Nanocurcumin and Curcumin-Loaded Nanoparticles in Antimicrobial Photodynamic Therapy: Mechanisms and Emerging Applications
Abstract
1. Introduction
2. Curcumin as a Natural Photosensitizer
3. Nanocurcumin (nCur), Curcumin Encapsulated or Conjugated Within Nanocarriers for aPDT
3.1. Nanocurcumin (nCur) for Enhanced aPDT
3.2. Curcumin Nanoconjugate and Nanocarriers for Enhanced aPDT
3.2.1. aPDT of Curcumin-Loaded Nanocarriers and Nanoconjugates
3.2.2. Structural and Functional Characteristics of Curcumin Nanocarriers
- i.
- Particle Size and Zeta Potential
- ii.
- Drug Loading Capacity and Release Kinetics
- iii.
- Toxicity and Biocompatibility
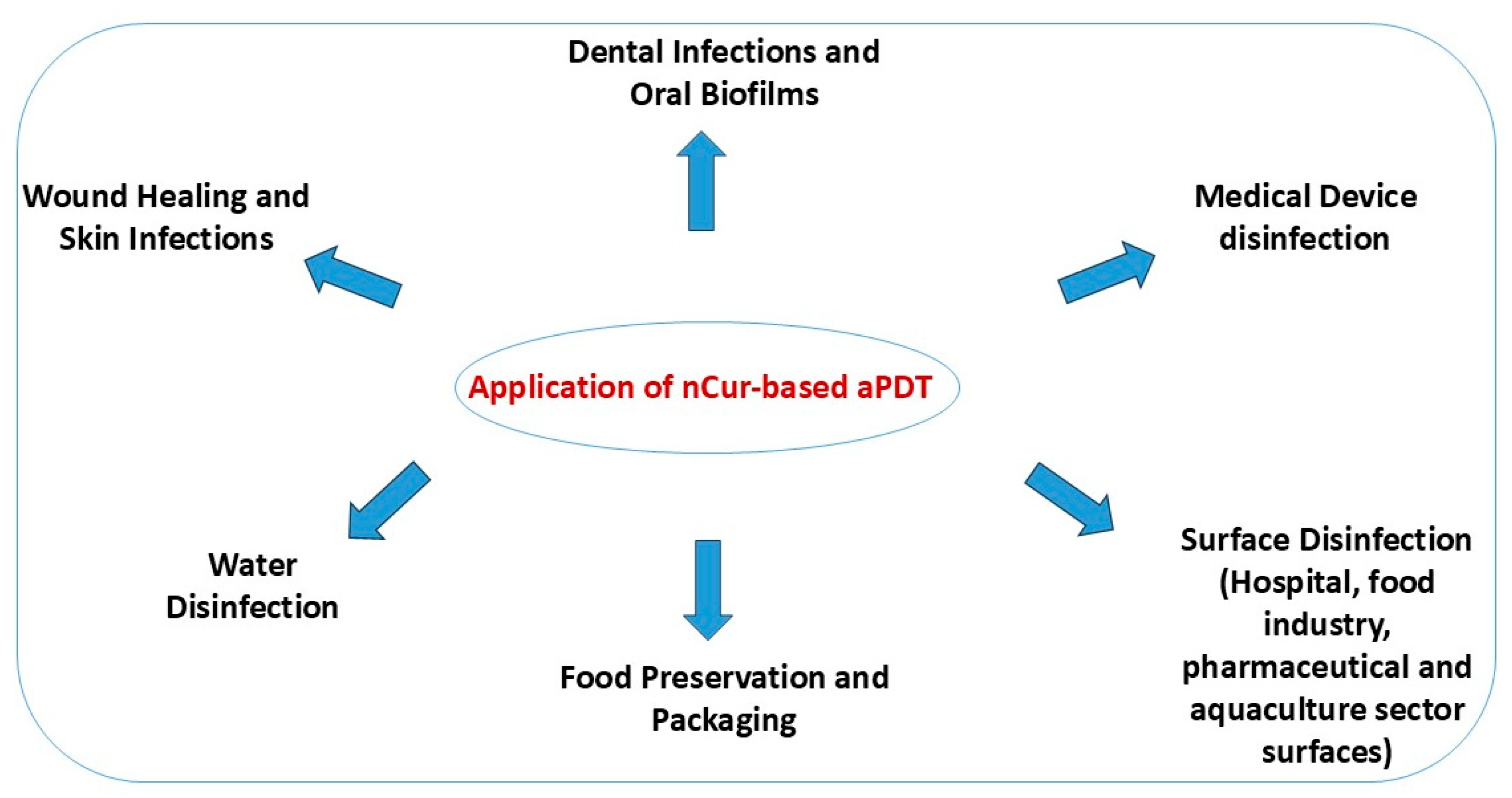
4. Applications of nCur and Curcumin-Based Nanocarriers in aPDT
4.1. Wound Healing and Skin Infections
4.2. Dental Infections and Oral Biofilms
4.3. Medical Device and Surface Disinfection
4.4. Water Disinfection
4.5. Food Packaging and Surface Disinfection
5. Conclusions and Future Perspectives
5.1. Challenges and Future Directions
5.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aslam, B.; Asghar, R.; Muzammil, S.; Shafique, M.; Siddique, A.B.; Khurshid, M.; Ijaz, M.; Rasool, M.H.; Chaudhry, T.H.; Aamir, A.; et al. AMR and Sustainable Development Goals: At a Crossroads. Glob. Health 2024, 20, 73. [Google Scholar] [CrossRef]
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Antimicrobial Resistance: A Concise Update. Lancet Microbe 2025, 6, 100947. [Google Scholar] [CrossRef] [PubMed]
- Afrasiyab; Zhou, R.; Raziq, K.; Xue, T.; Sun, D. Photodynamic Antibacterial Therapy by Metal Complex Mediators: A New Promise for Eliminating Drug-Resistant Infectious Microorganisms. Inorganica Chim. Acta 2025, 587, 122818. [Google Scholar] [CrossRef]
- Dube, E. Antimicrobial Photodynamic Therapy: Self-Disinfecting Surfaces for Controlling Microbial Infections. Microorganisms 2024, 12, 1573. [Google Scholar] [CrossRef]
- Da Silva, L.B.B.; Castilho, I.G.; de Souza Silva, F.A.; Ghannoum, M.; Garcia, M.T.; do Carmo, P.H.F. Antimicrobial Photodynamic Therapy for Superficial, Skin, and Mucosal Fungal Infections: An Update. Microorganisms 2025, 13, 1406. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.M.; Karam, K.; Khan, T.; Wahab, S.; Ullah, S.; Sadiq, M. Reactive Oxygen Species Induced Oxidative Damage to DNA, Lipids, and Proteins of Antibiotic-Resistant Bacteria by Plant-Based Silver Nanoparticles. 3 Biotech 2023, 13, 414. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial Photodynamic Therapy: Overview of a Promising Approach to Fight Antibiotic-Resistant Bacterial Infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Jin, S.; Feng, X. Curcumin, a Plant Polyphenol with Multiple Physiological Functions of Improving Antioxidation, Anti-Inflammation, Immunomodulation and Its Application in Poultry Production. J. Anim. Physiol. Anim. Nutr. 2024, 108, 1890–1905. [Google Scholar] [CrossRef]
- Xu, F.; Hu, J.; Sriboonvorakul, N.; Lin, S. Evaluation of Antimicrobial Photodynamic Activity of CuminUP60®- A Commercially-Available High-Soluble Curcumin. LWT—Food Sci. Technol. 2024, 205, 116490. [Google Scholar] [CrossRef]
- Lopresti, A.L. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef]
- Afrasiabi, S.; AL. Gburi, A.Q.K.; Ranjbar Omrani, L.; Chiniforush, N.; Moradi, Z. Evaluation of Riboflavin, Nanocurcumin, and Hydrogen Peroxide under Light Conditions: Reduction of Mature Dental Biofilms and Enamel Mineral Loss. Photodiagnosis Photodyn. Ther. 2024, 50, 104379. [Google Scholar] [CrossRef]
- Hassan, H.E.; Zaazou, M.H.; Sadony, D.M.; Mohamed, T.A. Effect of Diode Laser Irradiation and Application of Nanoparticle Herbal Endodontic Irrigation Solutions on Candida Albicans and Enterococcus Faecalis Bacteria. Egypt. J. Chem. 2024, 67, 331–340. [Google Scholar] [CrossRef]
- Aguilera, L.F.; Araujo, L.O.; Facchinatto, W.M.; Lima, R.G.; da Silva Pontes, M.; Pulcherio, J.H.V.; Caires, C.S.A.; de Oliveira, K.T.; de Oliveira, S.L.; Caires, A.R.L. Blue-Light Photoactivated Curcumin-Loaded Chitosan Nanoparticles Prepared by Nanoprecipitation and Ionic Gelation: A Promising Approach for Antimicrobial Photodynamic Inactivation. ACS Appl. Bio Mater. 2025, 8, 4055–4064. [Google Scholar] [CrossRef]
- Ferreira, S.; Grenho, L.; Fernandes, M.H.; Lima, S.A.C. Photoactivated Curcumin-Loaded Lipid Nanoparticles in Hydrogel: A Cutting-Edge Intracanal Medicament for Advanced Endodontic Therapy. Gels 2025, 11, 308. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Lee, R.J.; Xiang, G. Nano Encapsulated Curcumin: And Its Potential for Biomedical Applications. Int. J. Nanomed. 2020, 15, 3099–3120. [Google Scholar] [CrossRef]
- Mohanty, H.; Yadav, R.P. Nanoform of Curcumin: Expansion in Therapeutic Applications. Adv. Tradit. Med. 2024, 25, 39–55. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a Photosensitizer: From Molecular Structure to Recent Advances in Antimicrobial Photodynamic Therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Yang, R.; Jia, T.; Liu, C.; Geng, S. Curcumin-Mediated Antimicrobial Photodynamic Therapy for Inactivating Mycobacterium Abscessus: A Promising Approach for Non-Tuberculous Mycobacterial Skin Infections. Lasers Med. Sci. 2025, 40, 9. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Q.; Huang, Y.; Qi, M.; Yan, H.; Li, W.; Zhuang, H. Antibacterial Efficacy and Mechanisms of Curcumin-Based Photodynamic Treatment against Staphylococcus Aureus and Its Application in Juices. Molecules 2022, 27, 7136. [Google Scholar] [CrossRef] [PubMed]
