Semi-Interpenetrating Highly Conductive and Transparent Hydrogels for Wearable Sensors and Gesture-Driven Cryptography
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Formation Mechanism and Characterization of PPL Hydrogels
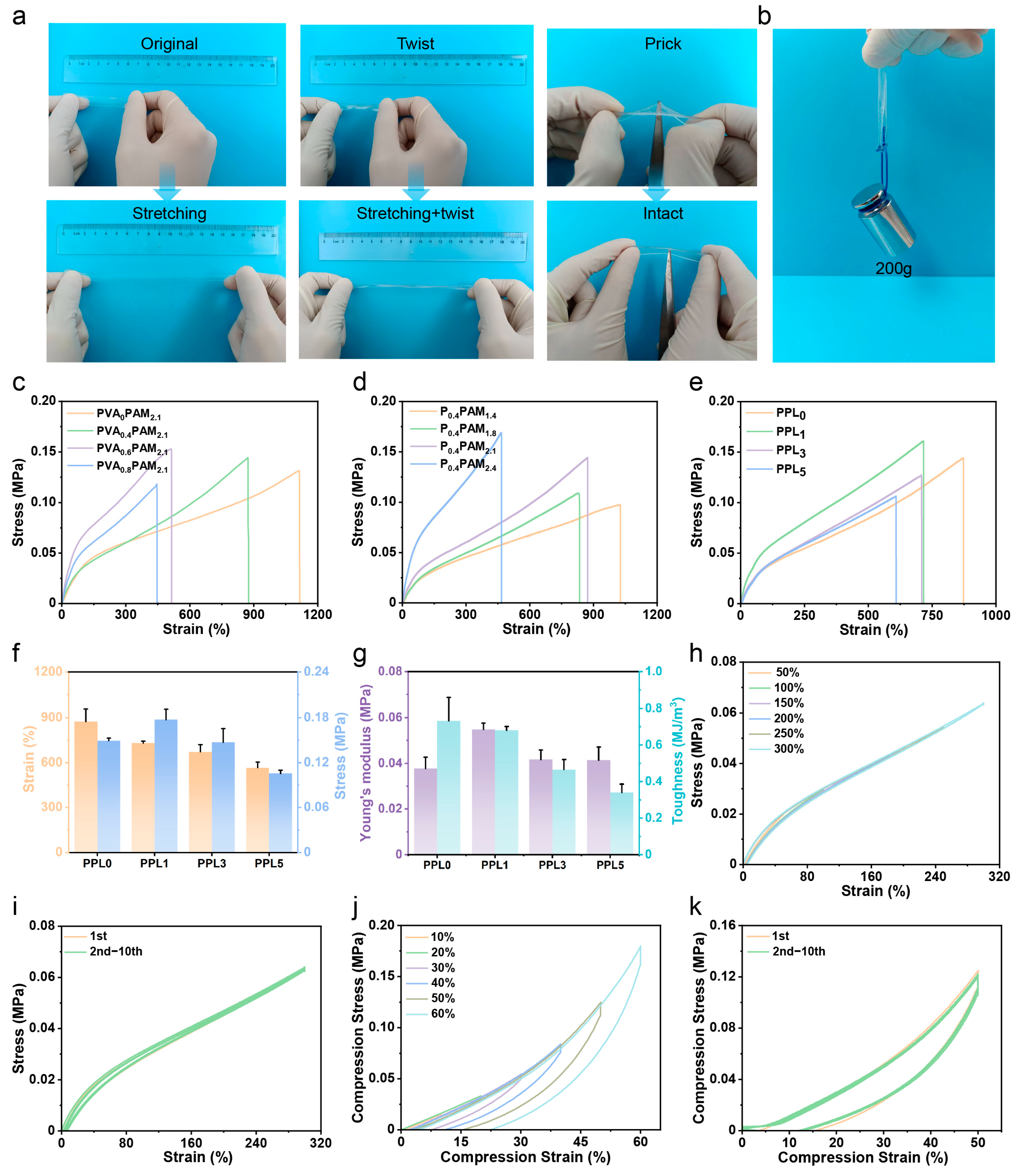
3.2. Mechanical Properties
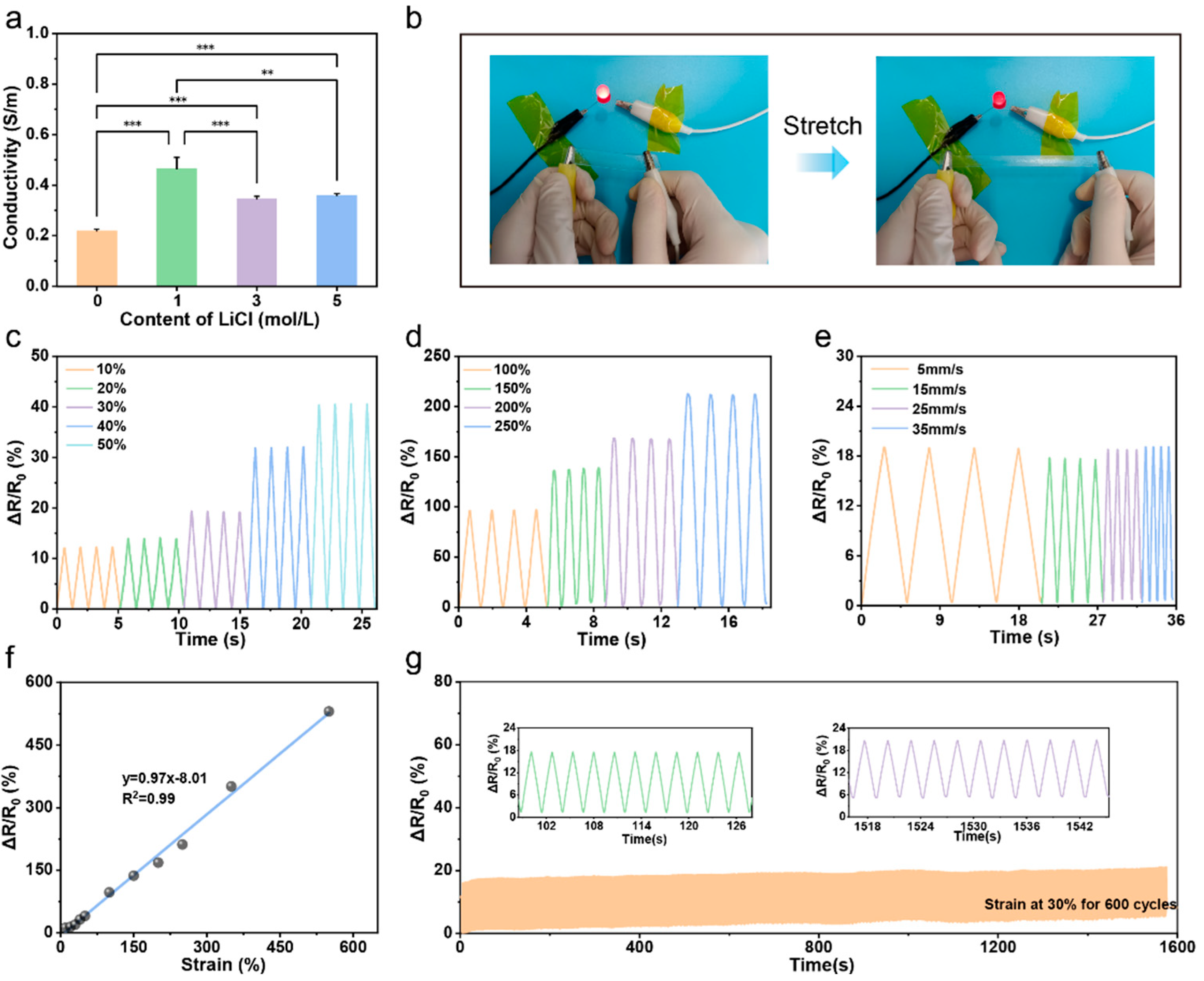
3.3. Conductivity and Sensing Properties of the PPL1 Hydrogel
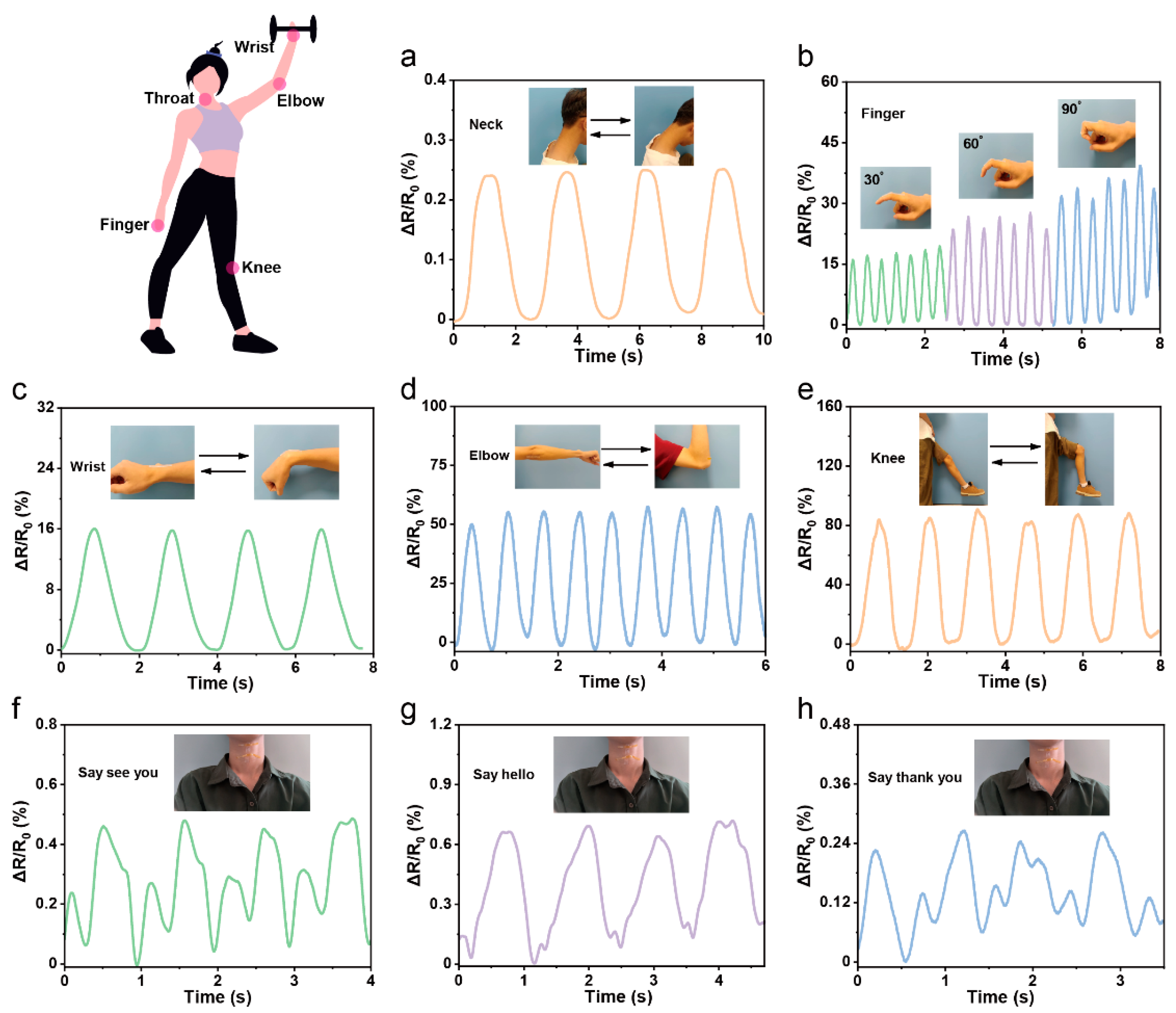
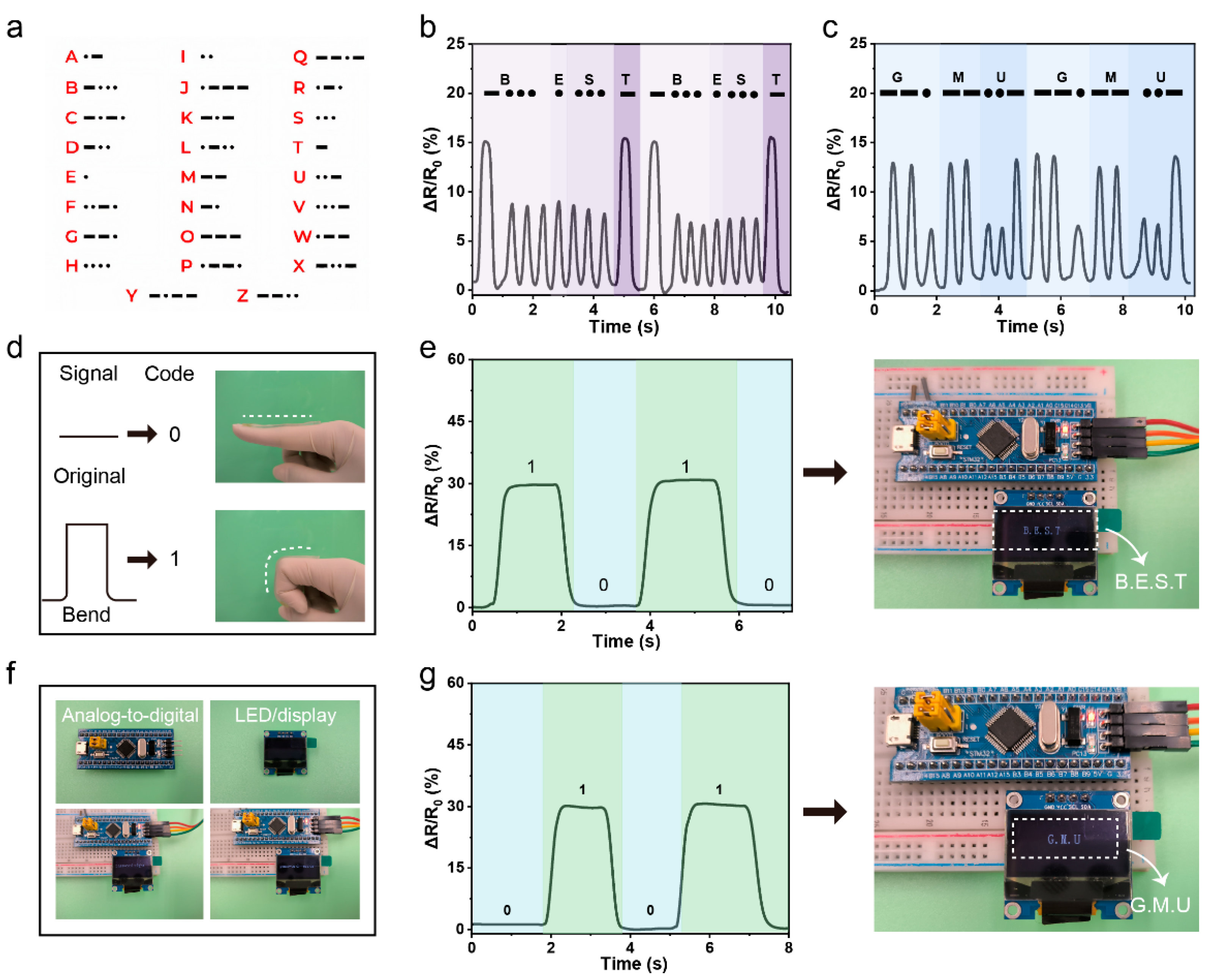
3.4. Application of the PPL1 Hydrogel Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, L.; Zhao, C.; Han, C.; Yang, Y.; Yang, F. Advancement of AI-assisted self-powered healthcare sensing systems. MedMat 2025, 2, 55–77. [Google Scholar] [CrossRef]
- Blachowicz, T.; Kola, I.; Ehrmann, A.; Guenther, K.; Ehrmann, G. Magnetic Micro and Nano Sensors for Continuous Health Monitoring. Micro 2024, 4, 206–228. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.; Dong, Y.; Teng, L.; Li, D.; Han, H.; Zhu, S.; Sun, X.; Zeng, Z.; Zeng, X.; et al. A Self-Powered, Skin Adhesive, and Flexible Human–Machine Interface Based on Triboelectric Nanogenerator. Nanomaterials 2024, 14, 1365. [Google Scholar] [CrossRef]
- Wu, R.; Zhu, T.; Ni, Y.; Wu, C.; Wang, W.; Zhao, K.; Huang, J.; Lai, Y. UV-Cured Dense Double Network Hydrogel via Multiple Dynamic Crosslinking for Stable Amphibious Motion Sensing. Adv. Funct. Mater. 2025, e15120. [Google Scholar] [CrossRef]
