Persistence of Anti-SARS-CoV-2 Antibodies Six Months after Infection in an Outbreak with Five Hundred COVID-19 Cases in Borriana (Spain): A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johns Hopkins University. COVID-19 Data in Motion: 12 May 2021. Available online: https://coronavirus.jhu.edu/ (accessed on 12 May 2021).
- Clapham, H.; Hay, J.; Routledge, I.; Takahashi, S.; Choisy, M.; Cummings, D.; Grenfell, B.; Metcalf, C.J.E.; Mina, M.; Barraquer, I.R.; et al. Seroepidemiologic study designs for determining SARS-COV-2 transmission and immunity. Emerg. Infect. Dis. 2020, 26, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lin, R.T.P.; Renia, L.; Ng, L.F.P. Serological approaches for COVID-19: Epidemiologic perspective on surveillance and control. Front. Immunol. 2020, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef] [PubMed]
- Hanna, S.J.; Codd, A.S.; Gea-Mallorqui, E.; Scourfield, D.O.; Richter, F.C.; Ladell, K.; Borsa, M.; Compeer, E.B.; Moon, O.R.; Galloway, S.A.E.; et al. T cell phenotypes in COVID-19—a living review. Oxf. Open Immunol. 2020, 2, iqaa007. [Google Scholar] [CrossRef]
- Egger, M.; Bundschuh, C.; Wiesinger, K.; Gabriel, C.; Clodi, M.; Mueller, T.; Dieplinger, B. Comparison of the Elecsys® Anti-SARS-CoV-2 immunoassay with the EDI™ enzyme linked immunosorbent assays for the detection of SARS-CoV-2 antibodies in human plasma. Clin. Chim. Acta 2020, 509, 18–21. [Google Scholar] [CrossRef]
- Dellière, S.; Salmona, M.; Minier, M.; Gabassi, A.; Alanio, A.; Le Goff, J.; Delaugerre, C.; Chaix, M.L.; Saint-Louis CORE (COvid REsearch) group. Evaluation of the COVID-19 IgG/IgM rapid test from Orient Gene Biotech. J. Clin. Microbiol. 2020, 58, e01233-20. [Google Scholar] [CrossRef]
- Yip, C.C.; Sridhar, S.; Cheng, A.K.; Leung, K.H.; Choi, G.K.; Chen, J.H.; Poon, R.W.; Chan, K.H.; Wu, A.K.; Chan, H.S.; et al. Evaluation of the commercially available LightMix® Modular E-gene kit using clinical and proficiency testing specimens for SARS-CoV-2 detection. J. Clin. Virol. 2020, 129, 104476. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, W.; Li, B.; Li, D.J.; Zhang, J.; Zhao, F. Association between ABO Blood Group System and COVID-19 susceptibility in Wuhan. Front. Cell. Infect. Microbiol. 2020, 10, 404. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, Z.; Li, P.; Yu, Q. Relationship between ABO blood group distribution and clinical characteristics in patients with COVID-19. Clin. Chim. Acta 2020, 509, 220–223. [Google Scholar] [CrossRef]
- Merzon, E.; Tworowski, D.; Gorohovski, A.; Vinker, S.; Golan Cohen, A.; Green, I.; Frenkel-Morgenstern, M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. FEBS J. 2020, 287, 3693–3702. [Google Scholar] [CrossRef]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef]
- Lapierre, Y.; Rigal, D.; Adam, J.; Josef, D.; Meyer, F.; Greber, S.; Drot, C. The gel test: A new way to detect red cell antigen-antibody reactions. Transfusion 1990, 30, 109–113. [Google Scholar] [CrossRef]
- Asif, M.; Groboske, S.E.; Leung, E.K.Y.; Yeo, K.J.; van Wijk, X.M.R. Evaluation of a new generation automated assay for 25-hydroxy vitamin D based on competitive protein binding. J. Appl. Lab. Med. 2019, 4, 247–253. [Google Scholar] [CrossRef]
- Greenland, S.; Pearl, J.; Robins, J.M. Causal diagrams for epidemiologic research. Epidemiology 1999, 10, 37–48. [Google Scholar] [CrossRef]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liskiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef] [Green Version]
- Fejt, V.; Krátká, Z.; Zelená, H.; Fürst, T. Age is not a disease: Evolution of protective antibodies against SARS-CoV-2 in seniors from the Břevnice nursing home. Cas. Lek. Cesk. 2020, 159, 303–311. [Google Scholar]
- Choe, P.G.; Kim, K.H.; Kang, C.K.; Suh, H.J.; Kang, E.; Lee, S.Y.; Kim, N.J.; Yi, J.; Park, W.B.; Oh, M.D. Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection. Emerg. Infect. Dis. 2021, 27, 928–931. [Google Scholar] [CrossRef]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020, 383, 1724–1734. [Google Scholar] [CrossRef]