- Ahrari, F.; Mazhari, F.; Ghazvini, K.; Fekrazad, R.; Menbari, S.; Nazifi, M. Antimicrobial Photodynamic Therapy against Lactobacillus Casei Using Curcumin, Nano-Curcumin, or Erythrosine and a Dental LED Curing Device. Lasers Med. Sci. 2023, 38, 260. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, H.; Su, R.; Li, P.; Wen, F.; Lv, Y.; Cai, J.; Su, W. Photoactivated Multifunctional Nanoplatform Based on Lysozyme-Au Nanoclusters-Curcumin Conjugates with FRET Effect and Multiamplified Antimicrobial Activity. J. Drug Deliv. Sci. Technol. 2022, 74, 103548. [Google Scholar] [CrossRef]
- Ensafi, F.; Fazlyab, M.; Chiniforush, N.; Akhavan, H. Comparative Effects of SWEEPS Technique and Antimicrobial Photodynamic Therapy by Using Curcumin and Nano-Curcumin on Enterococcus Faecalis Biofilm in Root Canal Treatment. Photodiagnosis Photodyn. Ther. 2022, 40, 103130. [Google Scholar] [CrossRef]
- Comeau, P.; Leite, M.L.; Manso, A. Development of a New Curcumin-Loaded Dental Varnish for Antimicrobial Photodynamic Therapy Application. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2025, 113, e35596. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.W.L.; Del Bianco Vargas Gouveia, S.; Oliveira, L.C.; de Freitas, R.N.; Dourado, N.G.; Sacoman, C.A.; Ribeiro, A.P.F.; Chaves-Neto, A.H.; Sivieri-Araújo, G.; de Toledo Leonardo, R.; et al. Comparative Analysis of Antimicrobial Activity and Oxidative Damage Induced by Laser Ablation with Indocyanine Green versus APDT with Methylene Blue and Curcumin on E. coli Biofilm in Root Canals. Odontology 2025. [Google Scholar] [CrossRef] [PubMed]
- Zupin, L.; Fontana, F.; Clemente, L.; Borelli, V.; Ricci, G.; Ruscio, M.; Crovella, S. Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer. Viruses 2022, 14, 2132. [Google Scholar] [CrossRef]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H. Anti-Infective Properties of the Golden Spice Curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Julia, G.; Mika, M.; Wr, O.; Siudem, P. Methods to Improve the Solubility of Curcumin from Turmeric. Life 2023, 13, 207. [Google Scholar] [CrossRef]
- Sohn, S.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin—An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef]
- Tsaplev, Y.B.; Lapina, V.A.; Trofimov, A.V. Curcumin in Dimethyl Sulfoxide: Stability, Spectral, Luminescent and Acid-Base Properties. Dye. Pigment. 2020, 177, 108327. [Google Scholar] [CrossRef]
- Damyeh, M.S.; Mereddy, R.; Netzel, M.E.; Sultanbawa, Y. An Insight into Curcumin-Based Photosensitization as a Promising and Green Food Preservation Technology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1727–1759. [Google Scholar] [CrossRef]
- Zhongfa, L.; Chiu, M.; Wang, J.; Chen, W.; Yen, W.; Fan-Havard, P.; Yee, L.D.; Chan, K.K. Enhancement of Curcumin Oral Absorption and Pharmacokinetics of Curcuminoids and Curcumin Metabolites in Mice. Cancer Chemother. Pharmacol. 2012, 69, 679–689. [Google Scholar] [CrossRef]
- Yan, S.; Liao, X.; Xiao, Q.; Huang, Q.; Huang, X. Photostabilities and Anti-Tumor Effects of Curcumin. RSC Adv. 2024, 14, 13694–13702. [Google Scholar] [CrossRef]
- Zhu, J.; Sanidad, K.Z.; Sukamtoha, E.; Zhang, G. Potential Roles of Chemical Degradation in the Biological Activities of Curcumin. Food Funct. 2017, 8, 907–914. [Google Scholar] [CrossRef]
- Wolnicka-glubisz, A.; Olchawa, M.; Duda, M.; Pabisz, P.; Wisniewska-becker, A. The Role of Singlet Oxygen in Photoreactivity and Phototoxicity of Curcumin. Photochem. Photobiol. 2023, 99, 57–67. [Google Scholar] [CrossRef]
- Rodrigues, F.M.S.; Tavares, I.; Aroso, R.T.; Dias, L.D.; Domingos, C.V.; de Faria, C.M.G.; Piccirillo, G.; Maria, T.M.R.; Carrilho, R.M.B.; Bagnato, V.S.; et al. Photoantibacterial Poly (Vinyl) Chloride Films Applying and Photosensitizers. Molecules 2023, 28, 2209. [Google Scholar] [CrossRef]
- Li, Z.; Shi, M.; Li, N.; Xu, R. Application of Functional Biocompatible Nanomaterials to Improve Curcumin Bioavailability. Front. Chem. 2020, 8, 589957. [Google Scholar] [CrossRef] [PubMed]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of Curcumin: An Emerging Paradigm for Improved Remedial Application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef] [PubMed]
- Munir, Z.; Molinar, C.; Banche, G.; Argenziano, M.; Magnano, G.; Cavallo, L.; Mandras, N.; Cavalli, R.; Guiot, C. Encapsulation in Oxygen-Loaded Nanobubbles Enhances the Antimicrobial Effectiveness of Photoactivated Curcumin. Int. J. Mol. Sci. 2023, 24, 15595. [Google Scholar] [CrossRef] [PubMed]
- Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Limami, Y.; Duval, R.E. Curcumin-Based Nanoparticles: Advancements and Challenges in Tumor Therapy. Pharmaceutics 2025, 17, 114. [Google Scholar] [CrossRef]
- Dube, E.; Okuthe, G.E. Biobased Organic Nanoparticles: A Promising Versatile Green Tool for Novel Antimicrobial Agents for Improved Safety. Int. J. Food Sci. 2025, 2025, 7955106. [Google Scholar] [CrossRef] [PubMed]
- Ahrari, F.; Nazifi, M.; Mazhari, F.; Ghazvini, K.; Menbari, S.; Fekrazad, R.; Babaei, K.; Banihashemrad, A. Photoinactivation Effects of Curcumin, Nano-Curcumin, and Erythrosine on Planktonic and Biofilm Cultures of Streptococcus Mutans. J. Lasers Med. Sci. 2024, 15, e7. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Ranjbar Omrani, L.; Noroozian, M.; Ghorbanzadeh, Z.; Bahador, A. In Vitro Antibacterial Activity and Durability of a Nano-Curcumin-Containing Pulp Capping Agent Combined with Antimicrobial Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2021, 33, 102150. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Talaei, N.; Bahador., A. Evaluation of Antimicrobial Effects of Photo-Sonodynamic Antimicrobial Chemotherapy Based on Nano-Micelle Curcumin on Virulence Gene Expression Patterns in Acinetobacter Baumannii. Infect. Disord. Drug Targets 2022, 22, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle Processing: Understanding and Controlling Aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Ababneh, A.M.; Al-Holy, M.; Al-Nabulsi, A.; Osaili, T.; Abughoush, M.; Ayyash, M.; Holley, R.A. A Review of Bacterial Biofilm Components and Formation, Detection Methods, and Their Prevention and Control on Food Contact Surfaces. Microbiol. Res. 2024, 15, 1973–1992. [Google Scholar] [CrossRef]
