Abstract
In recent years, the field of drug delivery has seen a significant shift towards the exploration and utilization of nanoparticles (NPs) as versatile carriers for therapeutic agents. With its ability to provide exact control over NPs’ characteristics, microfluidics has emerged as a potent platform for the efficient and controlled synthesis of NPs. Microfluidic devices designed for precise fluid manipulation at the micro-scale offer a unique platform for tailoring NP properties, enabling enhanced control over NP properties such as size, morphology, and size distribution while ensuring high batch-to-batch reproducibility. Microfluidics can be used to produce liposomes, solid lipid nanoparticles, polymer-based NPs, and lipid-polymer hybrid NPs, as well as a variety of inorganic NPs such as silica, metal, metal oxide, quantum dots, and carbon-based NPs, offering precise control over composition and surface properties. Its unique precision in tailoring NP properties holds great promise for advancing NP-based drug delivery systems in both clinical and industrial settings. Although challenges with large-scale production still remain, microfluidics offers a transformative approach to NP synthesis. In this review, starting from the historical development of microfluidic systems, the materials used to create the systems, microfabrication methods, and system components will be discussed in order to provide the reader with an overview of microfluidic systems. In the following, studies on the fabrication of nanoparticles such as lipid NPs, polymeric NPs, and inorganic NPs in microfluidic devices are included.
1. Introduction
Nanotechnology, or the production of systems/devices at the molecular level, is a multidisciplinary scientific field that has shown significant development in recent years. The potential applications of nanotechnology cover a very wide area and a wide variety of applications. These range from communications and robotics to construction, defense, education, and beyond. However, one of the most important contributions of nanotechnology to humanity is that it enables the creation of new and effective medical treatments [1]. Named nanomedicine by the National Institutes of Health, these groundbreaking technological advances span a number of subfields. These include nano drug delivery systems, gene therapies, vaccine studies, tissue engineering, the creation of artificial receptors, and the development of medical imaging systems [2].
Until the early 1970s, the intravenous administration of pharmaceutical suspensions consisting of solid particles dispersed in liquid was considered impractical due to the risk of embolism. However, the development of drug-loaded nanoparticle suspensions, also referred to as nanopharmaceuticals, revolutionized this problem. Nanopharmaceuticals include nano-sized powdered drugs, drug delivery systems, and vehicles. Developments in the fields of nanomedicine and nanopharmaceuticals have enabled the use of active substances (such as single molecule drugs, proteins, nucleic acids, etc.) that are difficult to use as drug active ingredients due to their insolubility or inability to be directed to specific parts of the body. Therapeutic agents that were incompatible with conventional drug formulations in the past can now be “nano-formulated” to precisely target specific biological sites due to improved pharmacokinetics/pharmacodynamics or accurate intracellular delivery. These methods, currently referred to as ‘drug targeting’ or ‘nano drug delivery systems’, have the potential to reduce toxicity by directing the active ingredient to target tissues and cells, and increase the concentration of the drug in the intended site of action, thereby increasing efficacy and improving patient compliance. Nanotechnology, and in particular the field of nanopharmaceuticals, has brought about a revolutionary change in the field of pharmaceutical applications [3,4]. In the field of nanomedicine, the use of nanoparticles (NPs) offers remarkable potential for diseases that are difficult to treat effectively using conventional therapies. However, fully realizing systems with this significant potential requires a rigorous approach: precise design, manufacturing, and rigorous testing in appropriate models are essential [5,6]. NPs for the intended purpose must be specifically designed with full regulation of their size, shape, composition, and characteristics such as surface charges and chemistry [7]. Many production methods have been developed since nanoparticles first appeared. Generally, the synthesis methods of nanomaterials (NMs) can be categorized into two approaches: top-down and bottom-up. In the top-down approach, bulk materials are meticulously downsized into nano-sized particles, resulting in NMs that are susceptible to oxidation and prone to dense agglomeration. This method potentially alters the inherent physical and surface properties of NMs by introducing surface defects and crystallographic irregularities. In contrast, the bottom-up approach, often using wet chemical synthesis, is used as the predominant method for producing NMs. In particular, the widespread use of this approach can be attributed to its simplicity, adaptability, use of cost-effective chemicals, and capacity to produce stable NMs at appreciable production rates compared to other methods. However, the limitations of this method stem from the difficulty of precisely controlling the reaction kinetics and achieving the desired NM properties [8]. Traditional nanoparticle production methods often struggle to consistently replicate the size, size distribution, and quality of nanomaterials from one batch to another, and rapid screening and the optimization of synthesis conditions are difficult to implement. In addition, scaling up production procedures to the quantities required for product development and optimization creates a separate set of challenges [9]. Given the inherent shortcomings of these traditional methods, the search for new alternatives for the production of nanoparticles has emerged. The search for a superior approach has led researchers to produce NPs using microfluidic technologies that offer the prospect of improving performance and overcoming the drawbacks observed in previous methods (Figure 1).
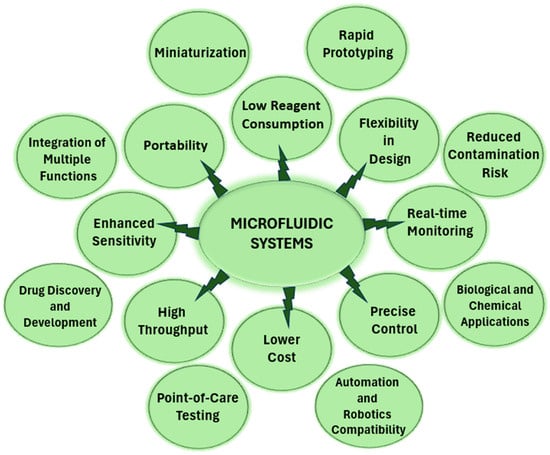
Figure 1.
Advantages of microfluidic systems.
Microfluidic technology involves the manipulation and processing of small liquid volumes ranging from 10−9 to 10−18 L through channels ranging in size from tens to hundreds of microns [10]. Microfluidic technology enables the meticulous management of the particle-forming environment and the minimal use of reagents in a continuous flow model with distinct flow characteristics not found in conventional production methods [8]. The power of microfluidics, which allows masterful control over microchannel architectures, flow and mixing velocities, interfacial viscosity, heat, and mass transfer processes, facilitates the synthesis of diverse micro/nanoparticles with distinctive properties [11]. The specific features of microfluidic applications, such as quick sample handling and fine liquid control in analyses, have made them favorable candidates to substitute conventional nanoparticle manufacturing processes. Micro- and nanoparticles synthesized in carefully designed microfluidic devices show significant potential for controlling size, shape, and size distribution. They also offer new ways to create sophisticated engineering nanostructures and innovative material systems by extending synthesis conditions (e.g., temperature, pressure, and reagents) to situations that are difficult to achieve with conventional techniques [9].
The primary focus in this review is the role of microfluidic devices in producing various nanoparticles such as lipids, polymers, and others. First, we will briefly touch upon microfluidic devices and their historical development. Next, the materials used in microfluidic devices, microfabrication methods and microfluidic system components will be discussed. Finally, we aim to provide readers with a brief understanding of nanoparticle production, particularly using microfluidic techniques, by summarizing current research in this field.
2. Historical Perspective on Microfluidic Systems
The history of microfluidics relates to many other fields and technologies that contributed to its development before it became an independent science. Even hundreds of years before the concept of microfluidics emerged, researchers were studying the properties of small amounts of body fluids and trying to extract information from them and understand their behavior. Researchers such as Hippocrates (400 BC), Galen (200 AD), and Theophilus (700 AD) placed urine samples in vials known as “matulae”, examined the color and appearance of urine, and tried to match various disorders and symptoms using diagrams. In 1717, Jurin published his work explaining the rise and suspension of water in capillaries [12]. Researchers such as James Jurin (1684–1750), Jean Léonard Marie Poiseuille (1799–1869), and Gotthilf Heinrich Ludwig Hagen (1797–1884) carried out studies to describe the behavior of fluids [13,14]. In 1839, Hagen published his work on water flow in cylindrical tubes [15]. Between 1838 and 1841, Poiseuille shared the results of his studies on the flow of water through glass tubes and the effects of tube length, tube diameter, temperature, and pressure [16,17]. The result of these studies was the famous Hagen–Poiseuille equation describing the pressure drop in a fluid flowing through a cylindrical pipe, which was later theoretically justified by George Stokes (1819–1903). This formed the basis of the theory describing the behavior of fluids at reduced scales [18]. In 1879, Lord Rayleigh (1842–1919) published his observations on the physics of droplet formation [19]. Rayleigh’s invention revealed that as a liquid flows through an orifice, it undergoes hydrodynamic instability, resulting in the formation of droplets. These droplets cumulatively exhibit a lower surface area and reduced surface tension than the initial fluid flow [20].
The events leading to the emergence of microfluidic technology continued with the development of materials and material-processing technologies. In 1859, John Walter Osborne (1828–1902) patented the photolithography process [21]. Photolithography, also known as optical lithography, is a type of printing using light-sensitive materials [22]. The field then continued to evolve with the development of soft lithography. The first example of the so-called “soft” method, which uses elastomeric stamps, molds, and conformable photo masks, is believed to have been applied in 1993 by Kumar et al. [23,24]. These methods have played an important role in the development of microfluidic devices, especially as they allow for the fine processing of polymers such as polydimethylsiloxane (PDMS). At this point, the development of polymer science should also be mentioned. In 1901, the concept of silicone was introduced by Frederic Kipping (1863–1949) [25]. This is an important development as silicone has been the primary material used to fabricate microfluidic devices for a long time. However, other materials were later used to overcome the disadvantages of using silicone, such as high cost and opaque structure. As the materials used and their applications evolved, more specific terminology was developed, and the most widely known silicone became PDMS [26,27]. Currently, PDMS is one of the most widely used materials for fabricating microfluidic devices/systems in research laboratories [28].
The factors that most influenced the development of microfluidics can be summarized in four points: microelectronics, molecular analysis, biodefense, and molecular biology. It can be said that the developments caused by these four factors led to a leap in microfluidics [10].
The foundation of microfluidics can be traced back to developments in the field of microelectronics. In 1947, Walter Brattain (1902–1987), John Bardeen (1908–1991), and William Shockley (1910–1989) invented the transistor in response to the need to improve the reliability of mechanical relay systems in telephone lines. Building on this work, in 1955, Jules Andrus and Walter L. Bond began adapting existing photolithographic techniques to produce much finer and more detailed designs on silicon [29,30]. This is an important development as the photolithography technique becomes a standard in microelectronics manufacturing. The year 1959 is considered to be the beginning of microtechnologies and nanotechnologies, especially because of RP Feynman’s speech “There is plenty of room at the bottom” at the American Physical Society meeting at Caltech. In his speech, Feynman emphasized the importance of studies in micro and nano dimensions [31]. In 1964, Jack Kilby took these technologies to the next level by explaining how many different components, including transistors, capacitors, and resistors, can be built into a single silicon crystal and create an oscillator circuit. This breakthrough in integrated circuits revolutionized microelectronics and heralded the beginning of the “silicon age” when companies competed to develop compact and secure electronic devices [29]. These developments gave birth to a new technology that has had a major impact on the microfluidics industry: inkjet printing. In 1965, Richard Sweet combined flow principles and materials science advances and applied them to inkjet printing technology [20,29]. In 1977, Bassous et al. took this technology even further and showed, through photolithography, that many inkjet nozzles could be produced in a single silicon wafer [32]. This process also showed that silicon could be used as a material for the mass production of microfluidic devices. In 1979, Terry et al. published the paper “A gas chromatographic air analyzer fabricated on a silicon wafer” [33]. Although concepts such as “lab-on-a-chip” or “micro total analysis system” did not yet exist at that time, this device is now recognized as the first example in this field. It is also considered to be the first publication in which microfluidics was recognized as a field in its own right [29]. The overarching goal and concept of the broad field of “lab-on-a-chip” was that microtechnology for the treatment of fluids would become as ubiquitous as “microelectronics”. The first breakthroughs in the development of what is now called “microfluidics” were based on miniaturization, i.e., reducing the size and complexity of analytical systems [34]. The science of miniaturization, which aims to miniaturize traditional macroscopic devices into microscopic devices, initially began with the development of miniature silicon-based electronic devices, and was later extended to the fabrication of mechanical devices known as microelectromechanical systems (MEMS) [35]. When these small devices were designed to handle liquids at sub-milliliter scales, they became known as microfluidic devices. The fabrication of microfluidic devices utilized the microelectronics manufacturing infrastructure as a subset of MEMS technology adapted for fluid processing. Silicon was predominantly used as the primary substrate material for microfluidic devices. The direct applicability of photolithography and related technologies (including thin film deposition and etching) to microfluidics, which have been highly successful in silicon microelectronics and MEMS, has played a crucial role in advancing this field. Silicon and glass, which were mostly used in the early stages of microfluidic system studies, were replaced by polymer materials in the 2000s due to their unsuitability for the analysis of biological samples for various reasons. The majority of experimental work in microfluidic devices has predominantly utilized a polymer called PDMS. PDMS has unique properties different from silicon, so it would be wrong to characterize microfluidic devices as mere replicas of silicon microelectronic devices. As an optically transparent, soft elastomer, PDMS has become a crucial material for early-stage discovery research and engineering, offering exceptional versatility for testing new concepts and assembling valuable components such as pneumatic valves. However, microelectronic techniques have played an essential role in the advancement of microfluidics. As the area has progressed, materials such as glass, steel, and silicon have re-emerged as viable options for building specialized systems that require chemical and thermal stability. Silicon and glass, in particular, are prized for their mechanical strength, which is highly valued in the field of nanofluidics, where hard-walled channels are highly advantageous [10,28]. MEMS technology has continued to advance with the development of devices for applications in medicine and life sciences. Biological microelectromechanical systems (BioMEMS) were coined to describe such devices and systems. BioMEMS are differentiated from traditional MEMS devices. BioMEMS may not have every component normally found in MEMS, and may include modernized definitions and applications [36]. The provision of a comprehensive solution from sample application to the presentation of analytical data is one of the goals of developing microfluidic systems. A microfluidic system is sometimes known as a lab-on-a-chip (LOC), biochip, or micro total analysis system (μTAS) for this reason. LOC and μTAS applications were first primarily utilized in the biomedical, food, and environmental domains, but more recently, they have also found usage in the synthesis of novel chemical compounds and the creation of nanostructures. Currently, microfluidic chips may be used to study bodily fluids, cells, tissues, and even complete organs. Both tiny start-ups and big pharmaceutical and biomedical corporations are developing an increasing number of microfluidic-based products that are already hitting the market [13]. The most important analytical developments affecting microfluidics technology are microanalytical methods such as gas phase chromatography, high-pressure liquid chromatography (HPLC), and capillary electrophoresis (CE). These methods have made it possible to obtain fast and highly sensitive data using very small amounts of samples [10]. In 1957, Golay’s research on gas chromatography [37] and Van Deemter’s research on liquid chromatography in 1956 [38] led to the realization that to maintain a high level of performance, the diameter of the column had to be reduced, so columns were produced in the micrometer range [39]. In 1993, Andreas Manz et al. published a paper describing the production of a miniaturized capillary electrophoresis-based chemical analysis system [40]. One year after the publication of this work, the first International Conference on Micro-Collective Analysis Systems took place in Enschede, the Netherlands, and capillary electrophoresis has become popular for separating various biomolecules [13]. As a result of the innovative perspective of this miniaturization, many researchers have invested considerable time and effort to develop microfluidic devices for liquid handling, liquid metering, liquid mixing, separation of liquids, etc. [39].