- Ripperger, T.J.; Uhrlaub, J.L.; Watanabe, M.; Wong, R.; Castaneda, Y.; Pizzato, H.A.; Thompson, M.R.; Bradshaw, C.; Weinkauf, C.C.; Bime, C.; et al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity 2020, 53, 925–933.e4. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Favresse, J.; Eucher, C.; Elsen, M.; Gillot, C.; Van Eeckhoudt, S.; Dogné, J.M.; Douxfils, J. Persistence of anti-SARS-CoV-2 antibodies depends on the analytical kit: A report for up to 10 months after infection. Microorganisms 2021, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Contant, P.; Embong, A.K.; Kanagaiah, P.; Chaves, F.A.; Yang, H.; Branche, A.R.; Topham, D.J.; Sangster, M.Y. S Protein-Reactive IgG and memory B cell production after human SARS-CoV-2 infection includes broad reactivity to the S2 subunit. mBio 2020, 11, e01991-20. [Google Scholar] [CrossRef] [PubMed]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Everden, S.; Nikitas, N. A case of COVID-19 re-infection in the UK. Clin. Med. 2021, 21, e52–e53. [Google Scholar] [CrossRef] [PubMed]
- Jeffery-Smith, A.; Iyanger, N.; Williams, S.V.; Chow, J.Y.; Aiano, F.; Hoschler, K.; Lackenby, A.; Ellis, J.; Platt, S.; Miah, S.; et al. Antibodies to SARS-CoV-2 protect against re-infection during outbreaks in care homes, September and October 2020. Eurosurveillance 2021, 26, 2100092. [Google Scholar] [CrossRef]
- Harvey, R.A.; Rassen, J.A.; Kabelac, C.A.; Turenne, W.; Leonard, S.; Klesh, R.; Meyer, W.A., III; Kaufman, H.W.; Anderson, S.; Cohen, O.; et al. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern. Med. 2021, 181, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.F.; O’Donnell, D.; Stoesser, N.E.; Matthews, P.C.; Howarth, A.; Hatch, S.B.; Marsden, B.D.; Cox, S.; James, T.; Warren, F.; et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N. Engl. J. Med. 2021, 384, 533–540. [Google Scholar] [CrossRef]
- Hall, V.J.; Foulkes, S.; Charlett, A.; Atti, A.; Monk, E.J.M.; Simmons, R.; Wellington, E.; Cole, M.J.; Saei, A.; Oguti, B.; et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN). Lancet 2021, 397, 1459–1469. [Google Scholar] [CrossRef]
- Addetia, A.; Crawford, K.H.D.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.L.; Jerome, K.R.; Bloom, J.D.; Greninger, A.L. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020, 58, e02107-20. [Google Scholar] [CrossRef]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J.; et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef]
- Markmann, A.J.; Giallourou, N.; Bhowmik, D.R.; Hou, Y.J.; Lerner, A.; Martinez, D.R.; Premkumar, L.; Root, H.; van Duin, D.; Napravnik, S.; et al. Sex disparities and neutralizing antibody durability to SARS-CoV-2 infection in convalescent individuals. medRxiv 2021, 3. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, S.; Li, H.; Wang, Y.; Lu, Z.; Liu, Z.; Lai, Q.; Ji, Y.; Huang, X.; Li, Y.; et al. Viral and antibody kinetics of COVID-19 patients with different disease severities in acute and convalescent phases: A 6-month follow-up study. Virol. Sin. 2020, 35, 820–829. [Google Scholar] [CrossRef]
- Benner, S.E.; Patel, E.U.; Laeyendecker, O.; Pekosz, A.; Littlefield, K.; Eby, Y.; Fernandez, R.E.; Miller, J.; Kirby, C.S.; Keruly, M.; et al. SARS-CoV-2 antibody avidity responses in COVID-19 patients and convalescent plasma donors. J. Infect. Dis. 2020, 222, 1974–1984. [Google Scholar] [CrossRef]
- Shields, A.M.; Faustini, S.E.; Perez-Toledo, M.; Jossi, S.; Allen, J.D.; Al-Taei, S.; Backhouse, C.; Dunbar, L.; Ebanks, D.; Emmanuel, B.; et al. Serological responses to SARS-CoV-2 following non-hospitalised infection: Clinical and ethnodemographic features associated with the magnitude of the antibody response. medRxiv 2020, 16. [Google Scholar] [CrossRef]
- Ladhani, S.N.; Jeffery-Smith, A.; Patel, M.; Janarthanan, R.; Fok, J.; Crawley-Boevey, E.; Vusirikala, A.; De Olano, E.F.; Perez, M.S.; Tang, S.; et al. High prevalence of SARS-CoV-2 antibodies in care homes affected by COVID-19: Prospective cohort study, England. EClinicalMedicine 2020, 28, 100597. [Google Scholar] [CrossRef]