- Guo, Y.; Mao, Z.; Ran, F.; Sun, J.; Zhang, J.; Chai, G.; Wang, J. Nanotechnology-Based Drug Delivery Systems to Control Bacterial-Biofilm-Associated Lung Infections. Pharmaceutics 2023, 15, 2582. [Google Scholar] [CrossRef]
- Jacob, S.; Kather, F.S.; Morsy, M.A.; Boddu, S.H.S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A.B. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, P.; Chen, R.; Cai, X.; Zhang, W.; Fan, P.; Su, J. A Review of Curcumin-Mediated Photodynamic Bactericidal Technology for Food Preservation: Limitations and Improvement Strategies. Food Microbiol. 2025, 131, 104802. [Google Scholar] [CrossRef]
- Azimzadeh, M.; Greco, G.; Farmani, A.; Pourhajibagher, M.; Taherkhani, A.; Alikhani, M.Y.; Bahador, A. Synergistic Effects of Nano Curcumin Mediated Photodynamic Inactivation and Nano-Silver@colistin against Pseudomonas Aeruginosa Biofilms. Photodiagnosis Photodyn. Ther. 2024, 45, 103971. [Google Scholar] [CrossRef]
- Mirzahosseinipour, M.; Khorsandi, K.; Hosseinzadeh, R.; Ghazaeian, M.; Shahidi, F.K. Antimicrobial Photodynamic and Wound Healing Activity of Curcumin Encapsulated in Silica Nanoparticles. Photodiagnosis Photodyn. Ther. 2020, 29, 101639. [Google Scholar] [CrossRef]
- Silvestre, A.L.P.; dos Santos, A.M.; de Oliveira, A.B.; Ferrisse, T.M.; Brighenti, F.L.; Meneguin, A.B.; Chorilli, M. Evaluation of Photodynamic Therapy on Nanoparticles and Films Loaded-Nanoparticles Based on Chitosan/Alginate for Curcumin Delivery in Oral Biofilms. Int. J. Biol. Macromol. 2023, 240, 124489. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Plotino, G.; Chiniforush, N.; Bahador, A. Dual Wavelength Irradiation Antimicrobial Photodynamic Therapy Using Indocyanine Green and Metformin Doped with Nano-Curcumin as an Efficient Adjunctive Endodontic Treatment Modality. Photodiagnosis Photodyn. Ther. 2020, 29, 101628. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Azimi, M.; Haddadi-asl, V.; Ahmadi, H. Photodiagnosis and Robust Antimicrobial Photodynamic Therapy with Curcumin-Poly(Lactic-Co-Glycolic Acid ) Nanoparticles against COVID-19: A Preliminary in Vitro Study in Vero Cell Line as a Model. Photodiagnosis Photodyn. Ther. 2021, 34, 102286. [Google Scholar] [CrossRef]
- Xiang, Y.-l.; Tang, D.-y.; Yan, L.-l.; Deng, L.-l.; Wang, X.-h.; Liu, X.-y.; Zhou, Q.-h. Poly-L-Lysine Modified MOF Nanoparticles with PH/ROS Sensitive CIP Release and CUR Triggered Photodynamic Therapy against Drug-Resistant Bacterial Infection. Int. J. Biol. Macromol. 2024, 266, 131330. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Guan, J.; Wang, W.; Wang, L.; Su, J.; Fang, L. PH and Light-Responsive Polycaprolactone/Curcumin@zif-8 Composite Films with Enhanced Antibacterial Activity. J. Food Sci. 2021, 86, 3550–3562. [Google Scholar] [CrossRef]
- Meng, X.; Guan, J.; Lai, S.; Fang, L.; Su, J. PH-Responsive Curcumin-Based Nanoscale ZIF-8 Combining Chemophotodynamic Therapy for Excellent Antibacterial Activity. RSC Adv. 2022, 12, 10005–10013. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, W.; Gao, Q.; Lai, S.; Liu, Y.; Zhou, S.; Yan, Y.; Zhang, J.; Wang, H.; Wang, J.; et al. Intelligent Bacteria-Targeting ZIF-8 Composite for Fluorescence Imaging-Guided Photodynamic Therapy of Drug-Resistant Superbug Infections and Burn Wound Healing. Exploration 2024, 4, 20230113. [Google Scholar] [CrossRef] [PubMed]
- Henriques, J.; Pina, J.; Braga, M.E.M.; Dias, A.M.A.; Coimbra, P.; de Sousa, H.C. Novel Oxygen- and Curcumin-Laden Ionic Liquid@Silica Nanocapsules for Enhanced Antimicrobial Photodynamic Therapy. Pharmaceutics 2023, 15, 1080. [Google Scholar] [CrossRef]
- Guo, C.; Cheng, F.; Liang, G.; Zhang, S.; Duan, S.; Fu, Y.; Marchetti, F.; Zhang, Z.; Du, M. Multimodal Antibacterial Platform Constructed by the Schottky Junction of Curcumin-Based Bio Metal–Organic Frameworks and Ti3C2Tx MXene Nanosheets for Efficient Wound Healing. Adv. NanoBiomed Res. 2022, 2, 2200064. [Google Scholar] [CrossRef]
- Li, P.; Li, B.; Wang, C.; Zhao, X.; Zheng, Y.; Wu, S.; Shen, J.; Zhang, Y.; Liu, X. In Situ Fabrication of Co-Coordinated TCPP-Cur Donor-Acceptor-Type Covalent Organic Framework-like Photocatalytic Hydrogel for Rapid Therapy of Bacteria-Infected Wounds. Compos. Part B 2023, 252, 110506. [Google Scholar] [CrossRef]