- Mpofu, N.S.; Blachowicz, T.; Ehrmann, A.; Ehrmann, G. Wearable Electrospun Nanofibrous Sensors for Health Monitoring. Micro 2024, 4, 798–822. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Y.; Cuthbert, T.J.; Shokurov, A.V.; Chu, P.K.; Feng, S.P.; Menon, C. Hydrogels as Soft Ionic Conductors in Flexible and Wearable Triboelectric Nanogenerators. Adv. Sci. 2022, 9, 2106008. [Google Scholar] [CrossRef]
- Zhao, E.; Wang, T.; Wang, Y.; Zeng, F.; Chen, L.; Zhu, Z.; Tang, W. Active learning assisted piezoelectric materials synthesis on the basis of composite decision-making. MedMat 2024, 1, 95–103. [Google Scholar] [CrossRef]
- Dey, K.; Agnelli, S.; Sartore, L. Designing Viscoelastic Gelatin-PEG Macroporous Hybrid Hydrogel with Anisotropic Morphology and Mechanical Properties for Tissue Engineering Application. Micro 2023, 3, 434–457. [Google Scholar] [CrossRef]
- Lei, T.; Duan, X.; Zhao, H.; Ma, S.; Ma, X.; Wang, N.; Zhang, Q.; Wan, A.; Xia, Z.; Shou, W.; et al. A multifunctional flexible wearable hydrogel sensor with anti-swelling via supramolecular interactions for underwater motion detection and information transmission. Chem. Eng. J. 2025, 504, 158700. [Google Scholar] [CrossRef]