- Wendel, S.; Fontão-Wendel, R.; Fachini, R.; Candelaria, G.; Scuracchio, P.; Achkar, R.; Brito, M.; Reis, L.F.; Camargo, A.; Amano, M.; et al. A longitudinal study of convalescent plasma (CCP) donors and correlation of ABO group, initial neutralizing antibodies (nAb), and body mass index (BMI) with nAb and anti-nucleocapsid (NP) SARS-CoV-2 antibody kinetics: Proposals for better quality of CCP collections. Transfusion 2021, 61, 1447–1460. [Google Scholar] [CrossRef]
- Racine-Brzostek, S.E.; Yang, H.S.; Jack, G.A.; Chen, Z.; Chadburn, A.; Ketas, T.J.; Francomano, E.; Klasse, P.J.; Moore, J.P.; McDonough, K.A.; et al. Postconvalescent SARS-CoV-2 IgG and neutralizing antibodies are elevated in individuals with poor metabolic health. J. Clin. Endocrinol. Metab. 2021, 106, e2025–e2034. [Google Scholar] [CrossRef]
- Gerhards, C.; Thiaucourt, M.; Kittel, M.; Becker, C.; Ast, V.; Hetjens, M.; Neumaier, M.; Haselmann, V. Longitudinal assessment of anti-SARS-CoV-2 antibody dynamics and clinical features following convalescent from COVID-19 infection. Int. J. Infect. Dis. 2021, 28. [Google Scholar] [CrossRef]
- Frasca, D.; Reidy, L.; Cray, C.; Diaz, A.; Romero, M.; Kahl, K.; Blomberg, B.B. Influence of obesity on serum levels of SARS-CoV-2-specific antibodies in COVID-19 patients. PLoS ONE 2021, 16, e0245424. [Google Scholar] [CrossRef]
- De Giorgi, V.; West, K.A.; Henning, A.N.; Chen, L.; Holbrook, M.R.; Gross, R.; Liang, J.; Postnikova, E.; Trenbeath, J.; Pogue, S.; et al. Anti-SARS-CoV-2 Serology persistence over time in COVID-19 Convalescent Plasma Donors. medRxiv 2021, 10. [Google Scholar] [CrossRef]
- de Bernardis, E.; Busà, L. A putative role for the tobacco mosaic virus in smokers’ resistance to COVID-19. Med. Hypotheses 2020, 143, 110153. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Dal Negro, R.W.; Nisini, R. The first, holistic immunological model of COVID-19: Implications for prevention, diagnosis, and public health measures. Pediatr. Allergy Immunol. 2020, 31, 454–470. [Google Scholar] [CrossRef]
- Iwuji, K.; Islam, E.; Berdine, G.; Nugent, K.; Test, V.; Tijerina, A. Prevalence of coronavirus antibody among first responders in Lubbock, Texas. J. Prim. Care Community Health 2020, 11, 2150132720971390. [Google Scholar] [CrossRef] [PubMed]
- Calisti, R. SARS-CoV-2: Exposure to high external doses as determinants of higher viral loads and of increased risk for COVID-19. A systematic review of the literature. Epidemiol. Prev. 2020, 44, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Barassi, A.; Pezzilli, R.; Mondoni, M.; Rinaldo, R.F.; DavÌ, M.; Cozzolino, M.; Melzi D’Eril, G.V.; Centanni, S. Vitamin D in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with non-invasive ventilation support. Panminerva Med. 2021, 25. [Google Scholar] [CrossRef]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; di Ruffano, L.F.; et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020, 6, CD013652. [Google Scholar] [CrossRef]
- Afzal, N.; Tariq, N.; Raza, S.; Shakeel, D. Diagnostic accuracy of electro-chemiluminescence immunoassay anti-SARS-CoV-2 serological test. Cureus 2021, 13, e12588. [Google Scholar] [CrossRef]
- Kittel, M.; Findeisen, P.; Muth, M.C.; Thiaucourt, M.; Gerhards, C.; Neumaier, M.; Haselmann, V. Specificity testing by point prevalence as a simple assessment strategy using the Roche Elecsys® anti-SARS-CoV-2 immunoassay. Int. J. Infect. Dis. 2021, 105, 632–638. [Google Scholar] [CrossRef]
- Speletas, M.; Kyritsi, M.A.; Vontas, A.; Theodoridou, A.; Chrysanthidis, T.; Hatzianastasiou, S.; Petinaki, E.; Hadjichristodoulou, C. Evaluation of two chemiluminescent and three ELISA immunoassays for the detection of SARS-CoV-2 IgG antibodies: Implications for disease diagnosis and patients’ management. Front. Immunol. 2020, 11, 609242. [Google Scholar] [CrossRef]