- Elkhateeb, O.; Badawy, M.E.I.; Tohamy, H.G.; Abou-ahmed, H.; El-kammar, M.; Elkhenany, H. Curcumin-Infused Nanostructured Lipid Carriers: A Promising Strategy for Enhancing Skin Regeneration and Combating Microbial Infection. BMC Vet. Res. 2023, 19, 206. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Primavera, R.; Joyce, R.; Thakor, A.S.; Kevadiya, B.D. Cellular Uptake and Retention of Nanoparticles: Insights on Particle Properties and Interaction with Cellular Components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Dragicevic, N.; Predic-Atkinson, J.; Nikolic, B.; Pajovic, S.B.; Ivkovic, S.; Adzic, M. Nanocarriers in Topical Photodynamic Therapy. Expert Opin. Drug Deliv. 2024, 21, 279–307. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.M.; Fangueiro, R.; Ferreira, D.P. Drug Delivery Systems for Photodynamic Therapy: The Potentiality and Versatility of Electrospun Nanofibers. Macromol. Biosci. 2022, 22, 2100512. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, H.M.; AbuelEzz, N.Z.; Ali, A.A.; Abdelaziz, A.E.; Nada, D.; Abdelraouf, S.M.; Fouad, S.A.; Bishr, A.; Radwan, R.A. Newly Designed Curcumin-Loaded Hybrid Nanoparticles: A Multifunctional Strategy for Combating Oxidative Stress, Inflammation, and Infections to Accelerate Wound Healing and Tissue Regeneration. BMC Biotechnol. 2025, 25, 49. [Google Scholar] [CrossRef]
- Paolillo, F.R.; Gracielli, P.; Rodrigues, S.; Bagnato, V.S.; Alves, F.; Pires, L.; Corazza, A.V. The Effect of Combined Curcumin-Mediated Photodynamic Therapy and Artificial Skin on Staphylococcus Aureus—Infected Wounds in Rats. Lasers Med. Sci. 2021, 36, 1219–1226. [Google Scholar] [CrossRef]
- Jansirani, D.; Muthulakshmi, L.; Gopukumar, S.T.; Rajeswari, R. Diabetic Wound Healing Effects of Photodynamic Therapy Using Chitosan Nanoparticles Encapsulated with Natural Photosensitizers in Wistar Rats. Bionanoscience 2025, 15, 376. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kang, H.W. Engineering Pharmaceutical Nanocarriers for Photodynamic Therapy on Wound Healing: Review. Mater. Sci. Eng. C 2019, 105, 110110. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tang, Q.; Xu, Z.; Xu, Y.; Zhang, H.; Zheng, D.; Wang, S.; Tan, Q.; Maitz, J.; Maitz, P.K.; et al. Challenges and Innovations in Treating Chronic and Acute Wound Infections: From Basic Science to Clinical Practice. Burn. Trauma 2022, 10, tkac014. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Sakima, V.T.; Barbugli, P.A.; Cerri, P.S.; Chorilli, M.; Carmello, J.C.; Pavarina, A.C.; De Oliveira Mima, E.G. Antimicrobial Photodynamic Therapy Mediated by Curcumin-Loaded Polymeric Nanoparticles in a Murine Model of Oral Candidiasis. Molecules 2018, 23, 2075. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Pourakbari, B.; Bahador, A. Contribution of Antimicrobial Photo-Sonodynamic Therapy in Wound Healing: An in Vivo Effect of Curcumin-Nisin-Based Poly (L-Lactic Acid) Nanoparticle on Acinetobacter Baumannii Biofilms. BMC Microbiol. 2022, 22, 28. [Google Scholar] [CrossRef]
- Ghanemi, M.; Salehi-Vaziri, A.; Pourhajibagher, M.; Bahador, A. Physico-Mechanical and Antimicrobial Properties of an Elastomeric Ligature Coated with Reduced Nanographene Oxide-Nano Curcumin Subjected to Dual-Modal Photodynamic and Photothermal Inactivation against Streptococcus Mutans Biofilms. Photodiagnosis Photodyn. Ther. 2023, 44, 103866. [Google Scholar] [CrossRef]
- Bessa, L.J.; Botelho, J.; Machado, V.; Alves, R.; Mendes, J.J. Managing Oral Health in the Context of Antimicrobial Resistance. Int. J. Environ. Res. Public Health 2022, 19, 16448. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Salehi Vaziri, A.; Takzaree, N.; Ghorbanzadeh, R. Physico-Mechanical and Antimicrobial Properties of an Orthodontic Adhesive Containing Cationic Curcumin Doped Zinc Oxide Nanoparticles Subjected to Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2019, 25, 239–246. [Google Scholar] [CrossRef]
- Da Silva, F.C.; Rosa, L.P.; De Oliveira Santos, G.P.; Inada, N.M.; Blanco, K.C.; Araújo, T.S.D.; Bagnato, V.S. Total Mouth Photodynamic Therapy Mediated by Blue Led and Curcumin in Individuals with AIDS. Expert Rev. Anti. Infect. Ther. 2020, 18, 689–696. [Google Scholar] [CrossRef]
- Panhóca, V.H.; Florez, F.L.E.; Corrêa, T.Q.; Paolillo, F.R.; de Souza, C.W.O.; Bagnato, V.S. Oral Decontamination of Orthodontic Patients Using Photodynamic Therapy Mediated by Blue-Light Irradiation and Curcumin Associated with Sodium Dodecyl Sulfate. Photomed. Laser Surg. 2016, 34, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Peng, Y.; Hu, S.; Xu, J.; Yu, C.; Hua, L.; Zhou, S.; Liu, Q. Therapeutic Application of Curcumin and Its Nanoformulation in Dentistry: Opportunities and Challenges. AIMS Mol. Sci. 2025, 12, 148–172. [Google Scholar] [CrossRef]
- Gnanasekar, S.; Kasi, G.; He, X.; Zhang, K.; Xu, L.; Kang, E.T. Recent Advances in Engineered Polymeric Materials for Efficient Photodynamic Inactivation of Bacterial Pathogens. Bioact. Mater. 2023, 21, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, C.R.; Peddinti, B.S.T.; Kisthardt, S.C.; Scholle, F.; Spontak, R.J.; Ghiladi, R.A. Toward Universal Photodynamic Coatings for Infection Control. Front. Med. 2021, 8, 657837. [Google Scholar] [CrossRef]
- Moideen, S.K.; Anas, A.; Sobhanan, J.; Zhao, H.; Biju, V. Photoeradication of Aquatic Pathogens by Curcumin for Clean and Safe Drinking Water. J. Photochem. Photobiol. A Chem. 2022, 432, 114104. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Umesh Hebbar, H. Efficacy of Blue LED in Microbial Inactivation: Effect of Photosensitization and Process Parameters. Int. J. Food Microbiol. 2019, 290, 296–304. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Cao, S.; Tang, Y.; Wang, M.; Qu, C. Photodynamic Bactericidal Nanomaterials in Food Packaging: From Principle to Application. J. Food Sci. 2025, 90, e17606. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Linson, N.; Mavelil-Sam, R.; Maria, H.J.; Pothan, L.A.; Thomas, S.; Kabdrakhmanova, S.; Laroze, D. Poly(Lactic Acid)/Nanocellulose Biocomposites for Sustainable Food Packaging; Springer: Dordrecht, The Netherlands, 2024; Volume 31, ISBN 0123456789. [Google Scholar]