- Hubl, M.; Atta, R.M.; Kaufhold, R.; Wang, B.; Ngo, H.D. Nano-Materials-Based Printed Glucose Sensor for Use in Incontinence Products for Health-Care Applications. Micro 2023, 3, 521–536. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, R.; Huang, H.; Zhong, J.; Huang, Y.; Wu, R.; Luo, D.; Liu, X.; Ning, H.; Peng, J. Highly sensitive flexible graphene pressure sensor based on indocalamus leaves’ microstructure. Surf. Interfaces 2024, 48, 104304. [Google Scholar] [CrossRef]
- Mishra, R.B.; El-Atab, N.; Hussain, A.M.; Hussain, M.M. Recent Progress on Flexible Capacitive Pressure Sensors: From Design and Materials to Applications. Adv. Mater. Technol. 2021, 6, 2001023. [Google Scholar] [CrossRef]
- Tang, L.; Wu, S.; Xu, Y.; Cui, T.; Li, Y.; Wang, W.; Gong, L.; Tang, J. High toughness fully physical cross-linked double network organohydrogels for strain sensors with anti-freezing and anti-fatigue properties. Mater. Adv. 2021, 2, 6655–6664. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Lin, Y.-J.; Enriquez, E.; Peng, X.-F.; Turng, L.-S. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. [Google Scholar] [CrossRef]
- Ma, X.; Wang, W.; Cui, X.; Li, Y.; Yang, K.; Huang, Z.; Zhang, H. Machine Learning Assisted Self-Powered Identity Recognition Based on Thermogalvanic Hydrogel for Intelligent Security. Small 2024, 20, 2402700. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Wang, H.; Wang, C.; Gu, Y.; Xu, Y.; Lee, S.; Yokota, T.; Haick, H.; Someya, T.; et al. A 10-micrometer-thick nanomesh-reinforced gas-permeable hydrogel skin sensor for long-term electrophysiological monitoring. Sci. Adv. 2024, 10, eadj5389. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Zhang, Y.; Yang, J.; Zhang, Z.; Zhou, P.; Xu, Y.; Sun, Q.; Zheng, M.; Yan, W.; et al. A 2.7-μm-thick robust, permeable, and antifreezing hydrogel electrode for long-term ambulatory health monitoring. Sci. Adv. 2025, 11, eadt2286. [Google Scholar] [CrossRef]
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional Conductive Hydrogels for Bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Sun, Z.; Dong, C.; Chen, B.; Li, W.; Hu, H.; Zhou, J.; Li, C.; Huang, Z. Strong, Tough, and Anti-Swelling Supramolecular Conductive Hydrogels for Amphibious Motion Sensors. Small 2023, 19, 2303612. [Google Scholar] [CrossRef]
- Kougkolos, G.; Golzio, M.; Laudebat, L.; Valdez-Nava, Z.; Flahaut, E. Hydrogels with electrically conductive nanomaterials for biomedical applications. J. Mater. Chem. B 2023, 11, 2036–2062. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.-x.; Huang, C.-h.; Zhang, Z.-x.; Lan, Z.-y.; Zha, Y.-f.; Wang, A.-y.; Guo, L.; Wang, Y. Synchronously enhancing fracture toughness and mechanical strength of polyester blend composites via constructing dual-network structures of one-dimensional carbon nanofillers. Polymer 2025, 324, 128242. [Google Scholar] [CrossRef]