- Kohmer, N.; Westhaus, S.; Rühl, C.; Ciesek, S.; Rabenau, H.F. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. J. Clin. Virol. 2020, 129, 104480. [Google Scholar] [CrossRef]
- Müller, L.; Ostermann, P.N.; Walker, A.; Wienemann, T.; Mertens, A.; Adams, O.; Andree, M.; Hauka, S.; Lübke, N.; Keitel, V.; et al. Sensitivity of anti-SARS-CoV-2 serological assays in a high-prevalence setting. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1063–1071. [Google Scholar] [CrossRef]
- Khawaja, S.; Asif, M.; Naz Mukry, S.; Sultan Shamsi, T. Possible correlation of electrochemiluminescence based numerical cut off index value with concentration of anti-SARS-CoV-2 antibody: Is it worth reporting? J. Public Health Res. 2021, 10, 2079. [Google Scholar] [CrossRef]
- Peterhoff, D.; Glück, V.; Vogel, M.; Schuster, P.; Schütz, A.; Neubert, P.; Albert, V.; Frisch, S.; Kiessling, M.; Pervan, P.; et al. A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection 2021, 49, 75–82. [Google Scholar] [CrossRef]
- Figueiredo-Campos, P.; Blankenhaus, B.; Mota, C.; Gomes, A.; Serrano, M.; Ariotti, S.; Costa, C.; Nunes-Cabaço, H.; Mendes, A.M.; Gaspar, P.; et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur. J. Immunol. 2020, 50, 2025–2040. [Google Scholar] [CrossRef]
- Krammer, F. Correlates of protection from SARS-CoV-2 infection. Lancet 2021, 397, 1421–1423. [Google Scholar] [CrossRef]
- Furukawa, K.; Arii, J.; Nishimura, M.; Tjan, L.H.; Lystia Poetranto, A.; Ren, Z.; Aktar, S.; Huang, J.R.; Sutandhio, S.; Kurahashi, Y.; et al. Seroepidemiological survey of the antibody for severe acute respiratory syndrome coronavirus 2 with neutralizing activity at hospitals: A cross-sectional study in Hyogo Prefecture, Japan. JMA J. 2021, 4, 41–49. [Google Scholar] [CrossRef]
- Schaffner, A.; Risch, L.; Aeschbacher, S.; Risch, C.; Weber, M.C.; Thiel, S.L.; Jüngert, K.; Pichler, M.; Grossmann, K.; Wohlwend, N.; et al. Characterization of a pan-immunoglobulin assay quantifying antibodies directed against the receptor binding domain of the SARS-CoV-2 S1-Subunit of the spike protein: A population-based study. J. Clin. Med. 2020, 9, 3989. [Google Scholar] [CrossRef]
- Anichini, G.; Terrosi, C.; Gandolfo, C.; Gori Savellini, G.; Fabrizi, S.; Miceli, G.B.; Cusi, M.G. SARS-CoV-2 Antibody Response in Persons with Past Natural Infection. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Saadat, S.; Tehrani, Z.R.; Logue, J.; Newman, M.; Frieman, M.B.; Harris, A.D.; Sajadi, M.M. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA 2021, 325, 1467–1469. [Google Scholar] [CrossRef]
- Domènech-Montoliu, S.; Pac-Sa, M.R.; Vidal-Utrillas, P.; Latorre-Poveda, M.; Del Rio-González, A.; Ferrando-Rubert, S.; Ferrer-Abad, G.; Sánchez-Urbano, M.; Aparisi-Esteve, L.; Badenes-Marques, G.; et al. Mass gathering events and COVID-19 transmission in Borriana (Spain): A retrospective cohort study. PLoS ONE 2021, 16, e0256747. [Google Scholar] [CrossRef]
- Ley 14/1986, de 25 de abril, General de Sanidad (Law General of Health). Available online: https://www.boe.es/eli/es/l/1986/04/25/14/con (accessed on 2 August 2021).
- Ley 16/2003, de 28 de mayo, de cohesión y calidad del Sistema Nacional de Salud. (Law of Cohesion and Quality of the National System of Health). Available online: https://www.boe.es/eli/es/l/2003/05/28/16 (accessed on 2 August 2021).
- Ley 33/2011, de 4 de octubre, General de Salud Pública (Law General of Public Health). Available online: https://www.boe.es/eli/es/l/2011/10/04/33/con (accessed on 2 August 2021).
- Ministerio de Sanidad Acordado en Consejo Interterritorial del Sistema Nacional de Salud el 16 de julio de 2020. Plan de respuesta temprana en un escenario de control de la pandemia por el COVID-19.pdf (Ministry of Health Agreed in the Interterritorial Council of the National Health System on July 16, 2020”. Early response plan in a COVID-19 pandemic control scenario.pdf). Available online: https://www.mscbs.gob.es/profesional/saludPublica/alertasActual/nCOV/documetos/CoVID19_Plan_de_respuesta_temprana_escenario_control.pdf (accessed on 2 August 2021).
