Nanotechnology-Based Face Masks: Transforming the Cosmetics Landscape
Abstract
1. Introduction
2. Role of Nanotechnology in Cosmetics
3. Role of Macromolecules in Cosmetics
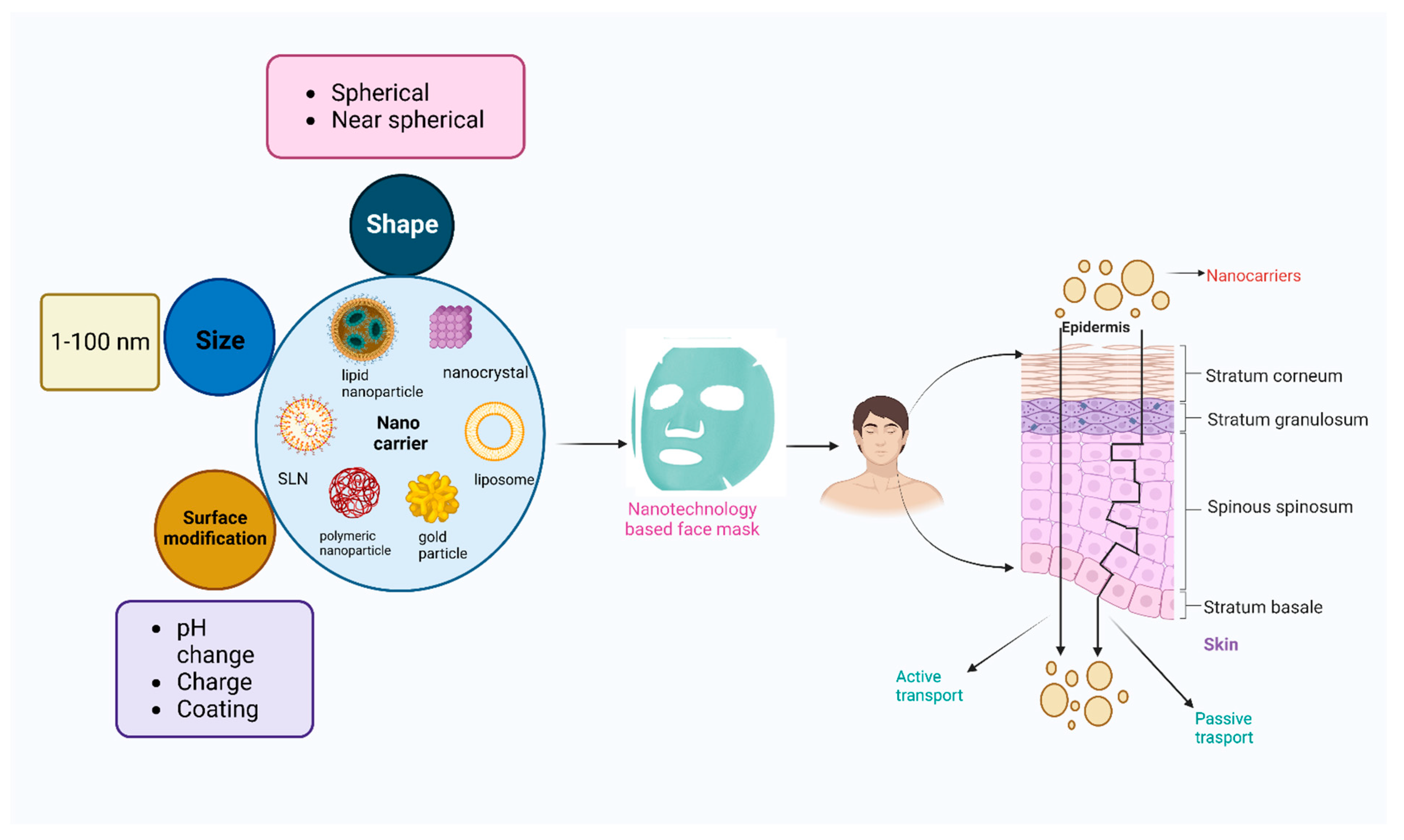
4. Novel Nanocarriers
4.1. Currently Used Nanocarriers in Face Masks
4.1.1. Liposomes and Other Lipid-Based Nanocarriers
4.1.2. Gold and Silver NPs
4.2. Future Prospects for the Use of Nanocarriers in Face Masks
4.2.1. Polymer Nanocarriers
4.2.2. Inorganic Nanocarriers
4.2.3. Solid Lipid Nanocarrier (SLN)
4.2.4. Nanofibers
4.2.5. Nanocrystals
4.2.6. Nanoemulsions
| Category | Examples | Ref. |
|---|---|---|
| UV filters |
| [19,89] |
| Bioactive molecules |
| [19,90] |
| Antiaging and moisturizing nanomaterials |
| [19,91] |
| Antibacterial and antifungal agents |
| [19,92] |
| Cleansing agents |
| [19,93] |
| Other uses |
| [19,94] |
5. Active Ingredients
5.1. Retinol (Vit-A)
5.2. Ascorbic Acid (Vit-C)
5.3. Carotenoids
5.4. Vitamin E (Tocopherol)
5.5. Coenzyme Q10 (Ubiquinone)
6. Skin Barriers and the Role of NPs in Resolving Skin Barrier Properties
7. Application of Nanoparticle-Based Face Masks
8. Advantages of Nanoparticle-Based Face Masks
9. Challenges
10. Future Prospects
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMullen, R.L.; Dell’Acqua, G. History of Natural Ingredients in Cosmetics. Cosmetics 2023, 10, 71. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E. Adverse Effects of Herbal Drugs in Dermatology. Br. J. Dermatol. 2000, 143, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Wong, N.K. Nanotechnology and Its Use in Imaging and Drug Delivery (Review). Biomed. Rep. 2021, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Alsabeelah, N.; Arshad, M.F.; Hashmi, S.; Khan, R.A.; Khan, S. Nanocosmeceuticals for the Management of Ageing: Rigors and Vigors. J. Drug Deliv. Sci. Technol. 2021, 63, 102448. [Google Scholar] [CrossRef]
- Ebrahimi, F. (Ed.) Nanocomposites—New Trends and Developments; InTech: Vienne, France, 2012; ISBN 978-953-51-0762-0. [Google Scholar]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.; Aldawsari, M.; Alalaiwe, A.; Mirza, M.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef]
- Shepard, M.; Brenner, S. Cutaneous Exposure Scenarios for Engineered Nanoparticles Used in Semiconductor Fabrication: A Preliminary Investigation of Workplace Surface Contamination. Int. J. Occup. Environ. Health 2014, 20, 247–257. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Amirkhani, M.A.; Zarrintaj, P.; Salehi Moghaddam, A.; Mehrabi, T.; Alavi, S.; Mollapour Sisakht, M. Skin Care and Rejuvenation by Cosmeceutical Facial Mask. J. Cosmet. Dermatol. 2018, 17, 693–702. [Google Scholar] [CrossRef]
- El-Atab, N.; Mishra, R.B.; Hussain, M.M. Toward Nanotechnology-Enabled Face Masks against SARS-CoV-2 and Pandemic Respiratory Diseases. Nanotechnology 2021, 33, 062006. [Google Scholar] [CrossRef]
- Raszewska-Famielec, M.; Flieger, J. Nanoparticles for Topical Application in the Treatment of Skin Dysfunctions—An Overview of Dermo-Cosmetic and Dermatological Products. Int. J. Mol. Sci. 2022, 23, 15980. [Google Scholar] [CrossRef]
- Sequeira, J.A.D.; Pereira, I.; Ribeiro, A.J.; Veiga, F.; Santos, A.C. Chapter 8—Surface Functionalization of PLGA Nanoparticles for Drug Delivery. In Handbook of Functionalized Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 185–203. ISBN 978-0-12-816787-8. [Google Scholar]
- Mu, L.; Sprando, R.L. Application of Nanotechnology in Cosmetics. Pharm. Res. 2010, 27, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Admin Nanoparticle Dispersions|Nanoparticle Formulations|Buy. Available online: https://avantama.com/shop/ (accessed on 23 October 2022).
- Yadwade, R.; Gharpure, S.; Ankamwar, B. Nanotechnology in Cosmetics Pros and Cons. Nano Express 2021, 2, 022003. [Google Scholar] [CrossRef]
- Fauzi, M.B.; Smandri, A.; Amirrah, I.N.; Kamaruzaman, N.; Salleh, A.; Mazlan, Z.; Sallehuddin, N.; Zulkiflee, I.; Jian, L.X.; Nor, F.M. Chapter 17—Nanomaterials for Aging and Cosmeceutical Applications. In Food, Medical, and Environmental Applications of Nanomaterials; Pal, K., Sarkar, A., Sarkar, P., Bandara, N., Jegatheesan, V., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 455–472. ISBN 978-0-12-822858-6. [Google Scholar]