- Lin, F.; Zhu, Y.; You, Z.; Li, W.; Chen, J.; Zheng, X.; Zheng, G.; Song, Z.; You, X.; Xu, Y. Ultrastrong and Tough Urushiol-Based Ionic Conductive Double Network Hydrogels as Flexible Strain Sensors. Polymers 2023, 15, 3219. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fan, Y.; Xiong, X.; Jiang, Q.; Li, P.; Jian, J.; Chen, L. Highly conductive and adhesive wearable sensors based on PVA/PAM/SF/PEDOT:PSS double network hydrogels. Appl. Phys. A 2024, 130, 157. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Xu, C.; Zhao, X.; Ren, C.; Wei, P.; Zhang, D.; Yin, Y.; Guan, F.; Zhai, W.; et al. A Seamlessly Integrated Sandwich-Structured Hydrogel for Supercapacitors and Multimodal Wearable Sensors Enabling Information Transmission. Adv. Funct. Mater. 2025, e12653. [Google Scholar] [CrossRef]
- Bi, D.; Qu, N.; Sheng, W.; Lin, T.; Huang, S.; Wang, L.; Li, R. Tough and Strain-Sensitive Organohydrogels Based on MXene and PEDOT/PSS and Their Effects on Mechanical Properties and Strain-Sensing Performance. ACS Appl. Mater. Interfaces 2024, 16, 11914–11929. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Li, S.; Zou, X.; Yin, H.; Huang, Y.; Dong, F.; Li, P.; Song, Y. Construction and characterization of highly stretchable ionic conductive hydrogels for flexible sensors with good anti-freezing performance. Eur. Polym. J. 2023, 186, 111827. [Google Scholar] [CrossRef]