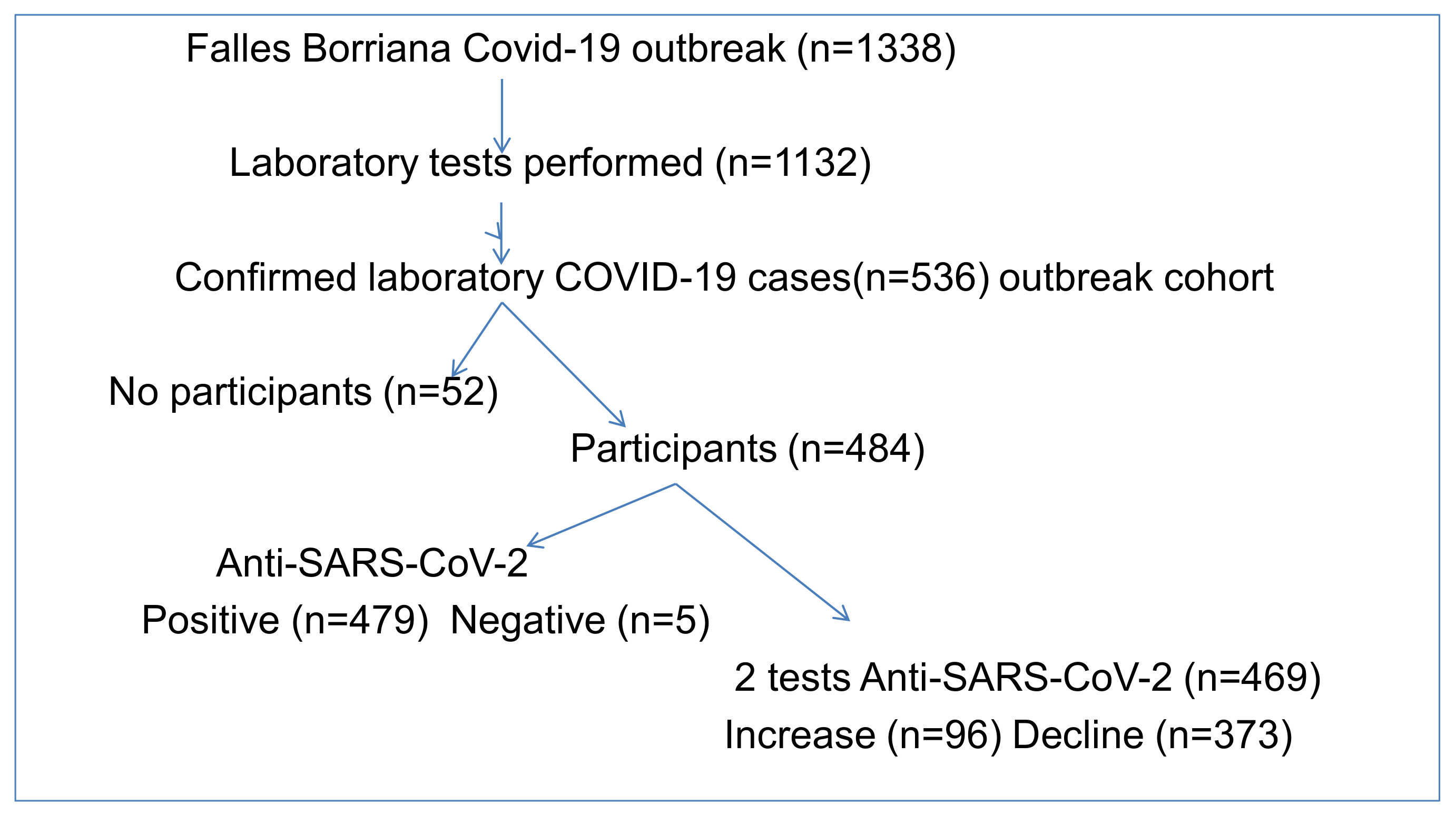
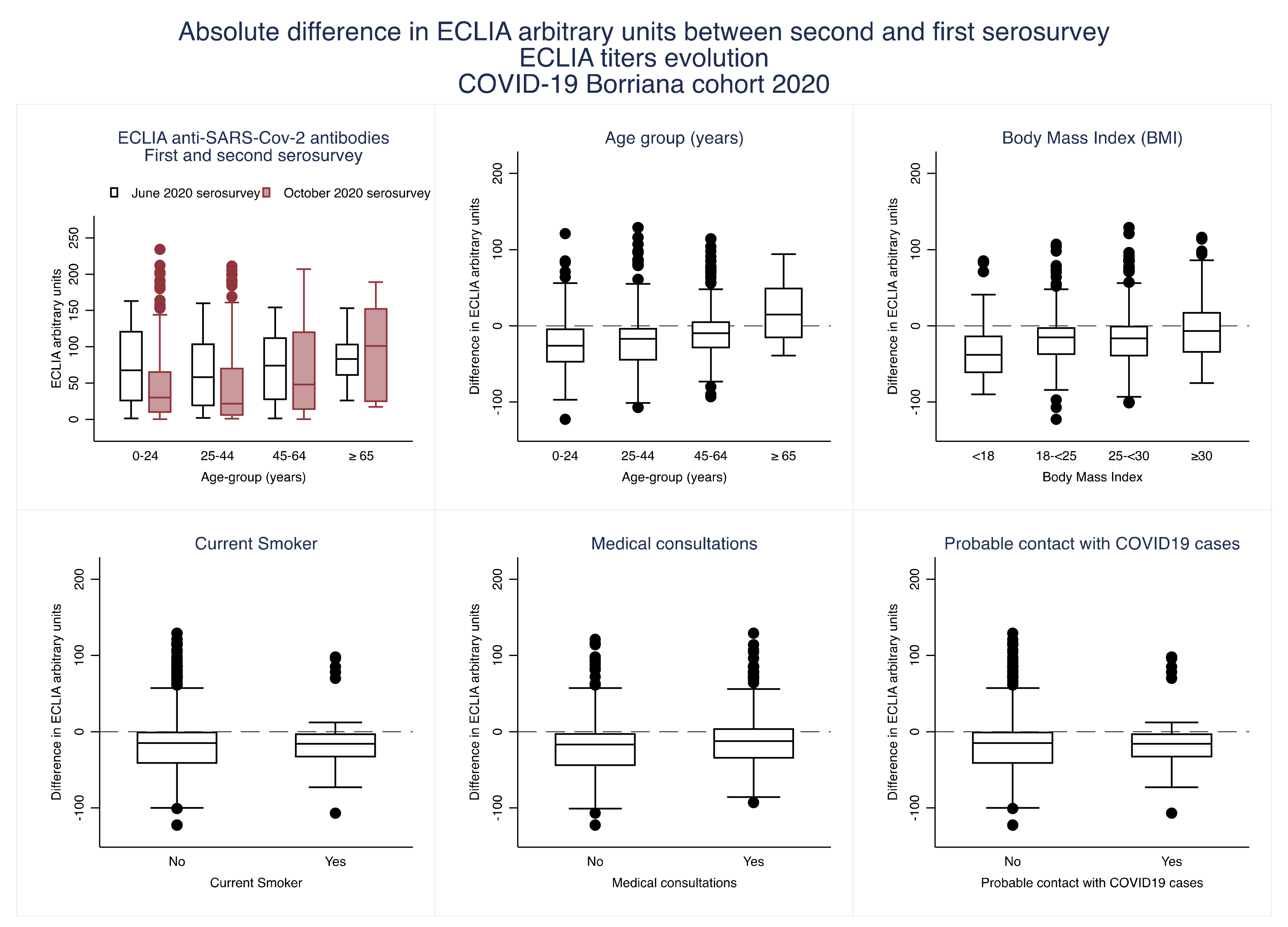
| Variables | Participant N = 484 (%) | No-Participants N = 52 (%) | p-Value |
|---|---|---|---|
| Female | 301 (62.2) | 32 (61.5) | 1.000 |
| Age mean ± Standard Deviation | 37.2 ± 17.1 | 33.5 ± 16.7 | 0.064 |
| 0–24 years | 143 (29.5) | 19 (36.5) | 0.483 |
| 25–44 | 157 (32.4) | 17 (32.7) | |
| 45–64 | 166 (34.3) | 16 (30.8) | |
| 65 and over | 18 (3.7) | 0 (0.0) | |
| Body mass index 1 Kg/m2 | |||
| <18.0 | 41 (8.5) | 6 (11.5) | 0.747 |
| 18.0–24.9 | 210 (43.7) | 24 (46.2) | |
| 25.0–29.9 | 148 (30.8) | 13 (25.0) | |
| ≥30.0 | 85 (17.7) | 9 (17.3) | |
| Occupation I-II 2,3 | 145 (30.1) | 16 (30.7) | 1.000 |
| Current smoker 4 | 65 (13.9) | 12 (23.1) | 0.221 |
| Physical exercise | 389 (80.4) | 24 (46.2) | 0.075 |
| Alcohol beverages 5 | 108 (23.0) | 10 (19.2) | 0.604 |
| Chronic illness 6 | 166 (34.6) | 14 (26.9) | 0.285 |
| COVID-19 | |||
| Family with COVD-19 case 7 | 303 (62.7) | 31 (59.6) | 0.554 |
| Probable contact COVID-19 case 8 | 390 (81.8) | 39 (75.0) | 0.349 |
| Assistance Mass Gathering events ≥ 2 and over 9 | 295 (61.0) | 16 (30.8) | 0.000 |
| Hospitalisations | 9 (1.9) | 3 (5.8) | 0.101 |
| PCR positive | 26 (5.4) | 13 (25.0) | NC 10 |
| Asymptomatic | 54 (11.2) | 10 (19.2) | 0.111 |
| Medical consultation | 208 (43.0) | 25 (48.1) | 0.556 |
| Variables | Negative Antibodies N = 5 (%) | Persistent Antibodies N = 479 (%) | RR 1 | 95% CI 2 | p-Value |
|---|---|---|---|---|---|
| Female | 4 (80.0) | 297 (62.0) | 0.41 | 0.05–3.66 | 0.426 |
| Age mean ± standard deviation | 24.0 ± 16.7 | 37.4 ± 17.1 | 0.95 | 0.90–1.01 | 0.106 |
| 0–24 years | 4 (80.0) | 139 (29.0) | 0.123 | ||
| 25–44 | 0 | 157 (32.8) | |||
| 45–64 | 1 (20.0) | 165 (34.4) | |||
| 65 and over | 0 | 18 (3.8) | |||
| O ABO 3 | 4 (80) | 195 (40.8) | 5.71 | 0.64–50.8 | 0.116 |
| Vitamin D ng/mL | 28.2 ± 8.9 | 29.8 ± 9.2 | 0.98 | 0.87–1.09 | 0.687 |
| Body mass index 4 Kg/m2 | 21.3 ± 2.7 | 25.0 ± 5.0 | 0.84 | 0.76–0.94 | 0.002 |