The influence of biodefense on microfluidic technology became prominent following the conclusion of the Cold War, when it was recognized that potentially deadly weapons that utilize chemical or biological agents posed significant military and terrorist risks. During the 1990s, the Defense Advanced Research Projects Agency (DARPA) launched initiatives aimed at developing microfluidic systems capable of detecting both chemical and biological hazards. This funding and support from DARPA played a pivotal role in expediting academic research in microfluidics and significantly contributed to the advancement of this technology [10].
The field of molecular biology has played an important role in the growth of microfluidics, especially with the genomics boom in the 1980s and the subsequent development of other microanalysis fields tied to molecular biology, including high-throughput DNA sequencing. These developments have created a demand for analytical techniques with significantly greater efficiency, sensitivity, and resolution than previously required in biology. This demand has extended to the development of commercial instruments for sequencing genomic nucleic acids and methods for handling very small liquid volumes. Microfluidics has emerged as a promising solution to overcome these challenges and has led to increased research activity in this field [10,34].
In summary, the advent of microfluidics has had a transformative impact on fields as diverse as biology, chemistry, analytical biochemistry, medical sciences, biotechnology, and tissue engineering. Microfluidics facilitates the manipulation and flow control of very small fluid volumes within complex channel networks. Widely adopted across scientific disciplines, microfluidic systems serve as fundamental tools for researchers and have established themselves as a multidisciplinary arena in science and engineering. Microfluidics has significantly enhanced drug discovery, drug delivery, biomedical research, and other lab-on-a-chip approaches, offering distinct advantages over traditional laboratory techniques by integrating various functions on a single microchip. This technology has made significant research contributions, especially in combining electronic, mechanical, and chemical processes in a miniaturized substrate. Furthermore, microfluidic devices enable the efficient production of micro- and nanoparticles characterized by exceptional morphology and size distribution. Microfluidics enhances a range of chemical reactions, often achieving higher yields and adapting to more demanding conditions than conventional methods [39]. Due to these factors, microfluidics’ utilization is on the rise across diverse fields, spanning fundamental domains like chemistry, biology, and physics, as well as encompassing a multitude of engineering disciplines [36]. The extensive influence of microfluidics in the scientific field is evident through the substantial and ongoing increase in publications across academic journals and conferences, a trend that has persisted from the mid-1990s to the present [10].
3. Microfluidic Devices
Microfluidic and lab-on-a-chip devices have been extensively studied and developed in recent years due to their inherent advantages of low chemical consumption, rapid analysis, biocompatibility, low cost, and automation in biological, biomedical, and analytical chemistry studies [41]. Microfluidics, an interdisciplinary field, has a wide range of applications, including environmental sensing, medical diagnostics, microscale genetic and proteomic analysis, drug discovery, drug delivery, high-throughput drug screening platforms, cell culture and manipulation platforms, microscale chemical production, combinatorial synthesis and assays, biosensors and pathogen detection systems, water purification, separation of various substances such as nanoparticles, microparticles, and cells, artificial organs, and micro propulsion microscale energy systems [42,43,44,45]. Emerging microfluidic technology has also led to rapid progress and success in the development of nanoparticle production. By taking advantage of the unique properties of fluid mechanics observed at micro dimensions, microfluidic technology can produce nanoparticles of appropriate size and precisely controllable shape that can be used for various applications in drug delivery, and in the biosciences, healthcare, and food industries [46]. In order to provide the reader with an overview of microfluidic devices, this section summarizes the types of materials used in the production of microfluidic devices, microfabrication methods, and the components that can be found in a microfluidic device, before moving on to the sections summarizing the studies where microfluidics are used in nanoparticle production.
3.1. Materials
This subsection presents various common materials used for fabricating microfluidic devices (microfabrication) with emphasis on their advantages and disadvantages.
Many materials have been used in the fabrication of microdevices, depending on the chosen technique, and devices have been fabricated on various substrates. Silicon, glass, polymers, hydrogels, paper, and various inorganic materials are among the materials commonly used to fabricate microfluidic systems [47,48].
One of the key steps in microfluidic applications is selecting the most suitable material for the device manufacturing and the process to be performed. To select the type of material for the microfluidic system to be used, many properties should be taken into account, such as surface functionalization potential, processability, ease of manufacture, surface charge, molecular adsorption, non-specific adsorption, electrical conductivity, cellular compatibility, biocompatibility, electroosmotic flow mobility, optical properties and transparency, air permeability, flexibility, the price of the material (especially if disposable devices are produced in processes where there is a risk of contamination), resistance to chemical compatibility with the solvents used (chemical stability), resistance to temperature changes and high temperatures (thermal stability), and resistance to pressure and other physical forces (mechanical stability). These properties must be chosen to be compatible with the properties of the reaction to be treated. Each material has both advantages and disadvantages depending on its intended use. It should not be forgotten that the materials used may influence the process. The miniaturization of the channels that form the basis of the microfluidic technique makes it necessary to pay special attention to the materials that make up the device [49]. In microfluidic systems in particular, shorter contact times, laminar flow, enhanced heat and mass transfer, and large surface-to-volume ratios result in unique phenomena that are not present in macro-scale systems [50]. Compared to macro-samples, in reactions taking place in the micro-scale environment, it is necessary to consider all variables that can influence the manufacturing process, including the wettability of the material of the device and the contact angle formed between the liquid phase and the microchannel.
The variety and quality of materials are constantly increasing due to developing technology and research. At the same time, these materials can also be combined in hybrid devices to utilize their advantages synergistically. However, the advancement of microfluidic device technology and their fabrication depends on the materials used, and requires a deeper evaluation and more experimental work. LOC systems, including microfluidics, MEMS/NEMS (nanoelectromechanical systems), and related microsystem families should focus more on challenges such as integration, standardization, economics of commercialization, and applicability of targeted systems, as well as improved functionality.
3.1.1. Silicon
From the early 1990s, silicon has been an important material used in creating microfluidic devices. Its widespread use in semiconductors is due to its well-documented attributes and advanced manufacturing techniques. Silicon has unique characteristics that make it the preferred choice for making microfluidic systems, particularly silicon microfluidic chips. These chips have exceptional surface stability, efficient thermal conductivity, resistance to organic solvents, semiconductor attributes, and the ability to integrate sensors and electronics onto a unified platform. They are readily available, easy to manufacture, adaptable to different designs, and compatible with various chemicals [51]. Despite its advantages, silicon has several limitations that must be considered when using it in practical applications. One of the most significant drawbacks of silicon is its inability to transmit visible light and ultraviolet radiation, making it incompatible with optical sensing in these spectral regions. This hinders its usefulness in detecting fluorescent labels, a commonly used technique in immunoassays. For in situ imaging, it is essential to ensure that the imaging portion of the device is not made from silicon. Moreover, the intrinsic hardness and brittleness of silicon create challenges when using active components like valves and pumps, or integrating such elements into a silicon-based platform [52]. Other limitations, such as the tendency of biological molecules to adsorb on silicon surfaces and its relatively expensive nature, have led to the exploration of different materials for fabricating microfluidic devices. However, today, the use of silicon for fabricating microfluidic platforms is found in biological applications such as medical diagnostics and organ-on-chip devices, especially for drug toxicity screening [50]. Surface micromachining, the synthesis of micromechanical structures from deposited thin films, or bulk micromachining, where the microstructure is synthesized by etching the substrate, can be used to produce silicon structures [39]. Most silicon-based devices, especially sensors based on electrical-based sensing methods, have a thermally grown SiO2 layer on the surface [47]. Silicon dioxide, or SiO2, is one of the top choices for microfabrication processes such as etching and deposition. It boasts impressive thermal and electrical insulation characteristics, making it a cost-effective option for molds. Its high selectivity to various silicon etchants is another advantage. Additionally, SiO2 is well suited for sealing microchannels. When paired with silicon nitride (Si3N4), it allows for the creation of complex three-dimensional structures via multi-step etching. Si3N4 is a formidable insulator that serves as an impermeable barrier against water and ion diffusion. Its exceptional thermal insulation properties make it ideal for suspending heater structures on silicon nitride membranes or fixtures. Furthermore, its resistance to oxidation and abrasives makes it an outstanding material for molds in deep etching processes. It is also commonly used as a high-strength electrical insulator [31].
3.1.2. Glass
Glass offers several distinct advantages over silicon, including outstanding optical transparency, compatibility with biological materials, lower cost, easy availability, and the potential for active component integration. As a result, glass substrate microreactors offer an alternative to their silicon-based counterparts. One of the primary strengths of glass in microfluidic systems is its optical transparency. Glass and quartz substrates blend smoothly with microfluidic practices due to their advantageous optical properties, making them well suited for biosensors utilizing optical sensing methods such as fluorescence or surface plasmon resonance (SPR) [39,47,48]. Glass-based microfluidic devices offer high solvent resistance and chemical robustness [53]. Glass is a remarkable material with outstanding qualities such as durability, electrical insulation, heat resistance, and potential for surface modification. Its compatibility with biological substances makes it highly valuable for biochemical analyses. Furthermore, its low coefficient of thermal expansion makes it an ideal material for microreactors designed to facilitate chemical reactions under extreme conditions. Glass can also be efficiently cleaned after experiments using chip heating or chemical washing. The ability to combine complementary elements made of glass or other materials makes it a preferred choice for microfluidic chip fabrication [50]. Although these materials are suitable for microfluidic arrays, the manufacturing techniques can be time-consuming, labor-intensive, and expensive. Although glass itself is an inexpensive material, the processing of glass into chips is expensive, time-consuming, labor-intensive, and in some cases requires preparation in clean rooms [49]. However, due to the poor O2/CO2 exchange, it is not suitable for long-term cell culture as it is impermeable to gases. The glass material, which is also hard and brittle, suffers from the difficulty of creating micro-pumps or valves on chips [51]. In terms of composition, glass is divided into soda-lime glass, borosilicate glass, and fused quartz. One of the most popular types of glass is soda-lime; however, it contains many contaminants such as aluminum, so microfluidic devices often use borosilicate. Due to its good optical properties, quartz or fused silica can sometimes also be used [47]. The main material used for these devices is borosilicate glass, which is made from boron trioxide and silica. It stands out because of its low coefficient of thermal expansion and its impressive resistance to thermal shocks. Recently, borosilicate glass capillaries have become a popular alternative to glass for creating nanoparticles. These capillaries work well with a variety of organic solvents. They are also very strong and resist physical deformation better than elastic polymeric devices, which allows them to operate stably and for a longer period of time [53]. In microfluidics and MEMS, Foturan is a material that is widely used. It is a photosensitive glass that contains silver oxides and cerium oxides, and is enriched with lithium-aluminosilicate. Foturan is a unique material that combines the characteristics of glass, such as thermal and hardness resistance, with the ability to create highly structured designs with substantial aspect ratios. The process of producing microfluidic arrays using glass or quartz substrates involves various microfabrication techniques such as plasma etching, reactive ion etching, lithography, etching, and lithography [39,51].