- Zeng, X.; Teng, L.; Wang, X.; Lu, T.; Leng, W.; Wu, X.; Li, D.; Zhong, Y.; Sun, X.; Zhu, S.; et al. Efficient multi-physical crosslinked nanocomposite hydrogel for a conformal strain and self-powered tactile sensor. Nano Energy 2025, 135, 110669. [Google Scholar] [CrossRef]
- Pu, L.; Yuan, Z.; Cai, Y.; Li, X.; Xue, Z.; Niu, Y.; Li, Y.; Ma, S.; Xu, W. Multiperformance PAM/PVA/CaCO3 Hydrogel for Flexible Sensing and Information Encryption. ACS Appl. Mater. Interfaces 2024, 16, 32762–32772. [Google Scholar] [CrossRef]
- Tong, R.; Chen, G.; Pan, D.; Qi, H.; Li, R.a.; Tian, J.; Lu, F.; He, M. Highly Stretchable and Compressible Cellulose Ionic Hydrogels for Flexible Strain Sensors. Biomacromolecules 2019, 20, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhang, J.; Zu, G.; Huang, J. Transparent, flexible, and multifunctional starch-based double-network hydrogels as high-performance wearable electronics. Carbohydr. Polym. 2021, 267, 118198. [Google Scholar] [CrossRef]
- Bian, Z.; Li, Y.; Sun, H.; Shi, M.; Zheng, Y.; Liu, H.; Liu, C.; Shen, C. Transparent, intrinsically stretchable cellulose nanofiber-mediated conductive hydrogel for strain and humidity sensing. Carbohydr. Polym. 2023, 301, 120300. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, M.; Xiao, J.; Lian, H. Ultrafast fabrication of deep eutectic solvent flexible ionic gel with high-transmittance, freeze-resistant and conductivity by frontal polymerization. J. Colloid Interface Sci. 2023, 650, 1382–1392. [Google Scholar] [CrossRef] [PubMed]