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef]
- Chavda, V.P.; Balar, P.C.; Bezbaruah, R.; Vaghela, D.A.; Rynjah, D.; Bhattacharjee, B.; Sugandhi, V.V.; Paiva-Santos, A.C. Nanoemulsions: Summary of a Decade of Research and Recent Advances. Nanomedicine 2024, 19, 519–536. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The Emerging Role of Nanotechnology in Skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.A.; Mohd-Nasir, H.; Ahmad, A.; Mohd. Setapar, S.H.; Peng, W.L.; Chuo, S.C.; Khatoon, A.; Umar, K.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Role of Nanotechnology for Design and Development of Cosmeceutical: Application in Makeup and Skin Care. Front. Chem. 2019, 7, 739. [Google Scholar] [CrossRef]
- Jatana, S.; DeLouise, L.A. Understanding Engineered Nanomaterial Skin Interactions and the Modulatory Effects of UVR Skin Exposure. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 61–79. [Google Scholar] [CrossRef]
- Katz, L.M.; Dewan, K.; Bronaugh, R.L. Nanotechnology in Cosmetics. Food Chem. Toxicol. 2015, 85, 127–137. [Google Scholar] [CrossRef]
- .Al-Abduljabbar, A.; Farooq, I. Electrospun Polymer Nanofibers: Processing, Properties, and Applications. Polymers 2023, 15, 65. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Afonso, C.R.; Hirano, R.S.; Gaspar, A.L.; Chagas, E.G.L.; Carvalho, R.A.; Silva, F.V.; Leonardi, G.R.; Lopes, P.S.; Silva, C.F.; Yoshida, C.M.P. Biodegradable Antioxidant Chitosan Films Useful as an Anti-Aging Skin Mask. Int. J. Biol. Macromol. 2019, 132, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Bujak, T.; Wasilewski, T.; Nizioł-Łukaszewska, Z. Role of Macromolecules in the Safety of Use of Body Wash Cosmetics. Colloids Surf. B Biointerfaces 2015, 135, 497–503. [Google Scholar] [CrossRef]
- Glatzel, S.; Laschewsky, A.; Lutz, J.-F. Well-Defined Uncharged Polymers with a Sharp UCST in Water and in Physiological Milieu. Macromolecules 2011, 44, 413–415. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, S.M.; Lee, Y.H.; Kim, W.J.; Park, J.K.; Park, Y.I.; Jang, W.J.; Shin, H.-D.; Synytsya, A. Macromolecules Isolated from Phellinus Pini Fruiting Body: Chemical Characterization and Antiviral Activity. Macromol. Res. 2010, 18, 602–609. [Google Scholar] [CrossRef]
- Nemes, D.; Kovács, R.; Nagy, F.; Mező, M.; Poczok, N.; Ujhelyi, Z.; Pető, Á.; Fehér, P.; Fenyvesi, F.; Váradi, J.; et al. Interaction between Different Pharmaceutical Excipients in Liquid Dosage Forms—Assessment of Cytotoxicity and Antimicrobial Activity. Molecules 2018, 23, 1827. [Google Scholar] [CrossRef] [PubMed]
- Zaid Alkilani, A.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Münch, S.; Wohlrab, J.; Neubert, R.H.H. Dermal and Transdermal Delivery of Pharmaceutically Relevant Macromolecules. Eur. J. Pharm. Biopharm. 2017, 119, 235–242. [Google Scholar] [CrossRef]
- Ahsan, H. The Biomolecules of Beauty: Biochemical Pharmacology and Immunotoxicology of Cosmeceuticals. J. Immunoass. Immunochem. 2019, 40, 91–108. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Amirkhani, M.A.; Zarrintaj, P.; Salehi Moghaddam, A.; Mehrabi, T.; Alavi, S.; Mollapour Sisakht, M. Skin Care and Rejuvenation by Cosmeceutical Facial Mask. J. Cosmet. Dermatol. 2018, 17, 693–702. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Lipid-Based Formulations in Cosmeceuticals and Biopharmaceuticals. Biomed. Dermatol. 2020, 4, 12. [Google Scholar] [CrossRef]
- Horrobin, D.F. Essential Fatty Acid Metabolism and Its Modification in Atopic Eczema. Am. J. Clin. Nutr. 2000, 71, 367S–372S. [Google Scholar] [CrossRef]
- Skibska, A.; Perlikowska, R. Signal Peptides—Promising Ingredients in Cosmetics. Available online: http://www.eurekaselect.com (accessed on 13 January 2025).
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.-B.; Shim, J.-H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides From Food and By-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef]
- Aramwit, P.; Bang, N. The Characteristics of Bacterial Nanocellulose Gel Releasing Silk Sericin for Facial Treatment. BMC Biotechnol. 2014, 14, 104. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, B. Development and Performance Study of a Natural Silk Fiber Facial Mask Paper. J. Eng. Fibers Fabr. 2020, 15, 1558925020975756. [Google Scholar] [CrossRef]