3.1.3. Polymers
In the past, microfluidic devices were primarily made of silicon and glass materials due to their excellent thermal conductance and resistance to temperature changes, respectively. However, due to the numerous disadvantages already mentioned, these materials have been increasingly substituted by polymers. The versatility of polymers has attracted the attention of researchers. This has led to a shift away from silicon and glass chips, and the widespread use of polymers in microfluidic device fabrication [50]. One of the biggest advantages of polymers is that they are cheap. Compared to other MEMS devices, microfluidic devices tend to be larger in size. This means that the cost of substrate materials is an important factor to consider. Polymers are a more cost-effective alternative to inorganic materials, and also offer the benefit of simpler and less expensive manufacturing techniques. Another advantage is that many different polymers with different surface chemistries are available and can be optimized according to the properties required for the desired application [31]. Polymers are available in transparent or semi-transparent varieties. Depending on the composition of the polymer, transparent or semi-transparent devices can be obtained, applicable to all processes where optical visibility is required [49]. Furthermore, easy manufacturing techniques and various surface modification methods are available to improve the efficiency of these devices. Techniques used to produce polymeric microfluidic channels and networks in a microfluidic system include soft lithography, UV laser ablation, hot embossing, injection molding, and direct micro-milling [39]. Polymers do not have a fixed melting temperature. Polymeric materials are softer and moldable above glass transit tempers. Their softness facilitates the manufacture of microanalytical system components, especially pumps and valves, using elastomers instead of hard materials [49]. The low cost, versatility, and ease of use of polymers have made them attractive materials for use in microfluidic systems. Polymers offer the advantage of biocompatibility compared to glass and silicon. Many polymers are compatible with tissue and blood, making them well suited for implantable microfluidic devices in drug delivery. These devices excel in applications, including DNA analysis, cell processing, clinical diagnostics, and polymerase chain reactions. They can also be used as photoresists or passivation layers in conventional microelectronic applications [31]. Polymer microfluidic platforms can be used in applications ranging from nanoparticle synthesis to fluid manipulation. Polymer-based microfluidic systems can provide reliable production under various temperature conditions, depending on the properties of the polymer used. Polymers are suitable for applications at room temperature or higher temperatures, and are very useful for large-scale production. However, polymers have some disadvantages, such as small molecule absorption, high sensitivity to organic solvents, and problems with vapor permeability, which can affect cell signaling dynamics [50]. One of the main issues with polymers is that they tend to exhibit natural fluorescence at low excitation wavelengths. This can pose a problem in microfluidic applications where fluorescence-based detection is used, potentially leading to a decrease in instrument sensitivity. Another challenge with polymers is that they generally possess a lower charge density due to the absence of ionizable groups commonly found in glass. As a result, achieving a stable and controllable electroosmotic flow, which relies heavily on a high surface charge density, can be more difficult with polymers compared to other materials. They cannot be used in microanalyses where the required surface properties are not compatible with the surface properties of the polymers, as in the case of electroosmosis [31].
Numerous polymers, including polypropylene, polycarbonate, polyethylene, polymethylmethacrylate (PMMA), and polystyrene, are widely utilized in both research and industrial settings. When it comes to creating microfluidic devices, the most commonly employed polymers include PDMS, PMMA, fluoropolymers, cyclo-olefin polymers, and copolymers (COPS/COCs), as well as thiol-ene polymers (TES). PDMS, also referred to as polydimethylsiloxane, has become particularly popular in recent years [48,50].
PDMS is the best example of the elastomer group that is frequently used in the production of microfluidics due to its mechanical properties such as flexibility. PDMS has attracted much attention for various reasons, and has been the most frequently used and reliable material in microdevice production since the beginning of the 21st century [52]. The favorable physical properties and easy manufacturing process of PDMS contribute to easy microfabrication at low cost. In addition, it is easy to mold. Multilayer channels, microvalves, or micropumps can be easily formed on PDMS chips. Using a multilayer design in PDMS-based microfluidic devices, internal channels can be customized and modified in both 2D and 3D [53]. PDMS is a crucial material in the production of highly flexible and biodegradable organ-on-a-chip platforms. Its optical transparency down to 280 nm allows high-quality microscopic imaging of cultured cells. Its inertness to biomolecules makes it ideal for creating micro-flow channels, while its biocompatibility, gas permeability, and autoclavability make it advantageous for biological studies. PDMS also inherently prevents cellular adhesion, enabling long-term on-chip cultures and fluorescence/chemiluminescence studies. All these properties make PDMS a valuable material for cellular research [48]. PDMS is a type of polymer with a remarkable tensile strength that enables it to tolerate high hydraulic pressure. This high-pressure tolerance is a crucial property required in microfluidic devices, especially in microfluidic-based nanoparticle synthesis, where optimal mixing requires a consistently high total flow rate. Despite these obvious advantages of PDMS-based microfluidic devices, they also have some significant disadvantages, such as poor solvent compatibility, surface fouling, and deformation problems under high-pressure conditions [52,53]. The porous nature of PDMS allows it to absorb small molecules, making it difficult to use organic solvents such as hexane, toluene, and chloroform. This is because these molecules can easily stick to the channel walls and cause swelling, leading to changes in the flow rate and concentration of the solution. Another issue with using PDMS is the evaporation of water along the channel walls, which can alter the solution’s concentration. Additionally, PDMS can create bubbles when gas flows through it, which can cause experimental errors and lead to inaccurate results. To address these problems, scientists have been exploring alternative polymeric materials with specific properties and intended uses for microfluidic applications. These materials may offer improved resistance to the absorption of small molecules and better chemical compatibility with organic solvents. However, despite these challenges, it is important to acknowledge that the introduction of PDMS has significantly advanced the practical development of microfluidic devices for both technological and biomedical research. Its unique properties, such as its optical transparency and ease of fabrication, have made it a popular choice for microfluidic device manufacturing [39].
The production of microchips also employs PMMA, an amorphous thermoplastic substance that offers certain benefits over PDMS. PMMA is appropriate for various applications because it has superior compatibility with solvents and does not absorb small molecules. Additionally, its thermoplastic nature means it can be easily fashioned using methods such as surface bonding or injection molding. PMMA has numerous desirable properties, including optical transparency, affordability, low permeability to gases, wettability of inner channel walls, and good mechanical characteristics. These attributes make it an exceptional choice for microfluidic research, particularly in developing micro-physiological systems and organ-on-chip devices. However, PMMA does have some factors to consider, such as a low-resolution limit, challenges in modifying surfaces, and difficulties bonding two substrates together. These factors should be taken into account when selecting materials for specific microfluidic applications [50,53].
Teflon, which is also referred to as polytetrafluoroethylene (PTFE), is a substance that provides clearness when it comes to optics and a moderate level of gas permeability. It is versatile enough to create valves with diaphragms, and it effectively prevents the absorption of molecules on the walls of channels. Additionally, PTFE has superior compatibility with solvents and is thermally stable because of its high melting point. This material is recommended for applications in cell cultures, experiments that require high precision, instruments that need to be kept very clean, and for the fabrication of valves and pumps. PTFE is also used in synthesis devices because it can withstand a wide range of chemicals and temperatures up to 240 °C. It is naturally resistant to the clogging of channels with aqueous solutions due to its hydrophobic characteristics. Despite its many benefits, current samples of fluoropolymer microdevices are seldom used in device fabrication because they do not provide for easy micro-modeling during fabrication and do not have the required flexibility [50,53].
3.1.4. Hydrogels
Hydrogels/hydrocolloids are three-dimensional networks of hydrophilic polymer chains that are porous and cross-linked. They allow small molecules and bioparticles to diffuse through them. These colloids are made up of hydrophilic polymers immersed in an aqueous medium. They have easily controlled pore sizes that can mimic the extracellular matrix (ECM). Unlike PDMS, which is suitable for tissue-level microfluidics, hydrocolloids are used as substrates for cell culture in tissue engineering. These colloids have a structural similarity to the extracellular matrix, making them useful for simulating physiological tissues in bio-microfluidic devices. Hydrogels can also be used to create microfluidic component functionalities such as semipermeable barriers and intelligent valves within a chip made of a harder material. The advantages of hydrocolloids include their low cytotoxicity, biocompatibility, biodegradability, high permeability, controllable pore size, and aqueous nature. They are also inexpensive and widely accessible. These properties make hydrocolloids ideal for encapsulating cells for 3D culture in tissue engineering research, for delivering solutions, cells, and other substances, and for sensors and actuators. However, using hydrogels as a primary production material is limited due to the difficulty in maintaining the integrity of the device produced with hydrogels [49,50].
3.1.5. Metals
Metals are a crucial category of materials when it comes to the production of MOEMS (microoptoelectromechanical systems) and MEMS, which are utilized in various applications, such as microfluidic devices. The use of metals in these applications is an essential factor in ensuring their reliability, efficiency, and longevity [39]. Metals are generally inexpensive, widely available, easily machinable, and withstand high working pressures, many strong chemicals (except strong acids), and high temperatures. They are mechanically and chemically strong and easy to clean. They are particularly suitable for use in the production of microfluidic systems when it is necessary to use materials that are incompatible with other materials [49,50]. The thermoelectric properties of metals can be used for temperature sensing [31]. When it comes to producing electromagnetic coils and sensors, microstamps, and structures that conduct magnetic fields, metallic parts are frequently employed. In spite of the multitude of advantages that metals offer, including their broad application in various industries, there exist certain drawbacks associated with micromachining that have impeded their widespread use in this particular area [48]. The most frequently utilized metals for microfluidic gadgets are Al, Cu, and Fe. Nonetheless, they are frequently blended with other metals in alloys to boost their chemical durability [50]. Aluminum is commonly employed for electrical connections and as a protective layer in surface micro-machining. Certain metals, such as permalloy, an iron-nickel alloy, are used for magnetic sensing and activation. Platinum and palladium, both possessing catalytic properties, are advantageous in chemical sensors and microreactors. In microdevice applications with elevated operating pressures, stainless steel can be a superior choice compared to silicon or glass [31].
3.1.6. Paper
Since 2007, paper-based microfluidics have been developed as a more sustainable and cheaper alternative system to polymer chips for microfluidic applications. Paper microfluidics is considered a slightly different technology than polymeric and inorganic microfluidics [47]. Microfluidic paper-based chips have been well received due to several advantages inherent in these devices. Paper-based devices are simple, inexpensive, and user-friendly. Apart from this, they have advantages such as biocompatibility, bio-affinity, biodegradability, easy handling, easy portability, mechanical properties including flexibility, lightweight and low thickness, high stacking, storage and handling capabilities, easy modification chemically or biologically, ease of manipulation and sterilization, high rate of disposable use, high porosity, high physical absorption, ability to absorb liquids by capillary action without any external power supply, and ability to operate without support equipment. Paper-based systems with colorimetric or electrochemical readings are becoming versatile and valuable for rapid diagnostic testing and medical screening in developing countries. Paper microfluidic systems can be operated on-site without technical infrastructure, making them a promising solution for field analysis or home testing. Microfluidic paper-based analytical devices (µPADs) are reliable and sensitive, making them useful in the fields of medicine, healthcare, and environmental monitoring [39,50]. However, despite these advantages, some disadvantages should also be mentioned. Paper has little resistance to high humidity conditions and mechanical forces, and is not easily applicable to manufacturing processes. When working with fluid samples that have low surface tension on microfluidic paper chips, the hydrophobicity of the channels may not be adequate. To prevent any sample leakage, it is essential to integrate hydrophobic barriers that are thick enough. It should be noted that the precision of colorimetric analyses on microfluidic paper chips is typically not very high, but this can be slightly enhanced through special channel design [49,50,51].
3.1.7. Epoxy
Microfluidic devices can be fabricated using epoxy resins, which are often used in combination with glass or silicon chips, but can also be used on their own to create organ-on-chip devices. These resins provide excellent stability at high temperatures, chemical resistance, and transparency, making them ideal for observing cell growth in biological samples. Additionally, they offer high resolution with small properties. However, a major drawback of using these materials is their high cost [50]. SU-8 is a type of photoresist that is epoxy-based and specifically designed for microelectronic applications where a thicker layer is needed that can withstand both thermal and chemical stress. The main components of SU-8 are Bisphenol A Novolac epoxy and an organic solvent, along with up to 10% hexafluoroantimonate-triaryl sulfonium salt. The viscosity of SU-8 varies depending on the amount of solvent used, which in turn affects the thickness of the layers it can create [39].
3.1.8. Ceramics
Inorganic materials used in microfluidic devices include low-temperature fired ceramics [47]. These ceramics have unique surface chemistry that enables them to withstand corrosive environments and high temperatures. However, their integration into a complete microsystem can be difficult due to their poor dimensional stability, porosity, and brittleness. Despite their limitations, aluminum oxide ceramics remain a promising material for microfluidic applications [50].
3.2. Microfabrication Methods
The field of microfluidic device fabrication initially relied on silicon micromachining, which involved creating microfluidic devices with integrated sensors and actuators from silicon substrates. Over time, the range of available microfabrication techniques has significantly expanded. These microtechnologies, primarily employing hard materials like silicon and glass, are categorized as hard technologies and involve processes such as etching, lithography, and deposition. Conversely, plastic microtechnologies, referred to as soft technologies, typically use polymer materials and offer the advantage of rapid prototyping. Soft technologies bring additional benefits such as surface effects, transparency, and a wider range of available materials. However, hard microfabrication technologies tend to be more complex and challenging to implement. These processes demand a highly controlled and clean environment. A clean room is meticulously regulated in terms of temperature, humidity, and airflow. This controlled setting is essential because, in dusty environments, standard micrometer-sized particles can adhere to surfaces, altering the microstructures being fabricated. To maintain cleanliness, preliminary surface preparation may be necessary before microfabrication. This preparation could involve the use of distilled water at various temperatures, different types of acids, or specific buffers, depending on the requirements for general cleaning, particle removal, oxide removal, and contaminant elimination [31].
The wide range of materials available for microfluidic device manufacturing has different properties that influence their behavior during processing. As a result, the manufacturing methods must be carefully adapted to the specific material properties and desired end product characteristics. Cost considerations also play an important role in selecting the appropriate manufacturing technique. Furthermore, it is crucial for the widespread adoption of chips that they can be manufactured in an accessible and scalable manner. As the field of microfluidics has progressed, numerous manufacturing techniques have emerged to create channels with the required size and properties. These techniques can be classified according to whether they involve material removal (referred to as removal techniques) or material deposition (known as deposition techniques). Another classification categorizes manufacturing methods according to the nature of the processes used and covers chemical, mechanical, laser-based, and various other processes. Starting a project with the right manufacturing technique can significantly accelerate project timelines and improve device performance. A significant amount of work in the field of microfluidics has been performed using soft lithography, with particular emphasis on soft lithography methods involving polydimethylsiloxane (PDMS). In addition to soft lithography, microfluidics engineers have developed various methods for fabricating sub-millimeter channels for a variety of reasons, including cost-effectiveness, faster turnaround times, availability of more affordable materials and tools, and the ability to offer greater functionality. These developments, coupled with the fabrication of microfluidic devices using a variety of materials and geometries, have unlocked new and advantageous physical behaviors and properties in microfluidic devices [54].