| <18.0 | 1 (20.0) | 40 (8.4) | 0.038 | ||
| 18.0–24.9 | 4 (80.0) | 206 (43.3) | |||
| 25.0–29.9 | 0 | 148 (31.1) | |||
| ≥30.0 | 0 | 85 (17.9) | |||
| Occupation I-II 5 | 1 (20.0) | 144 (30.3) | 0.57 | 0.07–5.15 | 0.624 |
| Current smoker 6 | 1 (20.0) | 64 (13.8) | 1.19 | 0.37–3.82 | 0.776 |
| Physical exercise | 4 (80.0) | 285 (59.5) | 2.70 | 0.30–24.08 | 0.653 |
| Alcohol consumption 7 | 1 (20.0) | 107 (23.0) | 0.83 | 0.29–7.44 | 0.874 |
| Chronic illness 8 | 0 | 166 (34.9) | 0.28 | 0.00–2.06 | 0.240 |
| COVID-19 disease | |||||
| Family with COVD-1 9 case 9 | 2 (40.0) | 301 (63.0) | 0.39 | 0.07–2.35 | 0.308 |
| Probable contact COVID-19 case 10 | 2 (40.0) | 388 (82.2) | 0.15 | 0.03–0.88 | 0.044 |
| Assistance Mass Gathering Events ≥ 2 and over 11 | 1 (25%) | 255 (60.6) | 0.22 | 0.02–2.10 | 0.189 |
| Hospitalisations | 0 | 9 (1.9) | 7.85 | 0.00–19.23 | 1.000 |
| PCR positive | 0 | 26 (5.4) | 2.61 | 0.00–57.60 | 1.000 |
| Asymptomatic | 1 (20.0) | 53 (11.1) | 1.99 | 0.23–17.62 | 0.448 |
| Medical consultation | 0 | 208 (43.4) | 0.19 | 0.00–1.45 | 0.121 |
| Illness duration | 4.3 ± 6.7 | 10.4 ± 17.5 | 0.93 | 0.76–1.13 | 0.457 |
| Post-COVID-19 | |||||
| Sequelae | 0 | 159 (33.2) | 0.30 | 0.00–2.23 | 0.273 |
| Health as before the disease 12 | 5 (100.0) | 397 (83.1) | 1.36 | 0.18-∞ | 0.732 |
| Recover health 13 | 5 (100.0) | 390 (81.6) | 1.50 | 0.20-∞ | 0.800 |
| Exposure post-COVID-19 | |||||
| Social contact | 4 (80.0) | 103 (78.5) | 1.09 | 0.12–9.79 | 0.935 |
| Gathering people | 0 | 25 (5.2) | 2.73 | 0.00–20.04 | 1.000 |
| Trip out of Borriana 14 | 1(20.0) | 109 (22.9) | 0.85 | 0.10–7.50 | 0.880 |
| Restaurant assistance | 0 | 142 (29.6) | 0.36 | 0.00–2.63 | 0.352 |
| Terrace assistance 15 | 3 (60.0) | 286 (59.8) | 2.19 | 0.37–13.04 | 0.400 |
| Factors | aRR | 95% CI 1 | p-Value | Pearson Goodness of Fit |
|---|---|---|---|---|
| Body mass index (Kg/m2) 2 | 0.87 | 0.77–0.99 | 0.037 | 0.817 |
| Age (years) 3 | 0.96 | 0.91–1.00 | 0.076 | 0.971 |
| Sex: Female 4 | 0.59 | 0.09–3.96 | 0.590 | 0.971 |