- Ferraris, C.; Rimicci, C.; Garelli, S.; Ugazio, E.; Battaglia, L. Nanosystems in Cosmetic Products: A Brief Overview of Functional, Market, Regulatory and Safety Concerns. Pharmaceutics 2021, 13, 1408. [Google Scholar] [CrossRef]
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Nowak, I. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Carriers for Cosmetic Ingredients. In Nanobiomaterials in Galenic Formulations and Cosmetics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–255. ISBN 978-0-323-42868-2. [Google Scholar]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical Characterization of Liposomes and Other Lipid Nanoparticles for Drug Delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef]
- Bansal, P.; Sardana, K.; Sharma, L.; Garga, U.C.; Vats, G. A Prospective Study Examining Isolated Acne and Acne with Hyperandrogenic Signs in Adult Females. J. Dermatol. Treat. 2021, 32, 752–755. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Capasso, C.; Donnarumma, M.; Cantelli, M.; Le Maître, M.; Monfrecola, G.; Emanuele, E. A Peel-off Facial Mask Comprising Myoinositol and Trehalose-Loaded Liposomes Improves Adult Female Acne by Reducing Local Hyperandrogenism and Activating Autophagy. J. Cosmet. Dermatol. 2017, 16, 480–484. [Google Scholar] [CrossRef]
- Tuchin, V.V.; Genina, E.A.; Bashkatov, A.N.; Simonenko, G.V.; Odoevskaya, O.D.; Altshuler, G.B. A Pilot Study of ICG Laser Therapy of Acne Vulgaris: Photodynamic and Photothermolysis Treatment. Lasers Surg. Med. 2003, 33, 296–310. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid Nanocarriers as Skin Drug Delivery Systems: Properties, Mechanisms of Skin Interactions and Medical Applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef]
- Raber, A.S.; Mittal, A.; Schäfer, J.; Bakowsky, U.; Reichrath, J.; Vogt, T.; Schaefer, U.F.; Hansen, S.; Lehr, C.-M. Quantification of Nanoparticle Uptake into Hair Follicles in Pig Ear and Human Forearm. J. Control Release 2014, 179, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-C.; Tsai, T.-H. Preparation and Characterization of Liposomal Coenzyme Q10 for in Vivo Topical Application. Int. J. Pharm. 2010, 395, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Khezri, K.; Saeedi, M.; Maleki Dizaj, S. Application of Nanoparticles in Percutaneous Delivery of Active Ingredients in Cosmetic Preparations. Biomed. Pharmacother. 2018, 106, 1499–1505. [Google Scholar] [CrossRef]
- Mensah, A.; Chen, Y.; Asinyo, B.K.; Howard, E.K.; Narh, C.; Huang, J.; Wei, Q. Bioactive Icariin/β-CD-IC/Bacterial Cellulose with Enhanced Biomedical Potential. Nanomaterials 2021, 11, 387. [Google Scholar] [CrossRef]
- Lauterbach, A.; Müller-Goymann, C.C. Applications and Limitations of Lipid Nanoparticles in Dermal and Transdermal Drug Delivery via the Follicular Route. Eur. J. Pharm. Biopharm. 2015, 97, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Bonina, F. Lipid Nanoparticles as Novel Delivery Systems for Cosmetics and Dermal Pharmaceuticals. Expert Opin. Drug Deliv. 2012, 9, 429–441. [Google Scholar] [CrossRef]
- Duarah, S.; Durai, R.D.; Narayanan, V.B. Nanoparticle-in-Gel System for Delivery of Vitamin C for Topical Application. Drug Deliv. Transl. Res. 2017, 7, 750–760. [Google Scholar] [CrossRef]
- Chavda, V.P.; Dawre, S.; Pandya, A.; Vora, L.K.; Modh, D.H.; Shah, V.; Dave, D.J.; Patravale, V. Lyotropic Liquid Crystals for Parenteral Drug Delivery. J. Control Release Off. J. Control Release Soc. 2022, 349, 533–549. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Silva, A.B.P.P.; Fagundes, L.L.; Raposo, N.R.B.; Ferreira, A.O.; Brandão, M.A.F.; Polonini, H.C. Development and Preliminary Cosmetic Potential Evaluation of Melaleuca Alternifolia Cheel (Myrtaceae) Oil and Resveratrol for Oily Skin. J. Dermatol. Res. Ther. 2016, 2, 032. [Google Scholar] [CrossRef]
- Bonato Alves Oliveira, L.; Oliveira, R.; Oliveira, C.; Raposo, N.; Brandão, M.; Ferreira, A.; Polonini, H. Cosmetic Potential of a Liotropic Liquid Crystal Emulsion Containing Resveratrol. Cosmetics 2017, 4, 54. [Google Scholar] [CrossRef]