- Sheng, L.; Li, X.; Wang, L. Photodynamic Inactivation in Food Systems: A Review of Its Application, Mechanisms, and Future Perspective. Trends Food Sci. Technol. 2022, 124, 167–181. [Google Scholar] [CrossRef]
- Wen, F.; Li, P.; Yan, H.; Su, W. Turmeric Carbon Quantum Dots Enhanced Chitosan Nanocomposite Films Based on Photodynamic Inactivation Technology for Antibacterial Food Packaging. Carbohydr. Polym. 2023, 311, 120784. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.F.; Tosati, J.V.; Tikekar, R.V.; Monteiro, A.R.; Nitin, N. Antimicrobial Activity of Curcumin in Combination with Light against Escherichia Coli O157:H7 and Listeria Innocua: Applications for Fresh Produce Sanitation. Postharvest Biol. Technol. 2018, 137, 86–94. [Google Scholar] [CrossRef]
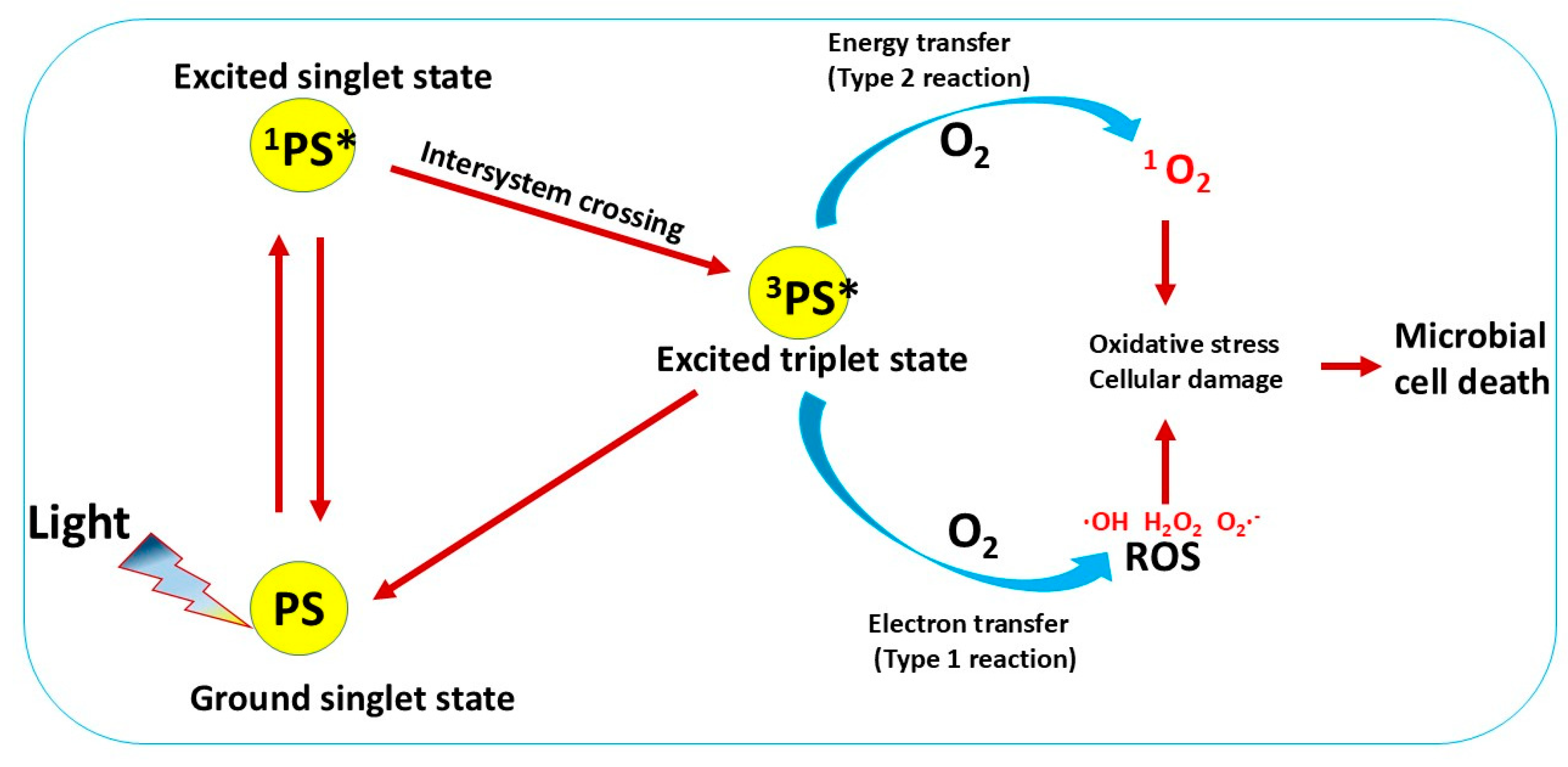


| Curcumin Concentration | Light Dosage | Effect | Ref |
|---|---|---|---|
| CuminUP60® vs. natural curcumin, 10.42–333.3 μM | light-emitting diode (LED, 425 ± 20 nm), 5.3 mW/cm2 for 30 min (9.54 J/cm2) | Both curcumin forms exhibited broad-spectrum bactericidal activity against multiple bacteria including S. aureus, Listeria monocytogenes, Escherichia coli, Vibrio parahaemolyticus, Shewanella putrefaciens, and Pseudomonas fluorescens. | [9] |
| 5 g/L and 3 g/L | LED (450 nm), 1200 mW/cm2, irradiance 738 mW/cm2 | At 3 g/L, curcumin reduced Lactobacillus casei by 99.5%, comparable to chlorhexidine (100%) and exceeding non-irradiated curcumin (60.3%). | [21] |
| 60 μg/mL (S. aureus), 100 μg/mL (E. coli) | Laser (405 nm), 14 mW/cm2, 40 min | Curcumin achieved 93.2% antibacterial rate against S. aureus and 42.6% against E. coli. | [22] |
| 5 mg/mL | LED (390–480 nm), 1000 ± 100 mW/cm2, 60 s (60 J/cm2) | E. faecalis count reduced by 1.35 log10 CFU/mL; more effective than curcumin alone(1.73 log10 CFU/mL), untreated control (3.03 log10 CFU/mL), and LED alone (2.90 log10 CFU/mL). | [23] |
| 0.25% and 10.0% Curcumin-loaded varnish | LED (440–460 nm), 19 mW/cm2 for 15–60 min (17.4–69.7 J/cm2) | Increased curcumin loading and light dose enhanced photoelimination of S. mutans and Candida albicans. | [24] |
| 100 μM | 50 W xenon lamp (400–780 nm), 48 J/cm2 | Complete inactivation of M. abscessus. | [19] |
| 10 µM | LED (440 ± 5 nm), 3.6 × 10−3 W/cm2, 1.296–2.592 J/cm2 | aPDT reduced S. aureus by 6.9 log CFU/mL in phosphate-buffered saline (PBS). Efficacy declined in fruit juices due to ROS quenching; pineapple was more responsive than mango; carrot juice showed no effect. At 2.592 J/cm2, no S. aureus was detectable in mango/pineapple juice. | [20] |
| 0.05% | LED (480 nm), 72 J/cm2 | In root canal treatment, curcumin-aPDT achieved 34% reduction in E. coli CFU, with 12% standard deviation. | [25] |
| 1–5 µM | Diode laser (445 nm), 0.25 W/cm2, 15 J/cm2 | Curcumin combined with blue light irradiation reduced SARS-CoV-2 viral load by >99% in vitro, despite the concentrations alone showing no antiviral activity | [26] |
| nCur Size | nCur Concentration | Light Dosage | Effect | Ref |
|---|---|---|---|---|
| - | 3 g/L | Blue LED emitting at 385–515 nm, output intensity of 1200 mW/cm2, irradiance, 738 mW/cm2 | aPDT significantly reduced Streptococcus mutans viability, with curcumin (3 g/L) achieving a 99.7% reduction, while nano-curcumin showed lower effectiveness, reaching 82.3% reduction | [42] |
| 9.5 ± 0.1 nm | 0.5% nCur in 0.9% sodium chloride solution | LED with wavelength of 450 nm, power density was 1 W/cm2, energy density was 60 J/cm2 and irradiation duration was 60 s | aPDT) achieved the greatest reduction in dual-species S. mutans and Lactobacillus acidophilus biofilms, with 40% reduction compared to nCur alone (24.53%). | [11] |
| 100 nm | - | Laser (980 nm), Power: 0.5 W, irradiation duration was 20 s | Laser-activated nCur demonstrated significant antimicrobial effects against both E. faecalis and C. albicans | [12] |
| - | nCur was added to Activa BioActive Base/Liner (ABBL) in 0.5%, 1%, 2%, and 5% (w/w) concentrations to fabricate nCur-ABBL disks | LED (435 ± 20 nm), output intensity 1000–1400 mW/cm2, dose 300–420 J/cm2, duration 5 min | After 60 days of aging, 5% nCur-ABBL disks activated by aPDT showed a 97.75% reduction in S. mutans colonies, while 2% nCur-ABBL discs showed a 96.25% reduction | [43] |
| - | 3 g/L |
LED (450 nm), power intensity of 1200 mW/cm2, irradiance 738 mW/cm2 | aPDT using nCur achieved a 96.5% reduction in L. casei biofilms, closely approaching the complete inhibition observed with the positive control, chlorhexidine digluconate (CHX, 100%). | [21] |
| - | 5 mg/mL | LED (390 to 480 nm), power density of 1000 ± 100 mW/cm2, irradiation time of 60 s, energy density was 60 J/cm2 | nCur-mediated aPDT significantly reduced E. faecalis count (1.22 log10 CFU/mL) versus curcumin alone (1.71), untreated control (3.03), and LED alone (2.90). | [23] |
| - | 3.1 mM. 6.2 mM and 12.5 mM | 28.7 mW/cm2 + 2 min laser, 45.2 mW/cm2 + 3 min laser and 45.2 mW/cm2 + 4 min laser | Significant reduction in Acinetobacter baumannii viability and modulating its virulence gene expression. | [44] |
| Curcumin Nanocarriers and Nanoconjugates | Curcumin Concentration | Light Dosage | Effect | Ref |
|---|---|---|---|---|
| Curcumin-loaded Chitosan (CT) NPs | 0.04 and 0.08 mg/mL | Blue light at 450 nm, light intensity 28.84 mW/cm2, irradiation time, 60 min | A reduction of more than 3 log10 CFU was observed in both S. aureus and E. coli | [13] |
| Curcumin loaded chitosan/sodium alginate NPs (Cur-CT-Alg NPs), 257.2 to 473.1 nm | - | LED (460 nm), Energy dose 15 J/cm2 for 11 min and 53 s | Cur-CT-Alg NPs combined with aPDT significantly reduced S. mutans biofilms by 3 log10 CFU/mL | [52] |
