of SARS-CoV-2-A review of current methods. J. Infect. Public Health 2020, 13, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Loeffelholz, M.J.; Tang, Y.W. Laboratory diagnosis of emerging human coronavirus infections-the state of the art. Emerg. Microbes Infect. 2020, 9, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.C.; Sharfstein, J.S.; Armstrong, K. The Need for More and Better Testing for COVID-19. JAMA 2020, 324, 2153–2154. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Haage, V.C.; Bleicker, T.; Schmidt, M.L.; Mühlemann, B.; Zuchowski, M.; Jo, W.K.; Tscheak, P.; Möncke-Buchner, E.; Müller, M.A.; et al. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: A single-centre laboratory evaluation study. Lancet Microbe 2021, 2, e311–e319. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 3, CD013705. [Google Scholar] [CrossRef] [PubMed]
- Mak, G.C.; Lau, S.S.; Wong, K.K.; Chow, N.L.; Lau, C.S.; Lam, E.T.; Chan, R.C.; Tsang, D.N. Evaluation of rapid antigen detection kit from the WHO Emergency Use List for detecting SARS-CoV-2. J. Clin. Virol. 2021, 134, 104712. [Google Scholar] [CrossRef] [PubMed]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.E.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2021, 2, e13–e22. [Google Scholar] [CrossRef]
- Oran, D.P.; Topol, E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review. Ann. Intemal Med. 2020, 173, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Buitrago-Garcia, D.; Egli-Gany, D.; Counotte, M.J.; Hossmann, S.; Imeri, H.; Ipekci, A.M.; Salanti, G.; Low, N. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020, 17, e1003346. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Pei, S.; Chen, B.; Song, Y.; Zhang, T.; Yang, W.; Shaman, J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2). Science 2020, 368, 489–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beetz, C.; Skrahina, V.; Förster, T.M.; Gaber, H.; Paul, J.J.; Curado, F.; Rolfs, A.; Bauer, P.; Schäfer, S.; Weckesser, V.; et al. Rapid Large-Scale COVID-19 Testing During Shortages. Diagnostics 2020, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- RKI. COVID-19: Entlassungskriterien aus der Isolierung. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Entlassmanagement-Infografik.pdf?__blob=publicationFile (accessed on 10 May 2021).
- Rasmussen, A.L.; Popescu, S.V. SARS-CoV-2 transmission without symptoms. Science 2021, 371, 1206–1207. [Google Scholar] [CrossRef] [PubMed]
- Pray, I.W. Performance of an Antigen-Based Test for Asymptomatic and Symptomatic SARS-CoV-2 Testing at Two University Campuses-Wisconsin, September–October 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 69, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/334253/WHO-2019-nCoV-Antigen_Detection-2020.1-eng.pdf?sequence=1&isAllowed=y (accessed on 25 April 2021).
- WHO. SARS-CoV-2 Antigen-Detecting RAPID diagnostic Tests: An Implementation Guide. 2020. Available online: https://www.who.int/publications/i/item/9789240017740 (accessed on 25 April 2021).
- EDCD. Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK. 2020. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Options-use-of-rapid-antigen-tests-for-COVID-19_0.pdf (accessed on 5 May 2021).