| O ABO blood group 5 | 5.52 | 0.61–49.57 | 0.127 | 0.971 |
| Occupation I-II 6 | 0.66 | 0.08–5.45 | 0.695 | 0.587 |
| Current smoker 7 | 2.43 | 0.14–41.87 | 0.541 | 0.072 |
| Physical exercise 7 | 3.05 | 0.36–25.71 | 0.305 | 0.999 |
| Alcohol consumption 7 | 1.07 | 0.10–11.77 | 0.954 | 0.398 |
| Chronic illness 8 | 0.42 | 0.00–3.35 | 0.456 | NC 9 |
| COVID-19 disease | ||||
| Family with COVD-19 case 10 | 0.49 | 0.08–2.85 | 0.428 | 0.540 |
| Probable contact COVID-19 case 11 | 0.18 | 0.04–0.82 | 0.027 | 1.000 |
| Assistance Mass Gathering Events ≥ 2 and over 12,13 | 0.20 | 0.02–1.78 | 0.150 | 0.458 |
| Medical consultation 14 | 0.26 | 0.00–2.05 | 0.227 | NC 9 |
| Illness duration 15 | 0.94 | 0.77–1.15 | 0.570 | 1.000 |
| Variables | Increase SARS-CoV-2 N = 96 (%) | Decline SARS-CoV-2 N = 373 (%) | p-Value |
|---|---|---|---|
| Female | 60 (62.5) | 236 (63.5) | 0.906 |
| Age mean ± standard deviation | 45.3 ± 18.0 | 34.9 ± 16.3 | 0.001 |
| 0–24 years | 15 (15.6) | 126 (33.8) | 0.000 |
| 25–44 | 24 (25.0) | 131 (35.1) | |
| 45–64 | 47 (49.0) | 108 (29.0) | |
| 65 and over | 10 (10.4) | 8 (2.1) | |
| O ABO 1 | 45 (46.9) | 149 (40.1) | 0.246 |
| Vitamin D ng/mL | 29.4 ± 9.0 | 30.0 ± 9.3 | 0.621 |
| Body mass index (Kg/m2) | 26.9 ± 5.0 | 24.5 ± 4.9 | 0.001 |
| <18.0 | 6 (6.3) | 38 (10.2) | 0.001 |
| 18.0–24.9 | 27 (28.1) | 176 (47.2) | |
| 25.0–29.9 | 35 (36.5) | 107 (28.7) | |
| ≥30.0 | 38 (40.0) | 55 (14.7) | |
| Occupation I-II 2 | 29 (30.2) | 107 (28.9) | 0.802 |
| Current smoker 3 | 7 (7.5) | 56 (15.6) | 0.003 |
| Physical exercise | 57 (59.4) | 222 (59.5) | 1.000 |
| Alcohol beverages 4 | 24 (25.8) | 82 (22.7) | 0.583 |
| Chronic illness 5 | 41 (43.2) | 123 (45.6) | 0.092 |
| COVID-19 | |||
| Family with COVD-19 case 6 | 63 (65.6) | 230 (61.8) | 0.555 |
| Probable contact COVID-19 case 7 | 83 (89.2) | 294 (79.7) | 0.036 |
| Assistance Mass Gathering Events ≥2 and over 8 | 61 (63.5) | 227 (60.9) | 0.724 |
| Hospitalisations | 3 (3.1) | 6 (1.6) | 0.398 |