| Curcumin-containing chitosan-shelled nanobubbles, (Cur-CT-NBs) | 743 ± 44 µg/mL of NBs | LED, (425 nm to 470 nm) for 3 h | Cur-CT-NBs showed the following MIC values: for E. coli, 46.4 µg/mL with and without LED; for S. aureus, 92.8 µg/mL without LED and 46.4 µg/mL with LED; and for E. faecalis, 46.4 µg/mL without LED and 23.2 µg/mL with LED. | [39] |
| Oxygen-loaded curcumin-containing nanobubbles, (Cur-Oxy-CT-NBs) | 743 ± 49 µg/mL of NBs | LED (425 nm to 470 nm) for 3 h | Cur-Oxy-CT-NBs exhibited enhanced antibacterial activity, with minimum inhibitory concentrations (MICs) as follows: for E. coli, 92.8 µg/mL without LED and 46.4 µg/mL with LED; for S. aureus, 46.4 µg/mL without LED and 11.6 µg/mL with LED; and for E. faecalis, 92.8 µg/mL without LED and 46.4 µg/mL with LED. | [39] |
| Curcumin-loaded solid lipid NPs (SLNPs- composed of cetyl palmitate and Tween® 80) within an alginate-based hydrogel matrix | 20% (v/v), and 50% (v/v) Cur-SLN in hydrogel | LED (430–490 nm) for 150 s | Photoactivated formulations reduced E. faecalis biofilms by 70%, outperforming non-photoactivated (45%) | [14] |
| Indocyanine green (ICG) doped with nano-curcumin and conjugated with Metformin (Met) (Cur-ICG-Met NPs), 60–100 nm | Cur-ICG-Met NPs consist of 100 mg of Cur NPs, 1000 μg/mL of ICG, and 10 mM of Met and 10 μL of Cur-ICG-Met NPs was used for aPDT | Diode Laser (810 nm), Energy density: 31.2 J/cm2 and power output capacity: 200 mW and LED (450 nm), Energy density: 60 J/cm2 and power output intensity: 500 mW/cm2 | The dual-wavelength irradiation technique (diode laser + LED) effectively reduced viable E. faecalis within biofilm structures, achieving a cell viability reduction ranging from 82.74% to 83.84%. | [53] |
| nCur-AgNPs-colistin (CL) (402.6 nm) | 250 µg/mL | LED light (450 ± 10 nm), Output Power: 200 mW, Power Density: 0.51 W/cm2, Exposure Time: 14 min (energy density was 476 J/cm2). | Reduced the formation of biofilm in P. aeruginosa | [50] |
| Lysozyme (Lys)-gold nanocluster (AuNCs)-Cur conjugate, (Lys-AuNCs-Cur) (Lys-AuNCs = 5 nm) | 10 to 60 μg/mL for S. aureus | Laser light (405 nm), intensity was 14 mW/cm2, exposure time 40 min | Lys-AuNCs-Cur exhibited greater antibacterial activity against S. aureus and E. coli than free curcumin and effectively eradicated MRSA. | [22] |
| Up to 100 μg/mL for E. coli | ||||
| Up to 100 μg/mL for MRSA | ||||
| Cur-silica NPs (36 to 40 nm) | 1 mg/mL | LED (465 nm), power density was 34 mW/cm2, irradiation time was 10 min, light dose was 20 J/cm2 | Cur–silica NPs caused a significant reduction of more than 6 log10 CFU in the growth of both S. aureus and P. aeruginosa planktonic cells. | [51] |
| Curcumin encapsulated in poly (lactic-co-glycolic acid) (PLGA) NPs (Cur@PLGA-NPs) | 10% weight (wt.) | Blue laser (450 ± 10 nm), output intensity of 1.6 W/cm2, 522.8 J/cm2, | Cur@PLGA-NPs demonstrated in vitro anti-COVID-19 activity by significantly reducing SARS-CoV-2 titers in infected plasma. | [54] |
| Curcumin encapsulated into zeolite imidazole framework-8 (ZIF-8) NPs, and coated with ethylated poly-L-lysine (PLL) and loaded with ciprofloxacin (CIP) (Cur@ZIF-8 NPs/PLL-CIP, 325.9 nm) | In vitro, the Cur concentration was 16 μg/mL at a Cur@ZIF-8 NPs/PLL-CIP dose of 500 μg/mL, while in vivo wound healing studies in mice used 8 μg/mL Cur at a NP dose of 250 μg/mL. | Blue light (430 nm), intensity of 10 mW/cm2 for 10 min in vitro and 20 min in vivo | Cur@ZIF-8 NPs/PLL-CIP enable ROS-responsive drug release and curcumin-triggered photodynamic therapy, effectively killing methicillin-resistant S. aureus (MRSA) (98.06%) and disrupting biofilms (99.83%) under 430 nm light with H2O2. In MRSA-infected mice, they accelerated wound healing (98.81% rate in 11 days) and restored normal skin structure, demonstrating strong synergistic antimicrobial and therapeutic effects. | [55] |
| Curcumin/polycaprolactone@zeolitic imidazolate framework-8 (Cur/PCL@ZIF-8, ~100–200 nm) composite film | Composite films contained ≥15% w/w Cur@ZIF-8 | Blue light (420–430 nm), intensity of 2.2 mW/cm2 | Composite films loaded with more than 15% (w/w) Cur@ZIF-8 demonstrated a 99.9% reduction in Escherichia coli and S. aureus, alongside a strong anti-adhesion effect. | [56] |
| Cur@ZIF-8 NPs (150 nm, while ZIF-8 alone was 80 nm) | 5 mg/mL | Blue light (420–430 nm), intensity of 2.2 mW/cm2 for 1 h | Cur@ZIF-8 NPs exhibited strong antibacterial activity against E. coli and S. aureus, which was significantly enhanced under blue light. The combination triggered ROS overproduction and K+ leakage, disrupting bacterial membranes. Cur@ZIF-8 NPs under light outperformed Cur alone, | [57] |
| Curcumin and aggregation-induced emission luminogen (TTD), encapsulated into ZIF-8 (Cur-TTD@ZIF-8 NPs, 360.84 nm) | 62.5 μg/mL (2MIC) against S. aureus and 125 μg/ mL (2MIC) against P. aeruginosa. | Blue light, 12 J/cm2 (20 mW/cm2 for 10 min) | Cur-TTD@ZIF-8 NPs provide bacteria targeting, fluorescence imaging, pH-responsive photodynamic therapy (PDT), and anti-inflammatory effects. Their positive charge enables targeting of P. aeruginosa and S. aureus, with red fluorescence aiding imaging. Under blue light, Cur release triggers ROS generation, yielding 99.94% and 99.84% antibacterial rates. In vivo, they reduced bacterial load, alleviated inflammation, and accelerated wound healing, promoting tissue regeneration, angiogenesis, collagen deposition | [58] |