- Khanh, N.C.; Thai, P.Q.; Quach, H.L.; Thi, N.A.H.; Dinh, P.C.; Duong, T.N.; Mai, L.T.Q.; Nghia, N.D.; Tu, T.A.; Quang, L.N.; et al. Transmission of SARS-CoV 2 During Long-Haul Flight. Emerg. Infect. Dis. 2020, 26, 2617–2624. [Google Scholar] [CrossRef] [PubMed]
- Betsch, C.; Sprengholz, P.; Siegers, R.; Eitze, S.; Korn, L.; Goldhahn, L.; Schmitz, J.M.; Giesler, P.; Knauer, G.; Jenny, M.A. Empirical evidence to understand the human factor for effective rapid testing against SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2021, 118, e2107179118. [Google Scholar] [CrossRef] [PubMed]
- Borovecki, A.; Mlinaric, A.; Horvat, M.; Smolcic, V.S. Informed consent and ethics committee approval in laboratory medicine. Biochem Med. 2018, 28, 030201. [Google Scholar] [CrossRef] [PubMed]
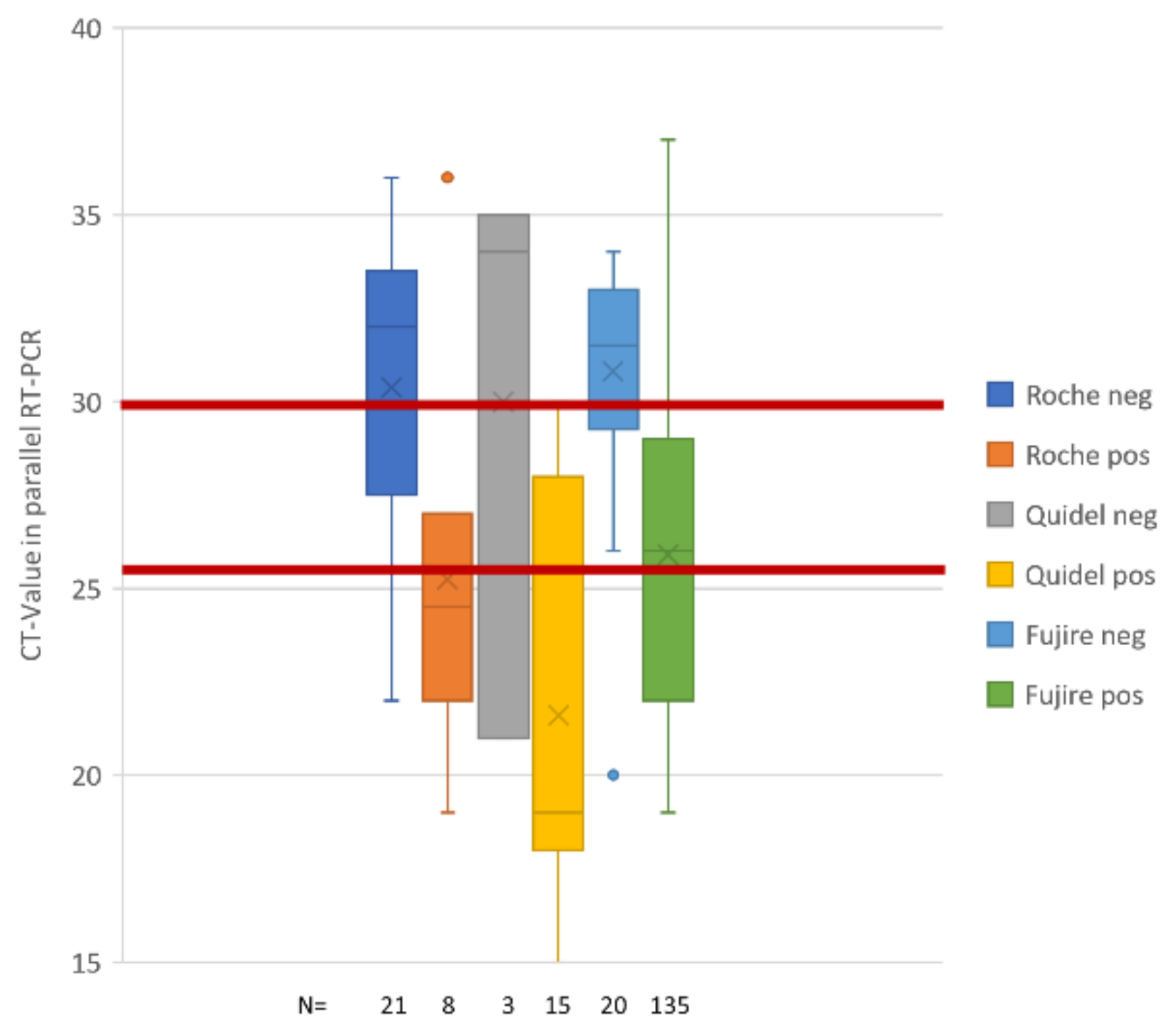


| Cycles | Roc-AG neg | Roc-AG pos | Q-AG neg | Q-AG pos | FRB-AG neg | FRB-AG pos |
|---|---|---|---|---|---|---|
| 19 | 1 | 8 | 8 | |||
| 20 | 1 | 1 | 8 | |||
| 21 | 1 | 9 | ||||
| 22 | 2 | 2 | 9 | |||
| 23 | 1 | 1 | 9 | |||
| 24 | 9 | |||||
| 25 | 13 | |||||
| 26 | 1 | 1 | 1 | 12 | ||
| 27 | 2 | 2 | 1 | 12 | ||
| 28 | 1 | 1 | 8 | |||
| 29 | 1 | 1 | 8 | |||
| 30 | 2 | 1 | 11 | |||
| 31 | 1 | 4 | 7 | |||
| 32 | 4 | 2 | 3 | |||
| 33 | 2 | 4 | 1 | |||
| 34 | 1 | 1 | 4 | 3 | ||
| 35 | 2 | 1 | 2 | |||
| 36 | 1 | 1 | 2 | |||
| 37 | 11 | 1 |
| Roc-AG neg | Roc-AG pos | Q-AG neg | Q-AG pos | FRB-AG neg | FRB-AG pos | |
|---|---|---|---|---|---|---|
| All cases | 32 | 8 | 3 | 10 | 20 | 135 |
| CT ≤ 26 cycles | 3 | 5 | 1 | 10 | 2 | 77 |
| CT ≤ 30 cycles | 9 | 7 | 1 | 10 | 6 | 116 |
| CT ˃ 30 cycles | 22 | 1 | 2 | 0 | 14 | 19 |
| Sensitivity of AG tests | Roc-AG | Q-AG | FRB-AG | |
|---|---|---|---|---|
| Sensitivity | 20.0% | 76.9% | 87.1% | |
| Identification of High risk for infectivity | Sensitivity ≤ 26 cycles | 62.5% | 90.9% | 97.5% |
| Identification of Moderate risk for infectivity | Sensitivity ≤ 30 cycles | 43.8% | 90.9% | 95.1% |
| Sensitivity ˃ 30 cycles | 4.3% | 0.0% | 57.6% | |
| RT-PCR positive samples | 40 | 13 | 155 |