- Gangwar, A.; Kumar, P.; Singh, R.; Kush, P. Recent Advances in Mupirocin Delivery Strategies for the Treatment of Bacterial Skin and Soft Tissue Infection. Future Pharmacol. 2021, 1, 80–103. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Nagasawa, T.; Kitagawa, A.; Nakamura, N.; Matsumoto, K.; Uchiwa, H.; Hirata, K.; Igarashi, R. New Nanotechnology for the Guided Tissue Regeneration of Skin--Potential of Lyotropic Liquid Crystals. Pharmazie 2006, 61, 112–116. [Google Scholar]
- Rajabalaya, R.; Musa, M.N.; Kifli, N.; David, S.R. Oral and Transdermal Drug Delivery Systems: Role of Lipid-Based Lyotropic Liquid Crystals. Drug Des. Dev. Ther. 2017, 11, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Musashi, M.; Coler-Reilly, A.; Nagasawa, T.; Kubota, Y.; Kato, S.; Yamaguchi, Y. Liquid Crystal Gel Reduces Age Spots by Promoting Skin Turnover. Cosmetics 2014, 1, 202–210. [Google Scholar] [CrossRef]
- Müller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured Lipid Carriers (NLC) in Cosmetic Dermal Products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef]
- AlZahabi, S.; Sakr, O.S.; Ramadan, A.A. Nanostructured Lipid Carriers Incorporating Prickly Pear Seed Oil for the Encapsulation of Vitamin A. J. Cosmet. Dermatol. 2019, 18, 1875–1884. [Google Scholar] [CrossRef]
- Freitas de Freitas, L.; Varca, G.H.C.; Dos Santos Batista, J.G.; Benévolo Lugão, A. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A.; et al. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Beneficial Prospect for Cancer Theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef] [PubMed]
- Manatunga, D.C.; Godakanda, V.U.; Herath, H.M.L.P.B.; de Silva, R.M.; Yeh, C.-Y.; Chen, J.-Y.; Akshitha de Silva, A.A.; Rajapaksha, S.; Nilmini, R.; Nalin de Silva, K.M. Nanofibrous Cosmetic Face Mask for Transdermal Delivery of Nano Gold: Synthesis, Characterization, Release and Zebra Fish Employed Toxicity Studies. R. Soc. Open Sci. 2020, 7, 201266. [Google Scholar] [CrossRef]
- Fathi-Azarbayjani, A.; Qun, L.; Chan, Y.W.; Chan, S.Y. Novel Vitamin and Gold-Loaded Nanofiber Facial Mask for Topical Delivery. Aaps PharmsciTech 2010, 11, 1164–1170. [Google Scholar] [CrossRef]
- Kahraman, E.; Güngör, S.; Özsoy, Y. Potential Enhancement and Targeting Strategies of Polymeric and Lipid-Based Nanocarriers in Dermal Drug Delivery. Ther. Deliv. 2017, 8, 967–985. [Google Scholar] [CrossRef] [PubMed]
- Yotsumoto, K.; Ishii, K.; Kokubo, M.; Yasuoka, S. Improvement of the Skin Penetration of Hydrophobic Drugs by Polymeric Micelles. Int. J. Pharm. 2018, 553, 132–140. [Google Scholar] [CrossRef]
- Hasanovic, A.; Zehl, M.; Reznicek, G.; Valenta, C. Chitosan-Tripolyphosphate Nanoparticles as a Possible Skin Drug Delivery System for Aciclovir with Enhanced Stability. J. Pharm. Pharmacol. 2009, 61, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Giulbudagian, M.; Rancan, F.; Klossek, A.; Yamamoto, K.; Jurisch, J.; Neto, V.C.; Schrade, P.; Bachmann, S.; Rühl, E.; Blume-Peytavi, U.; et al. Correlation between the Chemical Composition of Thermoresponsive Nanogels and Their Interaction with the Skin Barrier. J Control Release 2016, 243, 323–332. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, Y.; Choi, C.; Roh, S.; Kang, S.; Jang, M.; Nah, J. Retinol-Encapsulated Low Molecular Water-Soluble Chitosan Nanoparticles. Int. J. Pharm. 2006, 319, 130–138. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: Focus on Their Safety and Effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.D.; Pereira-Silva, M.; Veiga, F.; Ribeiro, A. Nanotechnology for the Development of New Cosmetic Formulations. Expert Opin. Drug Deliv. 2019, 16, 313–330. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
- Wissing, S.; Müller, R. Cosmetic Applications for Solid Lipid Nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef]
- Jenning, V.; Gysler, A.; Schäfer-Korting, M.; Gohla, S.H. Vitamin A Loaded Solid Lipid Nanoparticles for Topical Use: Occlusive Properties and Drug Targeting to the Upper Skin. Eur. J. Pharm. Biopharm. 2000, 49, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Elixirs, M. CBDerma-Repair® Nano-Fiber Mask—Moia Elixirs. Available online: https://www.moiaelixirs.com/cbderma-repair-nano-fiber-mask/ (accessed on 11 January 2023).