| Oxygen- and curcumin-laden ionic liquid@silica nanocapsules (Cur-IL@SiNCs, 15–70 nm and 280–350 nm) | 2 mg of Cur per gram of IL | Blue light LED Lamp, 5 W (450 nm) | Cur-IL@SiNCs in gelatin films showed a photodynamic antimicrobial effect under blue light, significantly reducing bacterial growth | [59] |
| Curcumin-based bioactive zinc-based metal–organic frameworks (Zn-MOF) and Ti3C2Tx MXene nanosheets (NSs) (Cur/Zn-MOF@Ti3C2Tx, lattice spacing of 0.22 nm) | 12.5 to 200 μg/mL | Near-infrared (NIR) light (808 nm), 1.0 W/ cm2 for 15 min | The Cur/Zn-MOF@Ti3C2Tx nanosheets combine photothermal therapy (49.7 °C, 38.2% efficiency), photodynamic ROS generation (•O2−, •OH, 1O2), and NIR-triggered release of Zn2+ and curcumin. It kills E. coli and S. aureus, inhibits bacterial growth for 24 h, and accelerates infected wound healing (>99% closure in 8 days). | [60] |
| Curcumin linked to tetrakis (4-carboxyphenyl) porphyrin TCPP (Cur-TCPP NPs) and co-coordinated with copper (Cu) and sodium alginate (SA) to form a covalent organic framework-like (COF-like) structure (800 nm long) | Cur-TCPP NPs were used at 10 mg/mL in hydrogel synthesis. For ROS detection, Cur-TCPP, and Cur-CuTCPP were tested at 0.5 mg/mL (as precursors: 112 mg TCPP, 72 mg Cur used). | Light (660 nm), 0.4 W/cm2 for 20 min | The Cur -TCPP NPs COF hydrogel exhibited strong antibacterial activity, achieving 99.95% and 99.8% eradication of S. aureus and E. coli, respectively. It increased bacterial membrane permeability, causing protein leakage. In vivo, it reduced TNF-α, elevated IL-10 and VEGF expression, enhanced collagen deposition, and accelerated wound healing and cell migration. | [61] |
| NPs | Particle Size, Zeta Potential | Drug Loading Rate and Release Kinetics | Toxicity | Ref |
|---|---|---|---|---|
| Curcumin-loaded Chitosan (CT) NPs | 122 ± 2 nm | Cur-CT NPs demonstrated a high curcumin encapsulation efficiency of 96%, indicating excellent drug loading capacity. They also exhibited a controlled and sustained release profile, with only 17% of curcumin released over 400 min, in contrast to 50% release from free curcumin within the same timeframe | - | [13] |
| Curcumin loaded chitosan/sodium alginate NPs (Cur-CT-Alg NPs) | 257.2 to 473.1 nm, −33.0 to −26.5 mV | The NPs showed high curcumin encapsulation efficiency (64.13–80.23%), with the highest at a CT:Alg ratio of 0.30:1. Higher ratios reduced EE% to ~33%. In simulated saliva (pH 7), Cur-CT-Alg NPs exhibited sustained release, with ~50% of curcumin released over 24 h, demonstrating effective drug retention. | The Cur-CT-Alg NPs showed excellent biocompatibility, and their use in aPDT achieved a 3-log10 CFU/mL reduction in S. mutans biofilms without toxicity. | [52] |
| Curcumin-containing chitosan-shelled nanobubbles, (Cur-CT-NBs) | 511 ± 25 nm +30 ± 2 mV. | Cur-CT-NBs and Cur-Oxy-CT-NBs demonstrated curcumin loading capacities of 4.3% and 5.3%, respectively, with high encapsulation efficiencies ranging from 80% to 88%. Curcumin was successfully incorporated into both the nanobubble core and the chitosan shell, with slightly higher EE and LC observed in Cur-Oxy-CT-NBs. In vitro release studies showed sustained release, with 69.14% and 66.98% of curcumin released from Cur-CT-NBs and Cur-Oxy-CT-NBs after 48 h, respectively. | Biocompatible oxygen- and curcumin-loaded NBs exhibited antimicrobial activity via ROS generation under LED irradiation, damaging bacterial membranes without harming human cells. Curcumin’s antioxidant effect within NBs reduced oxidative stress markers, supporting safe, eco-friendly antimicrobial applications. | [39] |
| Oxygen-loaded curcumin-containing nanobubbles, (Cur-Oxy-CT-NBs) | 521 ± 30 nm +31 ± 1 mV | |||
| Curcumin-loaded solid lipid NPs (SLNPs- composed of cetyl palmitate and Tween® 80) within an alginate-based hydrogel matrix | - | Curcumin-loaded SLNs were added to hydrogels at 20% and 50%, containing 9.5 µg and 24 µg of curcumin per 0.5 mL. Both gels showed slow, steady release over 72 h at body pH, with no burst effect. About 40% of curcumin was released from the 20% gel and 70% from the 50% gel. | Cytocompatibility tests with human gingival fibroblasts (hGFs) showed high cell viability for all formulations, including those exposed to aPDT. After 24 h and even 3 days, metabolic activity remained comparable to controls. Immunostaining confirmed normal fibroblast morphology. Prior studies support the safety of curcumin-PDT below 500 mg/L for dental applications. | [14] |
| Indocyanine green (ICG) doped with nano-curcumin and conjugated with Metformin (Met) (Cur-ICG-Met NPs) | 60–100 nm, −9.1 ± 1.3 mV | -- | - | [53] |
| nCur-AgNPs-colistin (CL) | 402.6 nm, nCur at 10.3 mV | - | An MTT assay confirmed the low cytotoxicity of nCur-AgNPs-colistin toward L929 fibroblasts, with cell viability ranging from 83.20% to 92.48% at FBIC concentrations for P. aeruginosa strains. Even at twice the FBIC, ~80% of cells remained viable. | [50] |
| Lysozyme (Lys)-gold nanocluster (AuNCs)-Cur conjugate, (Lys-AuNCs-Cur) (Lys-AuNCs) | 5 nm +42 mV | - | Lys-AuNCs-Cur exhibited excellent biocompatibility, showing minimal cytotoxicity toward normal human hepatocyte (L-02) cells, with over 90% viability even at 400 μg/mL. | [22] |
| Cur-silica NPs | 36 to 40 nm | Curcumin was loaded into silica NPs at a 10:1 SiO2-to-curcumin ratio, achieving 5% loading. At 50 μg/mL of nanoformulation, the effective curcumin concentration is approximately 2.5 μg/mL—20 times lower than the equivalent free curcumin dose. | Cur-silica NPs had a less cytotoxic/phototoxic effect on HDF fibroblast cells | [51] |