Material removal techniques involving chemical processes include wet etching, dry etching, and electrochemical discharge machining. These microfabrication techniques have long been widely used to fabricate glass and silicon microfluidic channels. Material deposition techniques involving chemical processes include silicon surface micromachining, lithography, inkjet 3D printing, powder 3D printing, direct writing, and two-dimensional virtual hydrophilic channels. Material removal techniques involving mechanical processes include techniques such as micro-milling, micro-grinding, micro-abrasive air-jet machining, micro-abrasive water jet machining, ultrasonic machining, and xurography. Material deposition techniques involving mechanical processes include injection molding and hot embossing. Material removal techniques involving laser-based processes are the photothermal process, ultra-short pulse process, absorbent material process, photochemical modification process, and laser direct machining. Material deposition techniques involving laser-based processes are selective laser sintering, stereolithography, and two-photon polymerization. Among other processes, the focused ion beam technique has been recorded as a material removing technique, while the forming process, soft lithography, layer-to-layer manufacturing, layer-on-layer manufacturing, fused deposition modeling, and 2.5-dimensional printing as material depositing techniques [50]. Since this review focuses on the use of microfluidic devices in nanoparticle production, we will discuss the methods used to produce PDMS-based devices, which are the most commonly used for nanoparticle production today. Other methods and more detailed descriptions are given elsewhere [54,55,56,57,58,59,60,61,62,63,64,65,66].
One of the most important methods for creating microchannel geometries and cavities in PDMS-based microfluidic devices is soft lithography. Depending on the resolution of the master mold, this technology enables fine microfluidic pattern resolution ranging from micrometers to nanometers. Typically, a negative mold is used into which PDMS is poured, filling the regions left open by the mold. The master mold used to pour the PDMS can be made from a variety of materials, with SU-8 being the most common choice. The fabrication of the master mold usually involves conventional photolithography techniques. In this process, the negative photoresist SU-8 is patterned onto a silicon wafer to form the ridges of the microchannel network. Alternative lithography methods such as reactive ion etching, electron beam lithography, wet etching, multiphoton lithography, direct laser writing, focused ion beam, and stereolithography can also be used to fabricate master molds. These methods enable high-resolution production, and some of them, such as direct laser writing, electron beams, and focused ion beams, do not require a physical mask, potentially reducing costs, although they tend to be slower. Master molds can also be produced using techniques such as the micromachining of materials such as PMMA, brass, glass, and steel, and 3D printing. While these processes are generally more cost-effective than the lithography methods, they may not achieve the same high resolution, limiting their applicability for commercial and research purposes. Advances in 3D printing technology have enabled relatively high-resolution master molds to be printed directly by a 3D printer. Once the master mold has been produced using any of the methods mentioned, it is typically fluorosilanized to make it hydrophobic. This hydrophobicity facilitates the peeling of the PDMS after the casting process. In the PDMS casting process, the crosslinker and curing agent are mixed; Dow and Corning’s Sylgard 184 is a common choice with a crosslinker-to-curing agent ratio of 1/10. Changing this ratio can change the hardness of the resulting PDMS. The mixture is degassed under vacuum to remove air bubbles and then cured in the master mold at a temperature higher than 70 °C, typically for times ranging from 15 min to 6 h. This process solidifies the polymer and transfers the pattern of the positive mold to the PDMS layer. PDMS exhibits ease of demolding, the ability to replicate nanoscale features, minimal shrinkage during curing (approximately 1%), and excellent elastic properties during these processes. Alternatively, microchannel arrays in the PDMS layer can be formed directly using a laser cutting machine. Inlets and outlets are drilled into the cast PDMS layer to provide access to the channels. The PDMS part with the channels is then bonded to a substrate, usually PDMS or other materials, to form the device. Oxygen or air plasma activation, ultraviolet/ozone (UVO) therapy, employing uncured or partially cured PDMS as an adhesive, or the corona discharge method are common methods for joining PDMS layers. These procedures guarantee a strong link between the layers. The device can then be modified on its surface and functionalized, utilizing a variety of techniques depending on the demands of the work [67,68].
3.3. System Components
The most basic components required to operate a microfluidic device are microchannels where the flow takes place, input and output ports to the microchannels, a pump that will allow the fluids to flow, and active or passive mixers that will allow them to mix. Apart from these, various internal or external components can be added to the system according to the requirements of the protocol to be applied. For example, valves, filters, micropumps, switches, lenses, membranes, heaters, motors, optical fibers, various other actuators and devices, various sensors for different applications, sealing gaskets, compressed gas lines, micro overpasses to prevent the mixing of liquids, and microflow meters are some of the components that can be used according to needs and purposes [69,70,71,72,73].
The main component of any microfluidic device is the channel network. Microchannels are channels with a hydraulic diameter of less than 1 mm, usually 1–99 μm. The channel structure can be formed using various design and fabrication methods. Microchannels can be in various shapes such as coil-shaped, rectangular, zigzag, or spiral. Although a simple channel network can be useful in some applications, for other applications it is necessary to add various components to the system to increase functionality [74,75].
The accurate control of liquid flow within microfluidic devices relies on various pumping schemes, which can be classified as either external or internal to the fluidic pathway. Each of these methods possesses distinct characteristics tailored to specific application requirements. Several types of external pump types are employed for microfluidic applications. Syringe pumps offer the advantage of delivering steady and precise flow rates. Peristaltic pumps are well suited for handling viscous and shear-sensitive fluids. Piezoelectric (diaphragm) pumps provide a rapid dynamic response, possess a simple structure, and are lightweight. Nanopump designs are ideal for handling very small fluid quantities, ensuring consistent continuous flow rates and allowing for the intermittent introduction or removal of several nanoliters of fluid per revolution. Pressure-driven pumps exhibit minimal time to achieve steadiness with no observed periodic fluctuations. High-performance liquid chromatography (HPLC) pumps, known for their high-pressure operation and increased flow rates, are also commonly used in microfluidics. However, due to possible valve failures, HPLC pumps might not be appropriate for viscous liquids [76,77,78,79,80,81,82]. On the other hand, the establishment and maintenance of flows are made possible by internal micropumps implanted into microfluidic chips. They produce a continuous laminar flow rather than a turbulent one, unlike micromixers. These micropumps depend on outside forces to function, frequently using optical forces for quick remote control and the ability to run numerous rotors at once. By depending on changes in internal pressure, passive pumping systems move fluid by using surface tension in tiny liquid drops. In order to provide effective fluid manipulation within microfluidic devices, the choice of pump type depends on certain system requirements, such as liquid characteristics, flow rate precision, and application needs [52,70,74].
From the point of view of nanoparticle production in microfluidic systems, the ability of this system to mix the liquids that are the formulation components will be essential. Therefore, various microfluidic mixing techniques are used. Micromixers are included in the channel network. Micromixers can be two- or three-dimensional and are usually classified as active or passive [50]. Most micromixers are passive devices. Passive micromixers employ their shape and geometry to create turbulence and chaotic advancement of the liquid, and the mixing of fluids occurs by diffusion. They do not require any external force to drive fluids or to orientate or segregate particles in the liquid. Given that mixing happens by diffusion, the effectiveness of mixing is influenced by the fluids’ contact area and the duration of their contact. Different designs of passive micromixers can be created to reduce laminar flow, increase the surface area of contact between the fluids, and enhance mixing efficiency. These designs can include T-type microreactors, Y-type microreactors, flow-focusing microreactors, co-flow microreactors, S-shaped microchannels, and staggered herringbone micromixers (Figure 2). In some microfluidic systems, various geometries can be combined. According to their mixing structure, these microreactors can also be divided into simple, split-and-recombined, curved, spiral, or multilayer configurations. Although it is simple to build superficial designs like T-type, Y-type, or cross micromixers, they perform poorly. Split-and-recombined micromixers are better for viscous and multiphase fluids, while helical and curved structures perform best at high flow rates. The potential of multilayer structures to offer outstanding mixing performance at both high and low flow rates in a few of milliseconds has recently gained a great deal of attention. Compared to active micromixers, passive ones are less expensive, more portable, and simpler to integrate into lab-on-a-chip systems. Passive mixers have significant drawbacks despite being simpler and more affordable. Increasing the contact surface of the liquids by prolonging the channel increases fluid resistance to the track, decreasing the efficiency of the microfluidic device. In an active micromixer, the mixing event is initiated or accelerated by an external force or physical field (such as magnetic, electrokinetic, thermal, acoustic, or ultrasonic), which enhances performance. In general, active stirrers are more effective than passive stirrers. However, given the undesirable complexity and additional expenses, the requirement to integrate an external force or field into the platform makes it a less practical alternative for rapid prototyping and commercial translation [52,83]. The optimal agitator type typically depends on the reagents to be mixed and the flow regime (i.e., Re number) during operation. While several mixers perform better at greater rates of flow, some perform better at slower rates of flow.
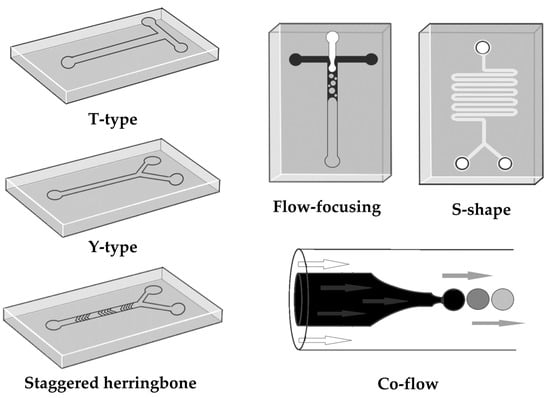
Figure 2.
Types of micromixers with different designs.
Another type of component that can be used to regulate flow properties are microvalves. A microvalve is a microscale valve, i.e., a two-port component that regulates the flow between sections within a microfluidic system. Microvalves serve as one of the fundamental parts of microfluidic devices and regulate fluid transfer. There are two different kinds of valves: active valves, which take energy to operate, and passive valves, which do not. The kind of valve a device uses is determined by the kind and quantity of control required for the application [84]. In recent years, Tesla valves have garnered significant attention as a notable example of valve technology. The distinctive architecture of Tesla valves is defined by their asymmetrical design and an arc-shaped channel. Tesla valve structures have found applications in various fields, leading to advancements in technologies like medical instruments, lab-on-a-chip systems, and chemosensors [85,86].
4. Advantages and Disadvantages
Thanks to their reduced size scale and larger surface-to-volume ratio, microfluidic technologies have several advantages over conventional macro-scale platforms, such as requiring less sample and reagent volume, operating at higher throughput, higher sensitivity, lower cost, easier portability due to their reduced size, ability to mimic biological systems, faster sample throughput, and decreased human error due to their potential to be automated and highly integrated [87,88,89,90]. The integration of microfluidic technologies into a ‘lab-on-a-chip’ provides a powerful solution for processing and analyzing samples on a single platform, thereby reducing the complexity, bioassay loss, and cross-contamination associated with multiple processing and transfer steps in more traditional benchtop workflows. Integrated multifunctional systems, created through their ability to be parallelized, enable better and faster process control [91]. Some flow characteristics not seen in conventional fluid handling platforms, such as the laminar flow exhibited by micro-sized channels, allow for some creative applications. The capacity of microfluidic devices to selectively collect and release certain cells, macromolecules, germs, and particles is one of their noteworthy characteristics. Microfluidic capture and release systems have a number of benefits over conventional batch analyzers, including non-contact operation, high sensitivity, accurate results, label-free identification, and reduced reagent needs [92]. Microfluidic devices provide an alternate method to traditional biochemical separations. It is beneficial to utilize technologies that make use of dielectrophoretic pressures to differentiate and manipulate cells as they do not need to be functionalized before use [93]. When it comes to the creation of drug-releasing nanoparticles, microfluidic technologies are a very useful method. Many of the problems associated with the limitations of conventional manufacturing techniques are effectively solved by these technologies. Micro/nanoparticles with specific properties such as small size, significant surface area, tunable surface chemistry, high drug loading capacity, and exceptional surface-to-volume ratios are becoming essential in the context of regulated and safe drug production. The production of nanoparticles using conventional techniques such as emulsion polymerization, dispersion polymerization, and spray drying often results in particles with drastic polydispersity, little functional diversity, poor reproducibility, and irregular shape. Microfluidic systems are unique in that they can produce smaller, more homogeneous particles. This accuracy is achieved through the careful regulation of liquid phase flow rates, resulting in higher drug loading levels and improved drug encapsulation efficiency (EE). The design of the structure of the microfluidic device, control of flow rates, manipulation of material composition, and selection of fluid viscosity can be used to precisely vary the size, structure, function, and distribution of particles. It is with this degree of control and accuracy that the efficient creation of a wide variety of particles with different compositions and properties, designed to meet the unique requirements of drug delivery systems and other applications in nanomedicine, is possible. Fundamentally, microfluidic technologies have transformed nanoparticle production, enabling the development of highly customized and precisely manufactured particles for a variety of applications [94,95]. The numerous advantages of microfluidic methods make them a key factor in developments in a variety of industries. These advantages include improved handling and storage safety, improved accuracy, flexibility, higher cost-effectiveness, and reduced raw material consumption due to integration capabilities. The ability of microfluidic systems to integrate seamlessly with a variety of elements such as heaters, sensors, and flow control elements offers a range of benefits including increased heat transfer, accurate temperature and feed flow control, increased repeatability, and real-time reaction monitoring. The use of microfabricated networks of specially designed microchannels and reservoirs enables the execution of several process steps and parallel reactions on a single chip. In addition, synthesis conditions such as temperature, pressure, and reagent compatibility are extended by microfluidic technologies, which is often not possible with conventional batch processes. This expanding field offers intriguing opportunities to create innovative material systems and complex nanostructures, and usher in a new era of innovation [9]. Particles created with microfluidic technologies can have advanced properties such as double partitioning, pH sensitivity, and controlled release [95]. However, there are several inescapable difficulties with micro/nanoparticle creation that microfluidics must overcome. Designing microsystems that can produce desirable materials under desired parameters (pressure, temperature, chemical compatibility, concentration, etc.) without clogging the system is the major problem when using microfluidics to synthesize nanoparticles. The manufacturing of devices with intricate geometries and combinations raises production costs and complicates the manufacturing process [9,94]. Highly competent technicians are needed for both microfluidic device design and production. Biologists and chemists are discouraged from using this technology in their laboratory due to the range of microfluidic devices [96]. Each material chosen to build the system has pros and cons, as has already been explained. Material selection and device construction should be carefully planned while taking the needs of the process into account. For instance, frequently used microfluidic chip components like PDMS can adsorb hydrophobic small molecule medicines, rendering them useless for drug research. It would be essential to reduce adsorption through surface modification to resolve this issue. For some systems, channel clogging during material production continues to be difficult.