| PCR positive | 8 (8.3) | 17 (4.6) | 0.198 |
| Asymptomatic | 7 (7.3) | 46 (12.3) | 0.206 |
| Medical consultation | 56 (58.3) | 144 (38.6) | 0.001 |
| Illness duration | 12.0 ± 15.0 | 10 ± 18.2 | 0.034 |
| Variables | aRR | 95% CI 1 | p-Value | Pearson Goodness of Fit |
|---|---|---|---|---|
| Body mass index (Kg/m2) 2 | 1.06 | 1.02–1.10 | 0.001 | 0.996 |
| Age (years) 3 | 1.03 | 1.02–1.04 | 0.000 | 0.991 |
| Current smoker 4 | 0.48 | 0.24–0.96 | 0.037 | 0.997 |
| Probable contact COVID-19 case 5 | 2.04 | 1.12–3.74 | 0.022 | 0.994 |
| Medical consultation 6 | 1.62 | 1.12–2.34 | 0.010 | 0.991 |
| Illness duration 7 | 0.99 | 0.99–1.01 | 0.543 | 0.966 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domènech-Montoliu, S.; Puig-Barberà, J.; Pac-Sa, M.R.; Vidal-Utrillas, P.; Latorre-Poveda, M.; Del Rio-González, A.; Ferrando-Rubert, S.; Ferrer-Abad, G.; Sánchez-Urbano, M.; Aparisi-Esteve, L.; et al. Persistence of Anti-SARS-CoV-2 Antibodies Six Months after Infection in an Outbreak with Five Hundred COVID-19 Cases in Borriana (Spain): A Prospective Cohort Study. COVID 2021, 1, 71-82. https://doi.org/10.3390/covid1010006
Domènech-Montoliu S, Puig-Barberà J, Pac-Sa MR, Vidal-Utrillas P, Latorre-Poveda M, Del Rio-González A, Ferrando-Rubert S, Ferrer-Abad G, Sánchez-Urbano M, Aparisi-Esteve L, et al. Persistence of Anti-SARS-CoV-2 Antibodies Six Months after Infection in an Outbreak with Five Hundred COVID-19 Cases in Borriana (Spain): A Prospective Cohort Study. COVID. 2021; 1(1):71-82. https://doi.org/10.3390/covid1010006
Chicago/Turabian StyleDomènech-Montoliu, Salvador, Joan Puig-Barberà, Maria Rosario Pac-Sa, Paula Vidal-Utrillas, Marta Latorre-Poveda, Alba Del Rio-González, Sara Ferrando-Rubert, Gema Ferrer-Abad, Manuel Sánchez-Urbano, Laura Aparisi-Esteve, and et al. 2021. "Persistence of Anti-SARS-CoV-2 Antibodies Six Months after Infection in an Outbreak with Five Hundred COVID-19 Cases in Borriana (Spain): A Prospective Cohort Study" COVID 1, no. 1: 71-82. https://doi.org/10.3390/covid1010006