| Curcumin encapsulated in poly (lactic-co-glycolic acid) (PLGA) NPs (Cur@PLGA-NPs) | The encapsulation efficiency (EE) of curcumin in Cur@PLGA NPs was 87.6 ± 3.25%, with a loading capacity (LC) of 8.42 ± 0.23%. | aPDT with 10% Cur@PLGA-NPs and blue laser was safe, showing no cell damage or plasma quality changes. | [54] | |
| Curcumin encapsulated into zeolite imidazole framework-8 (ZIF-8) NPs, and coated with ethylated poly-L-lysine (PLL) and loaded with ciprofloxacin (CIP) (Cur@ZIF-8 NPs/PLL-CIP) | 325.9 nm −4.57 mV at pH 7.4 and −6.19 mV at pH 5.5 | The drug loading capacity was 16.2% for CIP and 3.3% for Cur. At physiological pH (7.4), the 48 h release rates were 41.6% for Cur and 43.3% for CIP. Upon the addition of 2 mM H2O2, these rates increased to 66.1% for Cur and 64.7% for CIP. Under acidic conditions (pH 5.5) in the presence of H2O2, release rates further increased to 77.4% for Cur and 70.9% for CIP. | In vitro tests showed 69.1% HUVEC viability at 100 μg/mL, and only 2.1% haemolysis at 700 μg/mL, indicating good biocompatibility. In vivo, treated mice showed no weight loss or organ toxicity. Histological analysis revealed no tissue damage, and renal and hepatic function markers (AST, ALT, UREA, CREA, ALB) remained within normal ranges, confirming excellent biosafety. | [55] |
| Curcumin/polycaprolactone@zeolitic imidazolate framework-8 (Cur/PCL@ZIF-8) composite film | ~100–200 nm | 35% w/w Cur@ZIF-8 was loaded in PCL. Cur in the Cur/PCL film was rapidly released within 6 h, significantly exceeding that from the Cur/PCL@ZIF-8 composite film. Absorbance at 424 nm increased with time and higher Cur@ZIF-8 content, indicating sustained release. After 72 h, curcumin release under acidic conditions was nearly twice that under neutral conditions, demonstrating the film’s pH-responsive and controlled-release properties. | - | [56] |
| Cur@ZIF-8 NPs | 150 nm (while ZIF-8 alone was 80 nm) | Cur@ZIF-8 NPs exhibited a drug loading capacity of 11.57% and an encapsulation efficiency of 82.76%. Cur@ZIF-8 NPs exhibited pH-sensitive release behavior, maintaining high Cur retention rates at neutral to alkaline pH—93.38% at pH 7.4, 96.19% at pH 9, and 93.01% at pH 10 over 72 h—indicating excellent stability of Cur within the ZIF-8 framework. In contrast, under acidic conditions (pH 5.5 and 6.5), a burst release was observed during the initial 8 h, with cumulative Cur release reaching approximately 60% and 70%, respectively, demonstrating effective pH-triggered release for targeted antimicrobial applications. | - | [57] |
| Curcumin and aggregation-induced emission luminogen (TTD), encapsulated into ZIF-8 (Cur-TTD@ZIF-8 NPs) | 360.84 nm | Drug-loading capacity (DLC) was 5.12%, and drug-loading encapsulation (DLE) was 20.46%. The release of Cur from Cur-TTD@ZIF-8 NPs is pH-dependent, showing high retention at pH 7.5. At pH 5.5 and 6.5, a rapid burst release occurred within 1 h, reaching ~70% cumulative release due to the acid-sensitive disintegration of ZIF-8 | In vitro, the NPs maintained high cell viability in L929 (mouse fibroblast) and HUVEC (human umbilical vein endothelial) cells up to 250 μg/mL, with mild toxicity at 500 μg/mL due to excess Cur and Zn2+. Hemolysis assays showed no adverse effects on red blood cells. In vivo, treatment in rats (125 μg/mL for 7 days) showed no abnormalities in blood tests or organ histology, indicating good biocompatibility. | [58] |
| Curcumin-based bioactive zinc-based metal–organic frameworks (Zn-MOF) and Ti3C2Tx MXene nanosheets (NSs) (Cur/Zn-MOF@Ti3C2Tx) | lattice spacing of 0.22 nm | Curcumin from Cur/Zn-MOF@Ti3C2Tx under NIR irradiation showed rapid release in the first 2 h, reaching 10.09 μg/mL at 48 h, 2.1× higher than the non-irradiated group. Similarly, Zn2+ release peaked at 3.64 μg/mL. NIR irradiation significantly enhanced initial release rates of both curcumin and Zn2+ within 12 h | Cur/Zn-MOF@Ti3C2Tx exhibits excellent in vitro and in vivo biocompatibility, with >85% cell viability, <4% hemolysis, and minimal in vivo toxicity, even at 200 μg/mL. | [60] |
| Curcumin linked to tetrakis (4-carboxyphenyl) porphyrin TCPP (Cur-TCPP NPs) and co-coordinated with copper (Cu) and sodium alginate (SA) to form a covalent organic framework-like (COF-like) structure. | 800 nm long TCPP, Cur, and TCPP-Cur were negatively charged. However, CuTCPP-Cur was positively charged | - | The Cur-TCPP NPs COF hydrogel showed excellent cytocompatibility in vitro and no organ toxicity in vivo. Minimal copper ion release and negligible hemolysis further confirmed its good biocompatibility and biosafety. | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dube, E.; Okuthe, G.E. Nanocurcumin and Curcumin-Loaded Nanoparticles in Antimicrobial Photodynamic Therapy: Mechanisms and Emerging Applications. Micro 2025, 5, 39. https://doi.org/10.3390/micro5030039
Dube E, Okuthe GE. Nanocurcumin and Curcumin-Loaded Nanoparticles in Antimicrobial Photodynamic Therapy: Mechanisms and Emerging Applications. Micro. 2025; 5(3):39. https://doi.org/10.3390/micro5030039
Chicago/Turabian StyleDube, Edith, and Grace Emily Okuthe. 2025. "Nanocurcumin and Curcumin-Loaded Nanoparticles in Antimicrobial Photodynamic Therapy: Mechanisms and Emerging Applications" Micro 5, no. 3: 39. https://doi.org/10.3390/micro5030039
APA StyleDube, E., & Okuthe, G. E. (2025). Nanocurcumin and Curcumin-Loaded Nanoparticles in Antimicrobial Photodynamic Therapy: Mechanisms and Emerging Applications. Micro, 5(3), 39. https://doi.org/10.3390/micro5030039