5. Polymeric Nanoparticles
Polymer-based nanoparticles have been under scrutiny for drug delivery and imaging applications since the 1970s, and continue to be widely used in contemporary research due to their numerous advantages that contribute to effective drug delivery and targeting. They are favored because of qualities such as biocompatibility, stability, biodegradability, and readiness for preparation, which enables the exact control of NP characteristics and their length of action [97,98].
Polymer NPs are made using both natural and synthetic polymers; however, synthetic polymers provide a greater range of formulation options and more accurate batch-to-batch consistency. Poly(lactide-co-glycolide) (PLGA), poly(alkyl cyanoacrylate) (PACA), poly(ethylene oxide) (PEO), poly(lactic acid) (PLA), poly(L-lysine) (PLL), poly-caprolactone (PCL), and poly anhydrides are examples of synthetic polymers, whereas natural polymers include chitosan, gelatin, hyaluronic acid, and alginate. Among them, PLGA use is relatively widespread. The FDA-approved, biodegradable, and biocompatible polymer PLGA is being extensively used in sustained drug release strategies for the inclusion of both hydrophilic and lipophilic medicines due to its appropriate degrading characteristics and long clinical history [99,100].
The manufacturing processes of polymeric nanoparticles are relatively simple, and since polymers can be synthesized from a wide variety of monomers with different properties, engineered polymeric NPs can be produced in various shapes and sizes or with other surface properties such as porosity. This makes them highly adaptable and customizable for multiple applications, such as drug delivery [7,97]. Depending on the material and technique used, different final NP products with various structures and properties can be obtained [98]. Drug molecules can be encapsulated within the cores of the NP, entrapped or conjugated to polymer matrices, or bound to their surfaces after particle formation. Moreover, they can release therapeutic agents by triggering through the incorporation of specific stimulatory elements [7]. Polymeric nanostructured systems include nanospheres, nanocapsules, nanoemulsions, and nano gels. Nanospheres comprise a compact and homogeneous polymer matrix in which the substance is dispersed and trapped in polymeric networks or adhered to the surface. In contrast, nanocapsules are vesicular systems in which the drug is trapped in an oily liquid surrounded by a single polymeric layer. Well-designed nanocapsule shells protect the encapsulated drugs and release them in the internal structure at any time in response to external stimuli. Nanogels are three-dimensionally cross-linked polymer particles that combine the characteristics of hydrogels and colloidal particles. They are helpful for shielding medications from the outside environment and, through surface modification, can deliver pharmaceuticals to certain tissues and cells. A potential colloidal method for delivering and transporting chemicals is nanoemulsions. They are extensively employed in a variety of sectors, including agriculture, cosmetics, food, and medicines [94,97].
There are many methods described in the literature for the preparation of polymeric NPs. However, the most widely used processes are solvent emulsification-evaporation, solvent emulsification-diffusion, salting, and nanoprecipitation [97]. These conventional methods are usually based on the “batch method”, so they often lack control over mixing, nucleation and particle growth, resulting in the formation of NPs with large size, wide size distribution, and heterogeneous structure [100]. The clinical uses of nanoparticles (NPs) are constrained by issues with scalability, limited control over reaction parameters, large variation in nanoparticle size, poor reproducibility from batch to batch, and the need for significant amounts of chemicals and therapeutic agents. These issues are related to conventional batch synthesis methods. Microfluidic devices, on the other hand, provide a fresh strategy for producing and synthesizing polymeric nanoparticles that has definite benefits. By addressing many of the drawbacks of conventional batch procedures, microfluidic manufacturing may improve control over physicochemical parameters such nanoparticle size, size distribution, and shape. This innovation has great potential for developing polymer-based NPs’ therapeutic uses [101]. Microfluidic devices allow the preparation of monodisperse particles using any single or double emulsion technique. Versatile HFF (hydrodynamic flow-focusing) devices, stepped herringbone micromixers, two-phase gas-liquid microfluidic mixers, automatic microfluidic flow reactor, T-junction, co-flo microfluidic, and pulse jet micromixers have been developed in recent years to prepare synthetic polymer NPs. HFF is the most widely used method for synthesizing polymer NPs among all microfluidic methods. Compared with conventional bulk methods, the HFF method allows a better control of size, size distribution, and surface properties. In addition, using a microfluidic device can greatly improve the drug encapsulation efficiency of nanoparticles [94,100]. As an example of research carried out, Greco et al. developed a microfluidic mixing technique for creating chitosan-based nanoparticles capable of carrying various macromolecular cargos. They aimed to simplify the production of versatile nanoplatforms for delivering biologics. Chitosan, a cationic polysaccharide, was employed, and a microfluidic mixing chip, “Fluidic 186”, was used to form spherical and homogeneous nanosized structures. The optimal parameters for NP production were identified, resulting in NPs with a mean diameter of 70.94 nm, a low polydispersity index (PDI) of 0.13, and a positive surface charge of +14.38 mV. These findings demonstrate the potential of microfluidic mixing for efficient and controlled chitosan nanoparticle production with diverse applications in drug delivery [102]. Another study was published by Gimondi et al. This study utilized a microfluidic device to produce polymeric nanoparticles of various sizes, investigating the influence of crucial synthesis parameters such as polymer concentration, flow rates, and flow rate ratios. NPs with 30, 50, and 70 nm diameters were selected to examine size effects. The research demonstrated that NP size significantly impacted drug encapsulation, release profiles, and cytocompatibility. The cytocompatibility was studied on endothelial cells and macrophages. Smaller NPs exhibited lower drug content and faster release rates. The study also revealed that microfluidics could reliably generate NPs with controlled sizes, high monodispersity, and long-term stability. These findings highlight the importance of NP size in drug delivery applications, and underscore the potential of microfluidic platforms for precise NP synthesis and tailored drug delivery systems [103]. Saygili et al. utilized a microfluidic T-junction platform to synthesize functionalized polyacrylamide-alginate dual network hydrogels aimed at addressing cartilage repair challenges. The prepared hydrogels maintained mechanical stability under varying temperature and humidity conditions for three months. The nanoparticles prepared using the microfluidic platform for use in functionalization exhibited uniform, small sizes with low polydispersity indices. The functionalized hydrogels showed improved viscoelastic properties and exhibited remarkable cartilage regeneration potential in both in vitro and in vivo experiments [104]. Brzezinski et al. introduced a microfluidic-based method to produce (co)polymeric and hybrid NPs composed of (co)polymers and tannic acid using a microfluidic flow-driven glass-capillary device. The nanoparticles had tunable sizes ranging from 140 to 230 nm, controlled by varying phase flow rates and (co)polymer compositions. These NPs showed significant potential for controlled drug release, especially for the anticancer drug doxorubicin (DOX), and exhibited enhanced antitumor activity against breast cancer cells (MCF-7) in vitro. The study demonstrated the use of microfluidics in creating efficient nanocarriers with promising applications in anticancer therapy, and highlighted that the synergy between PCL macromolecules and tannic acid enhances drug delivery efficiency for chemotherapy against breast cancer [105]. Mahmoudi et al.’s study presents a microfluidic-based method for the production of alginate nanogels, utilizing a co-flow microfluidic chip. Computational fluid dynamics simulations were employed to optimize flow rate ratios and understand the flow behavior within the microchannels. By adjusting the FRR (flow rate ratio) and TFR (total flow rate), control over key physical properties of the nanogels, such as size and cargo release rate, was achieved. The results demonstrated that nanogels with average diameters ranging from 43 ± 4 to 125 ± 7 nm were produced at FRRs ranging from 0.01 to 0.2. Compared to conventionally synthesized nanogels, those synthesized via microfluidics exhibited smaller sizes and higher monodispersity, resulting in reduced burst release that shows a sustained release behavior. Overall, this study introduces a promising microfluidic method for synthesizing polymeric nanogels with applications in drug delivery and tissue engineering, showcasing the benefits of microfluidic synthesis over conventional bulk methods in achieving sustained drug release [106]. A summary of polymeric nanoparticle studies prepared using the microfluidic method is included in Table 1.

Table 1.
Polymeric NPs produced via microfluidic systems.
6. Lipid Nanoparticles
Lipid-based nanoparticles (LNPs), which range in size from 10 to 1000 nanometers and are mainly composed of lipids, serve as versatile drug delivery systems. These efficient carriers can be assembled from liquid or solid lipids, thus offering a wide range of options for modifying the properties of formulated nanoparticles. In the field of nanomedicine, liposomes and lipid nanoparticles, which exploit the high biocompatibility of lipid molecules, are widely used as drug delivery systems due to their distinctive properties such as self-assembly, degradability, simple synthesis, and surface functionalization. Besides drug delivery applications, they have various possible applications in cosmetics, food/nutrition, nutraceuticals, and the sustained release of active compounds. They can encapsulate a wide range of drug payloads. They can be selectively directed to specific cells or tissues through external modifications. Each type has distinctive characteristics that can be customized to suit the precise needs of the therapeutic cargo. Lipid nanoparticle (LNP) formulations frequently include a number of essential ingredients, including phospholipids, cholesterol, glycolipids that are positively charged, and lipids that have been PEGylated to imitate biological structures. LNPs have a structural framework thanks to phospholipids, which are essential for cell membranes and help release them from endosomes. This mixture is intended to improve the performance of LNPs in a variety of settings, including gene therapy and drug delivery [128,129].
Significant progress has been made by researchers in developing various lipid-based nanoparticles, each with its own unique advantages and characteristics. These nanoparticles can be classified into different subgroups based on their structure and composition. They usually appear as spherical vesicles with at least one lipid bilayer surrounding an internal compartment. One of these types is micelles. Micelles are structures in aqueous solutions where lipid monolayers spontaneously assemble to form a hydrophobic core. The hydrophobic cores of micelles are particularly suitable for encapsulating small hydrophobic molecules. In this way, they can increase the solubility of hydrophobic drugs and transport them effectively by providing stability. Liposomes stand out as an essential type of lipid-based nanoparticles. With one or more lipid bilayers around an aqueous core, liposomes have a special design that allows them to successfully encapsulate both hydrophobic and hydrophilic small molecules. Liposomes are excellent carriers for a variety of oligonucleotides, including siRNA, mRNA, plasmid DNA, and different medicinal compounds such as peptides, because of their versatility. Lipid nanoparticles (LNPs) are recognized for their remarkable stability and efficient intracellular delivery. Additionally, various ligands may be added to their surfaces to enable immune evasion and targeted administration, making them especially suited for nucleic acid-based therapeutics. However, liposomes have shown drawbacks in terms of the effectiveness of drug encapsulation and sensitivity to leakage. Solid lipid nanoparticles (SLNs), a novel strategy to improve drug encapsulation, were created in the 1990s as a solution to these difficulties. In contrast to liposomes, SLNs have a solid lipid core matrix encased in a surfactant shell. The structural stability and controlled release capabilities of the solid core matrix make SLNs advantageous for use in a variety of drug delivery applications. Physical stability, site-specific targeting, and regulated release are a few of their prominent benefits. However, SLNs continue to have challenges with drug loading and possible leakage [98,128].
Lipid nanocarriers help prepare personalized cancer vaccines and various vaccination approaches. They also serve as practical tools for delivering antiviral therapeutics to cells. Lipid nanoparticles have emerged as an important RNA delivery method, especially in recent years with the licensing of preventive vaccines for pandemics. Although LNPs have favorable characteristics such as convenient dimensions, stable storage, efficient payload, and controlled release rates, they face some limitations. In particular, they often fail to achieve high encapsulation efficiency and optimal scalability for nucleic acid delivery applications [130].