- Cosmetic Sheet Face Masks—Recent Trends. Scitech Patent Art. Available online: https://www.patent-art.com/knowledge-center/cosmetic-sheet-face-masks-recent-trends/ (accessed on 13 January 2023).
- Patel, V.; Sharma, O.P.; Mehta, T. Nanocrystal: A Novel Approach to Overcome Skin Barriers for Improved Topical Drug Delivery. Expert Opin. Drug Deliv. 2018, 15, 351–368. [Google Scholar] [CrossRef]
- Pyo, S.M.; Meinke, M.; Keck, C.M.; Müller, R.H. Rutin—Increased Antioxidant Activity and Skin Penetration by Nanocrystal Technology (smartCrystals). Cosmetics 2016, 3, 9. [Google Scholar] [CrossRef]
- Shegokar, R. What Nanocrystals Can Offer to Cosmetic and Dermal Formulations. In Nanobiomaterials in Galenic Formulations and Cosmetics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 69–91. ISBN 978-0-323-42868-2. [Google Scholar]
- dos Santos, P.P.; Andrade, L.d.A.; Flôres, S.H.; Rios, A.d.O. Nanoencapsulation of Carotenoids: A Focus on Different Delivery Systems and Evaluation Parameters. J. Food Sci. Technol. 2018, 55, 3851–3860. [Google Scholar] [CrossRef] [PubMed]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lutein Nanocrystals as Antioxidant Formulation for Oral and Dermal Delivery. Int. J. Pharm. 2011, 420, 141–146. [Google Scholar] [CrossRef]
- Scholz, P.; Keck, C.M. Flavonoid Nanocrystals Produced by ARTcrystal®-Technology. Int. J. Pharm. 2015, 482, 27–37. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Uter, W.; Gonçalo, M.; Yazar, K.; Kratz, E.M.; Mildau, G.; Lidén, C. Coupled Exposure to Ingredients of Cosmetic Products: III. Contact Dermat. 2014, 71, 162–169. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Falsafi, S.R.; Rostamabadi, M.M.; Assadpour, E.; Jafari, S.M. Electrospraying as a Novel Process for the Synthesis of Particles/Nanoparticles Loaded with Poorly Water-Soluble Bioactive Molecules. Adv. Colloid Interface Sci. 2021, 290, 102384. [Google Scholar] [CrossRef]
- Bhatia, E.; Kumari, D.; Sharma, S.; Ahamad, N.; Banerjee, R. Nanoparticle Platforms for Dermal Antiaging Technologies: Insights in Cellular and Molecular Mechanisms. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2022, 14, e1746. [Google Scholar] [CrossRef]
- Rozhin, A.; Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic Silver Nanoparticles: Synthesis and Application as Antibacterial and Antifungal Agents. Micromachines 2021, 12, 1480. [Google Scholar] [CrossRef]
- Javed, R.; Usman, M.; Tabassum, S.; Zia, M. Effect of Capping Agents: Structural, Optical and Biological Properties of ZnO Nanoparticles. Appl. Surf. Sci. 2016, 386, 319–326. [Google Scholar] [CrossRef]
- Wu, S.-H.; Hung, Y.; Mou, C.-Y. Mesoporous Silica Nanoparticles as Nanocarriers. Chem. Commun. 2011, 47, 9972–9985. [Google Scholar] [CrossRef]
- Spierings, N.M.K. Evidence for the Efficacy of Over-the-Counter Vitamin A Cosmetic Products in the Improvement of Facial Skin Aging: A Systematic Review. J. Clin. Aesthetic Dermatol. 2021, 14, 33–40. [Google Scholar]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the Treatment of Skin Aging: An Overview of Clinical Efficacy and Safety. Clin. Interv. Aging 2006, 1, 327–348. [Google Scholar] [CrossRef]
- Quan, T. Human Skin Aging and the Anti-Aging Properties of Retinol. Biomolecules 2023, 13, 1614. [Google Scholar] [CrossRef] [PubMed]
- Telang, P. Vitamin C in Dermatology. Indian Dermatol. Online J. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Sauermann, K.; Jaspers, S.; Koop, U.; Wenck, H. Topically Applied Vitamin C Increases the Density of Dermal Papillae in Aged Human Skin. BMC Dermatol. 2004, 4, 13. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Stinco, C.M.; Mapelli-Brahm, P. Skin Carotenoids in Public Health and Nutricosmetics: The Emerging Roles and Applications of the UV Radiation-Absorbing Colourless Carotenoids Phytoene and Phytofluene. Nutrients 2019, 11, 1093. [Google Scholar] [CrossRef]
- Darvin, M.E.; Fluhr, J.W.; Meinke, M.C.; Zastrow, L.; Sterry, W.; Lademann, J. Topical Beta-Carotene Protects against Infra-Red-Light-Induced Free Radicals. Exp. Dermatol. 2011, 20, 125–129. [Google Scholar] [CrossRef]
- Biesalski, H.K.; Obermueller-Jevic, U.C. UV Light, Beta-Carotene and Human Skin--Beneficial and Potentially Harmful Effects. Arch. Biochem. Biophys. 2001, 389, 1–6. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Alwakeel, S.S.; Moga, M.; Buvnariu, L.; Bigiu, N.; Zia-Ul-Haq, M. Application of Carotenoids in Cosmetics. In Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 747–756. ISBN 978-3-030-46459-2. [Google Scholar]