| Total | AG Negative | AG Failed | AG Positive | TP (PCR) | FP (PCR) | Borderline (PCR) | Failed (PCR) | |
|---|---|---|---|---|---|---|---|---|
| All AG tests | 36,758 | 36,441 | 100 | 217 | 165 | 42 | 6 | 4 |
| % from total | 99.14% | 0.27% | 0.59% | 0.45% | 0.11% | 0.02% | 0.01% | |
| Quidel-AG | 20,783 | 20,554 | 97 | 132 | 96 | 32 | 2 | 2 |
| % from total | 98.90% | 0.47% | 0.64% | 0.46% | 0.15% | 0.01% | 0.01% | |
| Fujirebio-AG | 15,975 | 15,887 | 3 | 85 | 69 | 10 | 4 | 2 |
| % from total | 99.45% | 0.02% | 0.53% | 0.43% | 0.06% | 0.03% | 0.01% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradas, L.; Viceconte, N.; Hannen, J.; Schölz, C.; Schubert, A.; Knote, R.; Wille, L.; Kleinow, P.; Heuer, A.; Weckesser, V.; et al. Comparison of SARS-CoV-2 Antigen Tests in Asymptomatic Testing of Passengers at German Airports under Time Constraints: Application of Three Different Antigen Test Formats. COVID 2021, 1, 546-554. https://doi.org/10.3390/covid1030046
Pradas L, Viceconte N, Hannen J, Schölz C, Schubert A, Knote R, Wille L, Kleinow P, Heuer A, Weckesser V, et al. Comparison of SARS-CoV-2 Antigen Tests in Asymptomatic Testing of Passengers at German Airports under Time Constraints: Application of Three Different Antigen Test Formats. COVID. 2021; 1(3):546-554. https://doi.org/10.3390/covid1030046
Chicago/Turabian StylePradas, Laura, Nikenza Viceconte, Jennifer Hannen, Christian Schölz, Axel Schubert, Robert Knote, Leon Wille, Peter Kleinow, Andreas Heuer, Volkmar Weckesser, and et al. 2021. "Comparison of SARS-CoV-2 Antigen Tests in Asymptomatic Testing of Passengers at German Airports under Time Constraints: Application of Three Different Antigen Test Formats" COVID 1, no. 3: 546-554. https://doi.org/10.3390/covid1030046
APA StylePradas, L., Viceconte, N., Hannen, J., Schölz, C., Schubert, A., Knote, R., Wille, L., Kleinow, P., Heuer, A., Weckesser, V., Hartkamp, J., Schaefer, S., & Bauer, P. (2021). Comparison of SARS-CoV-2 Antigen Tests in Asymptomatic Testing of Passengers at German Airports under Time Constraints: Application of Three Different Antigen Test Formats. COVID, 1(3), 546-554. https://doi.org/10.3390/covid1030046