Ultrasound, high shear or pre-homogenization, microemulsion-based preparations, and solvent evaporation are common procedures used in conventional methods for the production of lipid nanoparticles. Generally, these batch-mode mixing techniques include homogenizing particle size by operations like sonication and extrusion. The use of harsh conditions like high-energy homogenization and high-temperature evaporation, as well as the possibility of metal contamination from ultrasound, reduce the control over the ultimate lipid NP quality in terms of size and size distribution. These techniques can also result in batch manufacturing that is very variable across batches. The conversion of lipid NPs to therapeutic applications has been difficult due to this complexity and low yields. The synthesis of lipid NPs can be achieved more cheaply and with greater yields thanks to microfluidics, which provides answers to many of the issues with traditional manufacturing techniques. In a simple procedure, microfluidic manufacturing techniques may produce homogeneously sized nanoparticles with excellent drug encapsulation effectiveness, enabling the quick synthesis of significant amounts of these nanoparticles. Microfluidic devices provide precise control over the production of LNPs, offering continuous, controllable, and reproducible production processes. Microfluidic systems allow parallel reactions. They are also versatile, with various channel sizes, multiple production materials (e.g., polymers or glass), customizable device geometry and mixing patterns, and other modifiable and controllable features such as flow rate. This makes them suitable for producing lipid formulations with many properties. As a result of such advantages, the use of microfluidic techniques in producing LNPs has considerably increased in the last few years. Nucleic acids, such as RNA, and proteins may be effectively incorporated into lipid nanoparticles using microfluidic chip-based production techniques. A variety of biopharmaceuticals may be delivered using these nanoparticles. Various microfluidic designs have been developed for LNP production, including segmented flow micromixers, stepped herringbone micromixers, high-pressure micromixers, T-shaped microfluidic devices, glass capillary-based microfluidic chips, 2D and 3D invasive lipid nanoparticle production (iLiNP) devices, and flow-oriented micromixers [100,129,130,131,132]. The HFF approach stands out among these techniques as a straightforward and popular way for creating lipid nanoparticles with various compositions and functions [100]. It may feasible to achieve an encapsulation efficiency of almost 100% and maintain a constant, homogenous population of lipid nanoparticles, whether in a laboratory environment or on an industrial scale, by using quick mixing techniques like a stepped herringbone mixer [101]. As an example of the research carried out, van Der Meel et al. described a modular LNP platform that efficiently and stably co-encapsulates lipophilic taxane prodrugs and small interfering RNA (siRNA) targeting the androgen receptor, a critical driver in prostate cancer, using a T-junction microfluidic device. The combination therapy demonstrates additive therapeutic effects in vitro. The LNPs formed exhibit particle diameters around 50 nm, PDI ≤ 0.1, and nearly neutral zeta potentials. All formulations containing taxane prodrugs at concentrations ranging from 0.1 to 10 mol% consistently exhibited efficient entrapment of prodrugs and siRNA, with an average entrapment rate exceeding 90%. This high encapsulation efficiency was achieved by mixing the organic and aqueous solutions at a flow ratio of 1:3 (v:v) and a total flow rate of 28 mL min−1 using a T-junction microfluidic device. These findings highlight the potential of the co-encapsulation of siRNA and lipophilic prodrugs in LNPs prepared via microfluidic synthesis to enhance combination therapies in cancer treatment [133]. Leveraging microfluidics-based coaxial electrostatic spray technology, Wang et al. have successfully developed liposomes loaded with both paclitaxel (PTX) and DOX. These innovative liposomes exhibit uniform particle sizes, high drug loading capacities, and excellent stability. This PTX/DOX@cRGD-Lipo formulation demonstrates synergistic anti-tumor effects, promoting the enhanced apoptosis of tumor cells. Microscopy and flow cytometry analysis confirm the efficient uptake of these co-loaded targeted liposomes by MDA-MB-231 and 4T1 cells, followed by effective drug release. Moreover, in vivo studies reveal excellent targeting biodistribution and a higher rate of tumor growth inhibition in an orthotopic tumor mouse model. These promising results collectively suggest that dual drug-loaded targeted liposomes could offer a highly effective therapeutic strategy, and that microfluidic-based fabrication could play a crucial role in their development [134]. Fathordoobady et al. compared ultrasound and microfluidic methods for creating stable hempseed oil (HSO) nanoemulsions. Microfluidics outperformed ultrasound, producing smaller, stable nanoemulsions (<62.0 nm) at a 4:1 aqueous-to-organic flow ratio and 12 mL/min total flow rate. Comprehensive evaluations, including release behavior, transport, and toxicity tests, showed both methods as suitable for oral drug delivery. Optimization factors include surfactant concentration, flow rate ratio, and total flow rate. These nanoemulsions enhance poorly aqueous-soluble drug bioavailability as nanolipid carriers. Microfluidics offers stability advantages and promising drug delivery applications [135]. Li et al. used a single-step microfluidic process to form transferrin-conjugated lipid nanoparticles (Tf-LNPs), and compared it to a multi-step batch mixing (BM) method. The microfluidic setup utilized a PDMS-glass chip with three inlets and one outlet. The single-step microfluidic approach (Tf-LNPs-MF) produced smaller and more homogeneous Tf-LNPs compared to the multi-step BM method (Tf-LNPs-BM). Tf-LNPs-MF exhibited efficient cellular uptake in vitro and superior tumor inhibition in vivo, indicating enhanced siRNA delivery. This simplified microfluidic synthesis holds promise for the development of LNPs for use in cancer therapies [136]. Ran et al. used a one-step microfluidic approach to create a library of liposomes with different functionalities. By adjusting the flow rate ratio, the liposome size was controlled while maintaining consistent surface properties. Liposomes with an average diameter of approximately 70 nm and excellent serum stability were produced using an 8:1 FRR. This microfluidic method allowed the precise control of size, charge, and surface chemistry by mixing lipids in a microfluidic device. The dual-ligand liposomes showed enhanced cellular uptake and penetration into 3D tumor spheroids, and animal studies confirmed improved tumor accumulation and retention. This microfluidic method offers a promising platform for the development of functional liposomes and personalized therapies [137]. An overview of some of the other studies in which lipid-based nanoparticles were prepared by microfluidic methods is given in Table 2.

Table 2.
Lipid NPs produced via microfluidic systems.
7. Other Nanoparticle Types
In this section, the fabrication of inorganic nanoparticles such as silica NPs, metal NPs, metal oxide NPs, and quantum dots (QDs), and carbon-based nanoparticles such as fullerene, carbon nanotubes (CNTs), and graphene in microfluidic devices will be discussed.
Silica nanoparticles, metal nanoparticles like gold (AuNP) and silver (AgNP), metal oxide nanoparticles like iron oxide, and quantum dots like cadmium sulphide (CdS) and cadmium selenide (CdSe) are only a few examples of inorganic nanoparticles. In comparison to their organic counterparts, these inorganic nanoparticles frequently exhibit superior mechanical and chemical durability, lower polydispersity, more diversified surface chemistry and shape, and, in the case of porous nanoparticles, greater surface areas [88]. Numerous inorganic nanoparticle applications may be found in a wide range of industries, including optoelectronics, imaging, sensing, and catalysis. Due to their unique nanoscale physicochemical, optical, magnetic, and electrical capabilities, they are useful in medication delivery. Inorganic nanoparticles are valued for their distinctive qualities as well as the fact that they are non-toxic, biocompatible, hydrophilic, stable, and very adaptable in terms of their structures and geometries [87,90]. Due to their remarkable optical, photothermal, and magnetic capabilities, which make them useful in theranostics, thermotherapy, magnetically induced tumor targeting, and other applications, inorganic nanoparticles have attracted a lot of attention in drug delivery applications. These nanoparticles have shown promise as candidates, enabling the development of novel drug delivery methods that improve the accuracy of targeting cancer cells while reducing unfavorable side effects [100,101].
Metal-based nanoparticles (MNPs) are composed of pure metals such as iron (Fe), ruthenium (Ru), rhodium (Rh), palladium (Pd), silver (Ag), osmium (Os), iridium (Ir), platinum (Pt), gold (Au), copper (Cu), cobalt (Co), and nickel (Ni), or alloys such as FePt, NiPt, and NiPd. They are widely used in various biomedical applications due to their unique optical, mechanical, chemical, and catalytic properties [8,100,101]. Metal NPs find application in imaging methods thanks to their absorption capacity and fluorescence properties. They can also be biomarkers when combined with functional molecules such as antibodies and drugs. MNPs can be loaded with anticancer drugs to improve cancer treatment, guided using a magnetic field, used to generate heat in surrounding tissues, and used for localized destruction of tumor cells [98,101]. Metal nanoparticles, particularly Au and Ag NPs, have considerable appeal due to their biocompatibility and ease of surface functionalization for linking various biomolecules and therapeutic agents, and targeting ligands. This makes them up-and-coming tools for drug delivery. AuNPs, one of the most extensively investigated inorganic nanoparticle types, can be synthesized in various forms, including nanorods, nanocubes, nanoshells, nanospheres, and nanostars [98,100]. However, some limitations are associated with using MNPs due to their tendency to aggregate and precipitate when introduced into blood vessels. To overcome these limitations, MNPs can be coated with an organic biocompatible coating to form metal-organic nanoparticles (MONPs) [101]. Various approaches have been proposed for the production of metal NPs. An ideal platform should allow precise control over the reaction kinetics to obtain the desired metal nanoparticles. Microfluidic technologies have been studied to synthesize metal nanoparticles using various microfluidic architectures, such as helical flow inverters and emulsion droplet generation. Within a microfluidic system, properties such as morphology, shape, composition, size, and crystallinity of noble metal NMs can be precisely tuned using flow rate and duration, temperature, pH, and device geometry. The microfluidic approach offers improved yield, reliability, and precise control over NP properties. Compared to conventional batch processes, producing functionalized NPs in microfluidic reactors in a single step is easier [98,100]. Aftab et al. compared microscale and macroscale techniques for encapsulating Calotropis gigantea leaves extract (CaG) in silver-conjugated nanomatrices. The creation of smaller, more uniform silver nanoparticles was made possible by the microfluidic system, which also achieved a greater encapsulation efficiency (80.25%) as compared to the batch approach (52.5%). The CaG(POL/Ag) nanomatrices revealed strong cytotoxicity against MCF-7 cells and significant antioxidant activity. In comparison to the batch method’s 70% cytotoxicity, the microfluidic technology showed the maximum cytotoxicity among these formulations at an 80 g/mL concentration (90%). This suggests that the microfluidic system is more effective for creating size-controlled CaG(POL/Ag) nanomatrices, potentially as a new anti-cancer agent for further experiments [173].
Metal oxide nanoparticles (MOx) constitute an intriguing category of nanomaterials that find remarkable applications, especially in chemistry, physics, and materials science. Many metallic elements react with oxygen under numerous conditions to form metal oxides with various structural forms [174]. The majority of inorganic nanomedicines that have received FDA clearance use iron oxide nanoparticles. Their high responsiveness and difficulties with study and applications, however, have restricted their use. Iron oxide NMs are often synthesized via the coprecipitation technique, and the reaction temperature is a key factor in regulating the synthesis procedure. The rate at which the iron precursor and alkaline solution are mixed affects the quality of the NMs that are produced. Due to their ability to provide quick reagent mixing and exact temperature control, microfluidic devices provide an excellent platform for the production of iron oxide NMs. In addition, surface ligand changes have been successfully made possible via microfluidics, which improves the performance of these NMs in a variety of biological applications [98].
Mesoporous silica nanoparticles have distinct physicochemical features that define them as an individual entity. These adaptable nanocarriers may be adjusted in terms of size and shape, and can take the form of nanoribbons, nanohelices, nanotubes, and nanozigzags. Silica nanoparticles have a wide range of uses, including catalysis, adsorption, diagnostic sensors, photodynamic therapy (PDT), separation, and therapeutic drug administration, due to the tailorability of their optical, electrical, and mechanical characteristics. They are advantageous nanomaterials in a variety of scientific and technical applications due to their porous structure and adaptability [175]. Due to their exceptional biocompatibility, durability, and simplicity of surface functionalization, silica nanoparticles have drawn a lot of interest for application as drug delivery systems. Silica NPs are excellent carriers for a variety of applications because different active components, such as chemical therapies and imaging agents, can be placed onto or within them, either covalently or physically. Typically, the Stöber technique, a sol–gel process, or its variations are used in the production of silica nanoparticles. Microfluidics has been a promising technique in recent years for creating silica NPs specifically suited for biological applications. To create silica NPs, microfluidic systems have been used in a variety of geometries, including conventional flow-focusing, winding channels paired with T-junctions, and compartment micromixers. Microfluidics provides improved mixing efficiency and guarantees a more uniform reaction compared to conventional sol–gel batch procedures, giving it a clear advantage in the production of silica NPs with precise control over their size, shape, porosity, composition, and surface modification. This produces monodisperse silica nanoparticles at high production rates, which are crucial for biological applications [98,100]. Wacker et al. investigated droplet-based microfluidic synthesis of fluorescent silica nanoparticles (50–350 nm). This approach accelerates reactions and significantly improves size homogeneity compared to conventional bulk synthesis. Furthermore, incorporating FITC dye into silica nanoparticles using their method improves dye stability and reduces leakage [176].
Quantum dots are semiconductor nanoparticles that exhibit size- and composition-dependent optical and electronic (optoelectronic) properties. QDs are ultra-small, typically in the size range of 1.5 to 10.0 nm. The typical formula for QDs is MX, where M usually is cadmium (Cd) or zinc (Zn), and X is selenium (Se), sulfur (S), or tellurium (Te) [177,178]. Quantum dots are useful in a variety of applications due to their distinctive characteristics, which include variable size, prolonged lifespan, high brightness, limited luminescence spectra, and minimal autofluorescence. The electronics and pharmaceutical sectors have seen substantial growth in QD nanotechnology in recent years. For medication administration, gene therapy, in vivo imaging, cell tracking, labelling cellular proteins, tumor research, and detecting toxins and pathogens, QDs are being investigated in the field of biomedicine. In comparison to traditional batch procedures, microfluidics, a relatively new yet potent technology, allows fine control in the manufacturing of QDs. With the ability to fine-tune reaction conditions, microfluidic devices enable the manufacture of diverse QDs and QD-doped nanomaterials, which is essential for their performance. Different microfluidic methods have been created to manufacture QDs with various surface changes, compositions, forms, and photoluminescent capabilities, opening up new uses for them [98,100].