- Jiang, Q.; Christen, S.; Shigenaga, M.K.; Ames, B.N. Gamma-Tocopherol, the Major Form of Vitamin E in the US Diet, Deserves More Attention. Am. J. Clin. Nutr. 2001, 74, 714–722. [Google Scholar] [CrossRef]
- Nachbar, F.; Korting, H.C. The Role of Vitamin E in Normal and Damaged Skin. J. Mol. Med. 1995, 73, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Keen, M.A.; Hassan, I. Vitamin E in Dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef]
- Hernández-Camacho, J.D.; Bernier, M.; López-Lluch, G.; Navas, P. Coenzyme Q10 Supplementation in Aging and Disease. Front. Physiol. 2018, 9, 316577. [Google Scholar] [CrossRef]
- Dégboé, B.; Koudoukpo, C.; Agbéssi, N.; Elégbédé-Adégbitè, N.; Akpadjan, F.; Adégbidi, H.; Atadokpèdé, F. Acne on Pigmented Skin: Epidemiological, Clinical and Therapeutic Features in Dermatology in Benin. J.Cosmet. Dermatol. Sci. Appl. 2019, 09, 305–312. [Google Scholar] [CrossRef]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of Nanoparticles and Cell-Penetrating Peptides with Skin for Transdermal Drug Delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and Their Interactions with the Dermal Barrier. Dermatoendocrinol 2009, 1, 197–206. [Google Scholar] [CrossRef]
- Percival, S.L.; Emanuel, C.; Cutting, K.F.; Williams, D.W. Microbiology of the Skin and the Role of Biofilms in Infection. Int. Wound J. 2012, 9, 14–32. [Google Scholar] [CrossRef]
- Naves, L.B.; Dhand, C.; Venugopal, J.R.; Rajamani, L.; Ramakrishna, S.; Almeida, L. Nanotechnology for the Treatment of Melanoma Skin Cancer. Prog. Biomater. 2017, 6, 13–26. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Dufour, E.K.; Roberts, M.S. Nanotechnology, Cosmetics and the Skin: Is There a Health Risk? Ski. Pharmacol. Physiol. 2008, 21, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Labouta, H.I.; el-Khordagui, L.K.; Kraus, T.; Schneider, M. Mechanism and Determinants of Nanoparticle Penetration through Human Skin. Nanoscale 2011, 3, 4989–4999. [Google Scholar] [CrossRef]
- Raju, G.; Katiyar, N.; Vadukumpully, S.; Shankarappa, S.A. Penetration of Gold Nanoparticles across the Stratum Corneum Layer of Thick-Skin. J. Dermatol. Sci. 2018, 89, 146–154. [Google Scholar] [CrossRef]
- Ng, C.T.; Yong, L.Q.; Hande, M.P.; Ong, C.N.; Yu, L.E.; Bay, B.H.; Baeg, G.H. Zinc Oxide Nanoparticles Exhibit Cytotoxicity and Genotoxicity through Oxidative Stress Responses in Human Lung Fibroblasts and Drosophila Melanogaster. Int. J. Nanomed. 2017, 12, 1621–1637. [Google Scholar] [CrossRef]
- Park, K.H.; Ku, M.; Yoon, N.; Hwang, D.Y.; Lee, J.; Yang, J.; Seo, S. Effect of Polydiacetylene-Based Nanosomes on Cell Viability and Endocytosis. Nanotechnology 2019, 30, 245101. [Google Scholar] [CrossRef] [PubMed]
- Harisa, G.I.; Badran, M.M.; Alanazi, F.K.; Attia, S.M. An Overview of Nanosomes Delivery Mechanisms: Trafficking, Orders, Barriers and Cellular Effects. Artif. Cells Nanomed. Biotechnol. 2018, 46, 669–679. [Google Scholar] [CrossRef]
- Leite-Silva, V.R.; Sanchez, W.Y.; Studier, H.; Liu, D.C.; Mohammed, Y.H.; Holmes, A.M.; Ryan, E.M.; Haridass, I.N.; Chandrasekaran, N.C.; Becker, W.; et al. Human Skin Penetration and Local Effects of Topical Nano Zinc Oxide after Occlusion and Barrier Impairment. Eur. J. Pharm. Biopharm. 2016, 104, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current Advances in Lipid Nanosystems Intended for Topical and Transdermal Drug Delivery Applications. Pharmaceutics 2023, 15, 656. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, F.; Zhao, R.; Wang, C.; Hu, K.; Sun, Y.; Politis, C.; Shavandi, A.; Nie, L. Polyvinyl Alcohol/Sodium Alginate Hydrogels Incorporated with Silver Nanoclusters via Green Tea Extract for Antibacterial Applications. Des. Monomers Polym. 2020, 23, 118. [Google Scholar] [CrossRef]
- Elbehiry, A.; Al-Dubaib, M.; Marzouk, E.; Moussa, I. Antibacterial Effects and Resistance Induction of Silver and Gold Nanoparticles against Staphylococcus Aureus-induced Mastitis and the Potential Toxicity in Rats. MicrobiologyOpen 2018, 8, e00698. [Google Scholar] [CrossRef]