| Sample | PVA (g) | AM (g) | LiCl (M) | MBA (mg) | APS (mg) | Distilled H2O (mL) |
|---|---|---|---|---|---|---|
| PPL0 | 0.4 | 2.1 | 0 | 3 | 60 | 7 |
| PPL1 | 0.4 | 2.1 | 1 | 3 | 60 | 7 |
| PPL3 | 0.4 | 2.1 | 3 | 3 | 60 | 7 |
| PPL5 | 0.4 | 2.1 | 5 | 3 | 60 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Li, H.; Wei, Y.; Jiang, L.; Feng, H.; Zheng, Q. Semi-Interpenetrating Highly Conductive and Transparent Hydrogels for Wearable Sensors and Gesture-Driven Cryptography. Micro 2025, 5, 53. https://doi.org/10.3390/micro5040053
Li D, Li H, Wei Y, Jiang L, Feng H, Zheng Q. Semi-Interpenetrating Highly Conductive and Transparent Hydrogels for Wearable Sensors and Gesture-Driven Cryptography. Micro. 2025; 5(4):53. https://doi.org/10.3390/micro5040053
Chicago/Turabian StyleLi, Dan, Hong Li, Yilin Wei, Lu Jiang, Hongqing Feng, and Qiang Zheng. 2025. "Semi-Interpenetrating Highly Conductive and Transparent Hydrogels for Wearable Sensors and Gesture-Driven Cryptography" Micro 5, no. 4: 53. https://doi.org/10.3390/micro5040053
APA StyleLi, D., Li, H., Wei, Y., Jiang, L., Feng, H., & Zheng, Q. (2025). Semi-Interpenetrating Highly Conductive and Transparent Hydrogels for Wearable Sensors and Gesture-Driven Cryptography. Micro, 5(4), 53. https://doi.org/10.3390/micro5040053