Carbon-based nanomaterials constitute a diverse group of materials encompassing fullerenes, carbon nanotubes, graphene and its derivatives, graphene oxide, nanodiamonds, and carbon-based quantum dots. Due to their outstanding structural qualities and exceptional mechanical, electrical, thermal, optical, and chemical capabilities, these materials have attracted significant research interest across a variety of fields, notably in biomedical applications [179]. The variety of CNT-based biomedical applications has significantly increased as a result of recent advancements in microfluidic-based CNT production. Numerous carbon-based nanomaterials have been produced using microfluidic devices that use vortex flow. They have made it possible to synthesize carbon dots, which are generally nanometers in size and produced from multi-walled CNTs, as well as single-walled CNT toroids, lateral CNT slices, and surface decorating of CNTs with noble metal nanomaterials. These developments show potential for a number of CNT-based biological applications [98].
As a whole, recent years have seen a great deal of interest in the creation of carbon- and inorganic-based nanoparticles (NPs) utilizing microfluidic devices. A few benefits of microfluidics include effective mass and heat transmission, fine control of reaction parameters, temperature adjustment during flow, quick mixing, and the option to add more reactants later for the production of nanoparticles. The monodispersity of inorganic-based NPs relies on variables including reaction kinetics, mixes, reaction fluids, and temperature, all of which may be accurately controlled inside microfluidic devices, and necessitates this degree of control. As a consequence, microfluidics shortens the time needed to prepare inorganic NPs while retaining their quality and giving important new information about how reactions work. The continuous synthesis method also shows significant promise for future use in a variety of manufacturing sectors [180]. An overview of some of the other types of nanoparticles mentioned in this section prepared using microfluidic methods is given in Table 3.

Table 3.
Other types of NPs produced via microfluidic systems.
8. Conclusions and Future Prospects
The emerging science of microfluidics provides a potential route for the controlled production of engineered nanoparticles for biological purposes. Conventional NP production methods produce NPs with broad size distributions and batch-to-batch variations in physicochemical properties, often requiring additional chemical and physical treatments. Microfluidics, on the other hand, by precisely controlling microscale fluid dynamics, can enhance the fine-tuning of the NPs’ physicochemical properties such as composition, size, shape, and surface ligands during production, providing greater homogeneity, limited size distribution, good reproducibility, enhanced encapsulation efficiency, and scalability. Thus, some limitations seen in conventional methods are eliminated. As a result, microfluidics can enable the production of better characterized NPs. Creating well-characterized NPs dramatically benefits from the low surface-to-volume ratio, which is the distinctive feature of microfluidics. Researchers have closely studied microfluidic technologies to accurately control the size, shape, and structure of functional NPs, resulting in high-quality synthesis with excellent reproducibility. These well-designed delivery vehicles can optimize drug delivery efficiency and release profiles. Tuning parameters include flow rate regulation, temperature control, device geometry, size adjustments, and auxiliary device elements. However, the creation of NMs for biological applications on a microfluidic platform is anticipated to get greater attention in future studies in microfluidics. The survey of alternate materials and production techniques for system components, including chip materials and micromixers, is becoming increasingly popular in microfluidics. Although there have been advancements, the science of microfluidics is still constantly developing, necessitating a more thorough understanding of the NP generation process. Examining the preliminary phases of NP structure and composition assessment, technical considerations, device fabrication methodologies, and scalable production are necessary to achieve this. Here, we provide an overview of the critical properties of microfluidics to produce NPs for potential use in biomedical applications, and highlight the advantageous rationale behind microfluidics.
Author Contributions
Conceptualization, A.B. (Aysenur Bezelya), B.K. and A.B. (Asuman Bozkır); investigation, A.B. (Aysenur Bezelya) and B.K.; resources, A.B. (Aysenur Bezelya) and B.K.; writing—original draft preparation, A.B. (Aysenur Bezelya) and B.K.; writing—review and editing, A.B. (Aysenur Bezelya), B.K. and A.B. (Asuman Bozkır); supervision, B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Emerich, D.F.; Thanos, C.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003, 3, 655–663. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P. Nanoparticles in drug delivery: Past, present and future. Adv. Drug Deliv. Rev. 2013, 65, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Bawa, R. Nanopharmaceuticals: Nanopharmaceuticals. Eur. J. Nanomed. 2010, 3, 34–40. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic devices: A tool for nanoparticle synthesis and performance evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef] [PubMed]
- Oroojalian, F.; Charbgoo, F.; Hashemi, M.; Amani, A.; Yazdian-Robati, R.; Mokhtarzadeh, A.; Ramezani, M.; Hamblin, M.R. Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Control. Release 2020, 321, 442–462. [Google Scholar] [CrossRef] [PubMed]
- Pondman, K.; Le Gac, S.; Kishore, U. Nanoparticle-induced immune response: Health risk versus treatment opportunity? Immunobiology 2022, 2022, 152317. [Google Scholar] [CrossRef]
- Illath, K.; Kar, S.; Gupta, P.; Shinde, A.; Wankhar, S.; Tseng, F.-G.; Lim, K.-T.; Nagai, M.; Santra, T.S. Microfluidic nanomaterials: From synthesis to biomedical applications. Biomaterials 2022, 280, 121247. [Google Scholar] [CrossRef]
- Marre, S.; Jensen, K.F. Synthesis of micro and nanostructures in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1183–1202. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, J.; Chen, C.; Wang, J.; Liu, G.; Nandakumar, K.; Li, Y.; Wang, L. Microfluidic Applications in Drug Development: Fabrication of Drug Carriers and Drug Toxicity Screening. Micromachines 2022, 13, 200. [Google Scholar] [CrossRef]
- Jurin, J., II. An account of some experiments shown before the Royal Society; with an enquiry into the cause of the ascent and suspension of water in capillary tubes. Philos. Trans. R. Soc. Lond. 1717, 30, 739–747. [Google Scholar]
- Castillo-León, J. Microfluidics and lab-on-a-chip devices: History and challenges. In Lab-on-a-Chip Devices and Micro-Total Analysis Systems: A Practical Guide; Castillo-León, J., Svendsen, W.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Sutera, S.P.; Skalak, R. The history of Poiseuille’s law. Annu. Rev. Fluid Mech. 1993, 25, 1–20. [Google Scholar] [CrossRef]
- Hagen, G. Ueber die Bewegung des Wassers in engen cylindrischen Röhren. Ann. Der Phys. 1839, 122, 423–442. [Google Scholar] [CrossRef]
- Poiseuille, J.L. Recherches Expérimentales sur le Mouvement des Liquides Dans les Tubes de Très-Petits Diamètres; Imprimerie Royale: Paris, France, 1844. [Google Scholar]
- Poiseuille, J. Ecoulement des Liquides: Societe Philomatique de Paris. Extr. Proces-Verbaux Seances Pendant I’Annee 1838, 3, 77–81. [Google Scholar]
- Stokes, G.G. On the theories of the internal friction of fluids in motion, and of the equilibrium and motion of elastic solids. In Classics of Elastic Wave Theory, Society of Exploration Geophysicists; Pelissier, M.A., Hoeber, H., van de Coevering, N., Jones, I.F., Eds.; Society of Exploration Geophysicists: Houston, TX, USA, 2007. [Google Scholar] [CrossRef]
- Rayleigh, L. On the capillary phenomena of jets. Proc. R. Soc. Lond. 1879, 29, 71–97. [Google Scholar]
- Walker, R. Evolution Flow: The Historical Background of Flow Cytometry. Flow Cytometry. 2013. Available online: https://bitesizebio.com/13693/historical-background-of-flow-cytometry/ (accessed on 24 October 2023).
- Scardamaglia, A. A legal history of lithography. Griffith Law Rev. 2017, 26, 1–27. [Google Scholar] [CrossRef]
- Tiginyanu, I.; Ursaki, V.; Popa, V. Nanoimprint lithography (NIL) and related techniques for electronics applications. In Nanocoatings and Ultra-Thin Films; Elsevier: Amsterdam, The Netherlands, 2011; pp. 280–329. [Google Scholar]
- Rogers, J.A.; Nuzzo, R.G. Recent progress in soft lithography. Mater. Today 2005, 8, 50–56. [Google Scholar] [CrossRef]
- Kumar, A.; Whitesides, G.M. Features of gold having micrometer to centimeter dimensions can be formed through a combination of stamping with an elastomeric stamp and an alkanethiol ‘‘ink’’followed by chemical etching. Appl. Phys. Lett. 1993, 63, 2002–2004. [Google Scholar] [CrossRef]
- Kipping, F.S.; Lloyd, L.L. XLVII.—Organic derivatives of silicon. Triphenylsilicol and alkyloxysilicon chlorides. J. Chem. Soc. Trans. 1901, 79, 449–459. [Google Scholar] [CrossRef]
- Colas, A.; Curtis, J. Silicone biomaterials: History and chemistry. In Biomaterials Science: An Introduction to Materials in Medicine, 2nd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemans, J.E., Eds.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2004; ISBN 9780080470368. [Google Scholar]
- Gerami, A.; Alzahid, Y.; Mostaghimi, P.; Kashaninejad, N.; Kazemifar, F.; Amirian, T.; Mosavat, N.; Ebrahimi Warkiani, M.; Armstrong, R.T. Microfluidics for porous systems: Fabrication, microscopy and applications. Transp. Porous Media 2019, 130, 277–304. [Google Scholar] [CrossRef]
- Lei, K.F. Introduction: The origin, current status, and future of microfluidics. In Microfluidics: Fundamental, Devices and Applications: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2018; pp. 1–18. [Google Scholar]
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Andrus, J.; Bond, W. Photoengraving in transistor fabrication. Transistor Technol. 1958, 3, 151–162. [Google Scholar]
- Panigrahi, P.K. Transport Phenomena in Microfluidic Systems; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Bassous, E.; Taub, H.; Kuhn, L. Ink jet printing nozzle arrays etched in silicon. Appl. Phys. Lett. 1977, 31, 135–137. [Google Scholar] [CrossRef]
- Terry, S.C.; Jerman, J.H.; Angell, J.B. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron Devices 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Whitesides, G. Microfluidics in late adolescence. arXiv 2018, arXiv:1802.05595. [Google Scholar]
- Grimmer, A.; Wille, R.; Grimmer, A.; Wille, R. Designing Meanders. In Designing Droplet Microfluidic Networks: A Toolbox for Designers; Springer: Berlin/Heidelberg, Germany, 2020; pp. 63–76. [Google Scholar]
- Kumar, C.S. Microfluidic Devices in Nanotechnology: Fundamental Concepts; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Golay, M.J. A performance index for gas chromatographic columns. Nature 1957, 180, 435–436. [Google Scholar] [CrossRef]
- Van Deemter, J.; Zuiderweg, F.; Klinkenberg, A.v. Longitudinal diffusion and resistance to mass transfer as causes of nonideality in chromatography. Chem. Eng. Sci. 1956, 5, 271–289. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Karimi, M. Biomedical Applications of Microfluidic Devices; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Harrison, D.J.; Fluri, K.; Seiler, K.; Fan, Z.; Effenhauser, C.S.; Manz, A. Micromachining a miniaturized capillary electrophoresis-based chemical analysis system on a chip. Science 1993, 261, 895–897. [Google Scholar] [CrossRef]
- Li, X.J.; Zhou, Y. Microfluidic Devices for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2021. [Google Scholar]
- Aryasomayajula, A.; Bayat, P.; Rezai, P.; Selvaganapathy, P.R. Microfluidic devices and their applications. In Springer Handbook of Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 487–536. [Google Scholar]
- Yeo, L.Y.; Chang, H.C.; Chan, P.P.; Friend, J.R. Microfluidic devices for bioapplications. Small 2011, 7, 12–48. [Google Scholar] [CrossRef]
- Karvelas, E.; Liosis, C.; Benos, L.; Karakasidis, T.; Sarris, I. Micromixing efficiency of particles in heavy metal removal processes under various inlet conditions. Water 2019, 11, 1135. [Google Scholar] [CrossRef]
- Salafi, T.; Zeming, K.K.; Zhang, Y. Advancements in microfluidics for nanoparticle separation. Lab Chip 2017, 17, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.B.; Goel, S. Microfluidic devices for synthesizing nanomaterials—A review. Nano Express 2020, 1, 032004. [Google Scholar] [CrossRef]
- Mumtaz, Z.; Rashid, Z.; Ali, A.; Arif, A.; Ameen, F.; AlTami, M.S.; Yousaf, M.Z. Prospects of Microfluidic Technology in Nucleic Acid Detection Approaches. Biosensors 2023, 13, 584. [Google Scholar] [CrossRef]