- Seo, Y.S.; Oh, S.-G. Controlling the Recombination of Electron-Hole Pairs by Changing the Shape of ZnO Nanorods via Sol-Gel Method Using Water and Their Enhanced Photocatalytic Properties. Korean J. Chem. Eng. 2019, 36, 2118–2124. [Google Scholar] [CrossRef]
- Peralta, M.F.; Guzmán, M.L.; Pérez, A.P.; Apezteguia, G.A.; Fórmica, M.L.; Romero, E.L.; Olivera, M.E.; Carrer, D.C. Liposomes Can Both Enhance or Reduce Drugs Penetration through the Skin. Sci. Rep. 2018, 8, 13253. [Google Scholar] [CrossRef]
- Jensen, L.B.; Petersson, K.; Nielsen, H.M. In Vitro Penetration Properties of Solid Lipid Nanoparticles in Intact and Barrier-Impaired Skin. Eur. J. Pharm. Biopharm. 2011, 79, 68–75. [Google Scholar] [CrossRef]
- Witika, B.A.; Bassey, K.E.; Demana, P.H.; Siwe-Noundou, X.; Poka, M.S. Current Advances in Specialised Niosomal Drug Delivery: Manufacture, Characterization and Drug Delivery Applications. Int. J. Mol. Sci. 2022, 23, 9668. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Berardesca, E.; Darlenski, R. Psoriasis and Dry Skin: The Impact of Moisturizers. In Treatment of Dry Skin Syndrome: The Art and Science of Moisturizers; Lodén, M., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 285–293. ISBN 978-3-642-27606-4. [Google Scholar]
- Borkow, G. Using Copper to Improve the Well-Being of the Skin. Curr. Chem. Biol. 2014, 8, 89. [Google Scholar] [CrossRef]
- Singh, T.G.; Sharma, N. Nanobiomaterials in Cosmetics: Current Status and Future Prospects. In Nanobiomaterials in Galenic Formulations and Cosmetics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 149–174. ISBN 978-0-323-42868-2. [Google Scholar]
- Ko, W.-C.; Wang, S.-J.; Hsiao, C.-Y.; Hung, C.-T.; Hsu, Y.-J.; Chang, D.-C.; Hung, C.-F. Pharmacological Role of Functionalized Gold Nanoparticles in Disease Applications. Molecules 2022, 27, 1551. [Google Scholar] [CrossRef]
- Marin, S.; Vlasceanu, G.M.; Tiplea, R.E.; Bucur, I.R.; Lemnaru, M.; Marin, M.M.; Grumezescu, A.M. Applications and Toxicity of Silver Nanoparticles: A Recent Review. Curr. Top. Med. Chem. 2015, 15, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Netto, G. Role of Solid Lipid Nanoparticles as Photoprotective Agents in Cosmetics. J. Cosmet. Dermatol. 2019, 18, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Biological Reactivity of Zinc Oxide Nanoparticles with Mammalian Test Systems: An Overview. Nanomedicine 2015, 10, 2075–2092. [Google Scholar] [CrossRef]
- Akın, N.; Mutlu Danacı, H. An Investigation into the Architectural Use of Nanotechnology in the Context of the Titanium Dioxide. Environ. Sci. Pollut. Res. 2021, 28, 64130–64136. [Google Scholar] [CrossRef]
- Baroli, B. Penetration of Nanoparticles and Nanomaterials in the Skin: Fiction or Reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef]
- Severino, P.; Fangueiro, J.F.; Chaud, M.V.; Cordeiro, J.; Silva, A.M.; Souto, E.B. Advances in Nanobiomaterials for Topical Administrations: New Galenic and Cosmetic Formulations. In Nanobiomaterials in Galenic Formulations and Cosmetics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–23. ISBN 978-0-323-42868-2. [Google Scholar]
- Patnaik, A. Recent Developments in Application of Nanofibers. Processes 2024, 12, 1894. [Google Scholar] [CrossRef]
- Lombardi Borgia, S.; Regehly, M.; Sivaramakrishnan, R.; Mehnert, W.; Korting, H.C.; Danker, K.; Röder, B.; Kramer, K.D.; Schäfer-Korting, M. Lipid Nanoparticles for Skin Penetration Enhancement-Correlation to Drug Localization within the Particle Matrix as Determined by Fluorescence and Parelectric Spectroscopy. J. Control Release 2005, 110, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Junyaprasert, V.B.; Singhsa, P.; Suksiriworapong, J.; Chantasart, D. Physicochemical Properties and Skin Permeation of Span 60/Tween 60 Niosomes of Ellagic Acid. Int. J. Pharm. 2012, 423, 303–311. [Google Scholar] [CrossRef]
- Shah, S.M.; Ashtikar, M.; Jain, A.S.; Makhija, D.T.; Nikam, Y.; Gude, R.P.; Steiniger, F.; Jagtap, A.A.; Nagarsenker, M.S.; Fahr, A. LeciPlex, Invasomes, and Liposomes: A Skin Penetration Study. Int. J. Pharm. 2015, 490, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-Y.; Fang, C.-L.; Liu, C.-H.; Su, Y.-H. Lipid Nanoparticles as Vehicles for Topical Psoralen Delivery: Solid Lipid Nanoparticles (SLN) versus Nanostructured Lipid Carriers (NLC). Eur. J. Pharm. Biopharm. 2008, 70, 633–640. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.-K.; Huang, R. Nanoparticles-induced Potential Toxicity on Human Health: Applications, Toxicity Mechanisms, and Evaluation Models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef]
- Canta, M.; Cauda, V. The Investigation of the Parameters Affecting the ZnO Nanoparticle Cytotoxicity Behaviour: A Tutorial Review. Biomater. Sci. 2020, 8, 6157–6174. [Google Scholar] [CrossRef]
- Singh, S. Zinc Oxide Nanoparticles Impacts: Cytotoxicity, Genotoxicity, Developmental Toxicity, and Neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A. Toxicity of Nanoparticles_ Challenges and Opportunities. Appl. Microsc. 2019, 49, 2. [Google Scholar] [CrossRef]
- Shah, P.; Lalan, M.; Jani, D. Toxicological Aspects of Carbon Nanotubes, Fullerenes and Graphenes. Curr. Pharm. Des. 2021, 27, 556–564. [Google Scholar] [CrossRef]
- Tang, S.C.N.; Lo, I.M.C. Magnetic Nanoparticles: Essential Factors for Sustainable Environmental Applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and Environmental Risks of Nanomaterials: Challenges and Future Needs. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Nhani, G.B.B.; Di Filippo, L.D.; de Paula, G.A.; Mantovanelli, V.R.; da Fonseca, P.P.; Tashiro, F.M.; Monteiro, D.C.; Fonseca-Santos, B.; Duarte, J.L.; Chorilli, M. High-Tech Sustainable Beauty: Exploring Nanotechnology for the Development of Cosmetics Using Plant and Animal By-Products. Cosmetics 2024, 11, 112. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]


| Nanoparticle | Property | Advantage | Reference |
|---|---|---|---|
| Gold | Anti-wrinkle Anti-pro radical Antioxidant | Gold particles act as anti-inflammatory and antioxidants by preventing oxidation, reducing acne and wrinkles, and helping to remove dead skin. | [130] |
| Silver | Antibacterial | Silver is extremely good at sterilizing and aids in the reduction in skin pore size and the treatment of acne. The antibacterial properties are due to the high reflectivity of silver. | [131] |
| Lipid nanoparticle | Antiaging As moisturizer | Liposomes have the capacity for sustained release, so it is beneficial to deliver active ingredients at the right time, which gives long-lasting effects. | [132] |
| Zinc oxide(coated) | Antibacterial | Coated zinc particles avoid direct skin contact, increasing their margin of safety. It adsorbs the protein on the nanoparticle surface, which may improve its bioavailability. | [133] |
| Titanium oxide (SiO2 = Al2O3 coated) | Antibiotic Antimicrobial | Because particles are only penetrated into the surface layer of the stratum corneum and are not penetrated deeply, adverse effects such as itching are avoided. | [134] |
| Silica | Antibacterial Antiaging | Improving product texture and providing a matt finish to the face mask. | [135] |
| Nanosphere and nanocapsule | Synthetic polymers act as antioxidants | Nanocapsules are reservoirs consisting of a liquid core encapsulated by a surfactant or coating that facilitates the sustained release of a drug. In contrast, nanospheres are polymeric matrices that store multiple active substances. It is the polymeric matrix that plays a crucial role in enhancing the properties of face masks that incorporate nanoparticles. | [136] |
| Nanofiber | High retardancy and antibacterial properties | It provides a high surface area to volume ratio, increases the pore size and permeability of active ingredients, and reduces the thickness of the mask. | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavda, V.P.; Solanki, H.K.; Vaghela, D.A.; Prajapati, K.; Vora, L.K. Nanotechnology-Based Face Masks: Transforming the Cosmetics Landscape. Micro 2025, 5, 11. https://doi.org/10.3390/micro5010011
Chavda VP, Solanki HK, Vaghela DA, Prajapati K, Vora LK. Nanotechnology-Based Face Masks: Transforming the Cosmetics Landscape. Micro. 2025; 5(1):11. https://doi.org/10.3390/micro5010011
Chicago/Turabian StyleChavda, Vivek P., Hetvi K. Solanki, Dixa A. Vaghela, Karishma Prajapati, and Lalitkumar K. Vora. 2025. "Nanotechnology-Based Face Masks: Transforming the Cosmetics Landscape" Micro 5, no. 1: 11. https://doi.org/10.3390/micro5010011
APA StyleChavda, V. P., Solanki, H. K., Vaghela, D. A., Prajapati, K., & Vora, L. K. (2025). Nanotechnology-Based Face Masks: Transforming the Cosmetics Landscape. Micro, 5(1), 11. https://doi.org/10.3390/micro5010011