- Kumar, C.S. Microfluidic Devices in Nanotechnology: Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Sommonte, F.; Denora, N.; Lamprou, D.A. Combining 3D printing and microfluidic techniques: A powerful synergy for nanomedicine. Pharmaceuticals 2023, 16, 69. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and applications of microfluidic devices: A review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Luo, M.; Yukawa, H.; Baba, Y. Micro-/nano-fluidic devices and in vivo fluorescence imaging based on quantum dots for cytologic diagnosis. Lab Chip 2022, 22, 2223–2236. [Google Scholar] [CrossRef]
- Popa, M.L.; Preda, M.D.; Neacșu, I.A.; Grumezescu, A.M.; Ginghină, O. Traditional vs. Microfluidic Synthesis of ZnO Nanoparticles. Int. J. Mol. Sci. 2023, 24, 1875. [Google Scholar] [CrossRef]
- Seo, H.; Jeon, L.; Kwon, J.; Lee, H. High-Precision Synthesis of RNA-Loaded Lipid Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2023, 2023, 2203033. [Google Scholar] [CrossRef]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.C.; Kamarapu, S.K. A review of current methods in microfluidic device fabrication and future commercialization prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Hwang, J.; Cho, Y.H.; Park, M.S.; Kim, B.H. Microchannel fabrication on glass materials for microfluidic devices. Int. J. Precis. Eng. Manuf. 2019, 20, 479–495. [Google Scholar] [CrossRef]
- Scott, S.M.; Ali, Z. Fabrication methods for microfluidic devices: An overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Razavi Bazaz, S.; Rouhi, O.; Raoufi, M.A.; Ejeian, F.; Asadnia, M.; Jin, D.; Ebrahimi Warkiani, M. 3D printing of inertial microfluidic devices. Sci. Rep. 2020, 10, 5929. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.; Soliman, E.A.; El-Bab, A.M.F.; Matsushita, Y.; Abdel-Mawgood, A.L. Fabrication and characterization of microfluidic devices based on boron-modified epoxy resin using CO2 laser ablation for bio-analytical applications. Sci. Rep. 2023, 13, 12623. [Google Scholar] [CrossRef]
- Jayarama, A.; Kannarpady, G.K.; Kale, S.; Prabhu, S.; Pinto, R. Chemical etching of glasses in hydrofluoric Acid: A brief review. Mater. Today Proc. 2022, 55, 46–51. [Google Scholar]
- Han, X.; Zhang, Y.; Tian, J.; Wu, T.; Li, Z.; Xing, F.; Fu, S. Polymer-based microfluidic devices: A comprehensive review on preparation and applications. Polym. Eng. Sci. 2022, 62, 3–24. [Google Scholar] [CrossRef]
- Gal-Or, E.; Gershoni, Y.; Scotti, G.; Nilsson, S.M.; Saarinen, J.; Jokinen, V.; Strachan, C.J.; af Gennäs, G.B.; Yli-Kauhaluoma, J.; Kotiaho, T. Chemical analysis using 3D printed glass microfluidics. Anal. Methods 2019, 11, 1802–1810. [Google Scholar] [CrossRef]
- Nie, J.; Fu, J.; He, Y. Hydrogels: The next generation body materials for microfluidic chips? Small 2020, 16, 2003797. [Google Scholar] [CrossRef]
- Alting, L.; Kimura, F.; Hansen, H.N.; Bissacco, G. Micro engineering. CIRP Ann. 2003, 52, 635–657. [Google Scholar] [CrossRef]
- Brousseau, E.; Dimov, S.S.; Pham, D.T. Some recent advances in multi-material micro-and nano-manufacturing. Int. J. Adv. Manuf. Technol. 2010, 47, 161–180. [Google Scholar] [CrossRef]
- Madou, M.J. Fundamentals of Microfabrication: The Science of Miniaturization; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Uriarte, L.; Herrero, A.; Ivanov, A.; Oosterling, H.; Staemmler, L.; Tang, P.T.; Allen, D. Comparison between microfabrication technologies for metal tooling. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2006, 220, 1665–1676. [Google Scholar] [CrossRef]
- Friend, J.; Yeo, L. Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 2010, 4, 026502. [Google Scholar] [CrossRef]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef]
- Deb, R.; Sarma, B.; Dalal, A. Magnetowetting dynamics of sessile ferrofluid droplets: A review. Soft Matter 2022, 18, 2287–2324. [Google Scholar] [CrossRef]
- Bragheri, F.; Vazquez, R.M.; Osellame, R. Microfluidics. In Three-Dimensional Microfabrication Using Two-Photon Polymerization; Elsevier: Amsterdam, The Netherlands, 2020; pp. 493–526. [Google Scholar]
- Battat, S.; Weitz, D.A.; Whitesides, G.M. An outlook on microfluidics: The promise and the challenge. Lab Chip 2022, 22, 530–536. [Google Scholar] [CrossRef]
- Ng, J.M.; Gitlin, I.; Stroock, A.D.; Whitesides, G.M. Components for integrated poly (dimethylsiloxane) microfluidic systems. Electrophoresis 2002, 23, 3461–3473. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Tsou, C. The implementation of a thermal bubble actuated microfluidic chip with microvalve, micropump and micromixer. Sens. Actuators A Phys. 2014, 210, 147–156. [Google Scholar] [CrossRef]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Kandlikar, S.; Garimella, S.; Li, D.; Colin, S.; King, M.R. Heat Transfer and Fluid Flow in Minichannels and Microchannels; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Li, H.; Liu, J.; Li, K.; Liu, Y. A review of recent studies on piezoelectric pumps and their applications. Mech. Syst. Signal Process. 2021, 151, 107393. [Google Scholar] [CrossRef]
- Zeng, W.; Li, S.; Wang, Z. Characterization of syringe-pump-driven versus pressure-driven microfluidic flows. In Proceedings of the 2015 International Conference on Fluid Power and Mechatronics (FPM), Harbin, China, 5–7 August 2015; pp. 711–715. [Google Scholar]
- Kartmann, S.; van Heeren, H.; Batista, E.; Akselli, B.; Silverio, V. MFMET A1.1.1 Literature and Market Research: Definitions, Characteristics, Specifications, Application and Function of Flow Control Components; Zenodo: Geneva, Switzerland, 2022. [Google Scholar]
- Silverio, V.; Metaxiotou, Z.; Batista, E.; Kartmann, S.; Ogheard, F.; Lötters, J. MFMET A1.1.2—Definitions Symbols and Vocabulary of Flow Control; Zenodo: Geneva, Switzerland, 2022. [Google Scholar]
- Batista, E.; Sousa, J.A.; Cardoso, S.; Silverio, V. Experimental testing for metrological traceability and accuracy of liquid microflows and microfluidics. Flow Meas. Instrum. 2020, 71, 101691. [Google Scholar] [CrossRef]
- Melvad, C.; Krühne, U.; Frederiksen, J. Design considerations and initial validation of a liquid microflow calibration setup using parallel operated syringe pumps. Meas. Sci. Technol. 2010, 21, 074004. [Google Scholar] [CrossRef]
- Darby, S.G.; Moore, M.R.; Friedlander, T.A.; Schaffer, D.K.; Reiserer, R.S.; Wikswo, J.P.; Seale, K.T. A metering rotary nanopump for microfluidic systems. Lab Chip 2010, 10, 3218–3226. [Google Scholar] [CrossRef] [PubMed]
- Kamat, V.; Dey, P.; Bodas, D.; Kaushik, A.; Boymelgreen, A.; Bhansali, S. Active microfluidic reactor-assisted controlled synthesis of nanoparticles and related potential biomedical applications. J. Mater. Chem. B 2023, 11, 5650–5667. [Google Scholar] [CrossRef]
- Oh, K.W.; Ahn, C.H. A review of microvalves. J. Micromech. Microeng. 2006, 16, R13. [Google Scholar] [CrossRef]
- Purwidyantri, A.; Prabowo, B.A. Tesla Valve Microfluidics: The Rise of Forgotten Technology. Chemosensors 2023, 11, 256. [Google Scholar] [CrossRef]
- Liosis, C.; Sofiadis, G.; Karvelas, E.; Karakasidis, T.; Sarris, I. A Tesla Valve as a Micromixer for Fe3O4 Nanoparticles. Processes 2022, 10, 1648. [Google Scholar] [CrossRef]
- Verma, A.; Bhattacharyya, S. Microfluidics-the state-of-the-art technology for pharmaceutical application. Adv. Pharm. Bull. 2022, 12, 700. [Google Scholar] [CrossRef]
- Ma, X.; Guo, G.; Wu, X.; Wu, Q.; Liu, F.; Zhang, H.; Shi, N.; Guan, Y. Advances in Integration, Wearable Applications, and Artificial Intelligence of Biomedical Microfluidics Systems. Micromachines 2023, 14, 972. [Google Scholar] [CrossRef]
- Fredrick, S.J.; Gross, E.M. Use of microelectrodes for electrochemiluminescent detection in microfluidic devices. Bioanalysis 2009, 1, 31–36. [Google Scholar] [CrossRef]
- Tsai, H.-F.; Podder, S.; Chen, P.-Y. Microsystem Advances through Integration with Artificial Intelligence. Micromachines 2023, 14, 826. [Google Scholar] [CrossRef] [PubMed]
- Surappa, S.; Multani, P.; Parlatan, U.; Sinawang, P.D.; Kaifi, J.; Akin, D.; Demirci, U. Integrated “lab-on-a-chip” microfluidic systems for isolation, enrichment, and analysis of cancer biomarkers. Lab Chip 2023, 23, 2942–2958. [Google Scholar] [CrossRef]
- Gong, L.; Cretella, A.; Lin, Y. Microfluidic systems for particle capture and release: A review. Biosens. Bioelectron. 2023, 2023, 115426. [Google Scholar] [CrossRef]
- Tan, S.J.; Yobas, L.; Lee, G.Y.H.; Ong, C.N.; Lim, C.T. Microdevice for the isolation and enumeration of cancer cells from blood. Biomed. Microdevices 2009, 11, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Gao, Y.; Wang, H. Recent advances in drug delivery system fabricated by microfluidics for disease therapy. Bioengineering 2022, 9, 625. [Google Scholar] [CrossRef]
- Forigua, A.; Kirsch, R.L.; Willerth, S.M.; Elvira, K.S. Recent advances in the design of microfluidic technologies for the manufacture of drug releasing particles. J. Control. Release 2021, 333, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fang, H.; Zhang, J.; Yan, S. Modular microfluidics for life sciences. J. Nanobiotechnol. 2023, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Leal, B.B.J.; Braghirolli, D.I.; Pranke, P. Production of nanostructured systems: Main and innovative techniques. Drug Discov. Today 2022, 2022, 103454. [Google Scholar] [CrossRef]
- Agha, A.; Waheed, W.; Stiharu, I.; Nerguizian, V.; Destgeer, G.; Abu-Nada, E.; Alazzam, A. A review on microfluidic-assisted nanoparticle synthesis, and their applications using multiscale simulation methods. Discov. Nano 2023, 18, 18. [Google Scholar] [CrossRef]
- Maged, A.; Abdelbaset, R.; Mahmoud, A.A.; Elkasabgy, N.A. Merits and advances of microfluidics in the pharmaceutical field: Design technologies and future prospects. Drug Deliv. 2022, 29, 1549–1570. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C.X. Microfluidic nanoparticles for drug delivery. Small 2022, 18, 2106580. [Google Scholar] [CrossRef]
- Fabozzi, A.; Della Sala, F.; di Gennaro, M.; Barretta, M.; Longobardo, G.; Solimando, N.; Pagliuca, M.; Borzacchiello, A. Design of functional nanoparticles by microfluidic platforms as advanced drug delivery systems for cancer therapy. Lab Chip 2023, 23, 1389–1409. [Google Scholar] [CrossRef]
- Greco, A.; Gabold, B.; Chen, S.; Wang, X.; Xu, Z.; Hartschuh, A.; Chiesa, E.; Genta, I.; Ried, C.L.; Merdan, T. Microfluidic mixing as platform technology for production of chitosan nanoparticles loaded with different macromolecules. Eur. J. Pharm. Biopharm. 2023, 188, 170–181. [Google Scholar] [CrossRef]
- Gimondi, S.; Guimarães, C.F.; Vieira, S.F.; Gonçalves, V.M.; Tiritan, M.E.; Reis, R.L.; Ferreira, H.; Neves, N.M. Microfluidic mixing system for precise PLGA-PEG nanoparticles size control. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102482. [Google Scholar] [CrossRef]
- Saygili, E.; Kaya, E.; Ilhan-Ayisigi, E.; Saglam-Metiner, P.; Alarcin, E.; Kazan, A.; Girgic, E.; Kim, Y.-W.; Gunes, K.; Eren-Ozcan, G.G. An alginate-poly (acrylamide) hydrogel with TGF-β3 loaded nanoparticles for cartilage repair: Biodegradability, biocompatibility and protein adsorption. Int. J. Biol. Macromol. 2021, 172, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Brzeziński, M.; Socka, M.; Makowski, T.; Kost, B.; Cieślak, M.; Królewska-Golińska, K. Microfluidic-assisted nanoprecipitation of biodegradable nanoparticles composed of PTMC/PCL (co) polymers, tannic acid and doxorubicin for cancer treatment. Colloids Surf. B Biointerfaces 2021, 201, 111598. [Google Scholar] [CrossRef]
- Mahmoudi, Z.; Mohammadnejad, J.; Bazaz, S.R.; Mehrizi, A.A.; Saidijam, M.; Dinarvand, R.; Warkiani, M.E.; Soleimani, M. Promoted chondrogenesis of hMCSs with controlled release of TGF-β3 via microfluidics synthesized alginate nanogels. Carbohydr. Polym. 2020, 229, 115551. [Google Scholar] [CrossRef]
- Kly, S.; Huang, Y.; Moffitt, M.G. Enhancement of Cellular Uptake by Increasing the Number of Encapsulated Gold Nanoparticles in Polymeric Micelles. J. Colloid Interface Sci. 2023, 652, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Li, X.; Xu, J.; Cheng, Y.; Zhang, M.; Shi, G. Continuous Preparation of Semiconducting Polymer Nanoparticles with Varied Sizes for Online Fluorescence Sensing via a Laser-Tailored 3D Microfluidic Chip. Anal. Chem. 2023, 95, 10422–10429. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Lalanne, P.; Weber-Vax, A.; Mutschler, A.; Lecommandoux, S. Controlling Polymersome Size through Microfluidic-Assisted Self-Assembly: Enabling’Ready to Use’formulations for biological applications. Int. J. Pharm. 2023, 2023, 123157. [Google Scholar] [CrossRef]
- Gimondi, S.; de Castro, J.V.; Reis, R.L.; Ferreira, H.; Neves, N.M. On the size-dependent internalization of sub-hundred polymeric nanoparticles. Colloids Surf. B Biointerfaces 2023, 225, 113245. [Google Scholar] [CrossRef]
- Behnke, M.; Klemm, P.; Dahlke, P.; Shkodra, B.; Beringer-Siemers, B.; Czaplewska, J.A.; Stumpf, S.; Jordan, P.M.; Schubert, S.; Hoeppener, S. Ethoxy acetalated dextran nanoparticles for drug delivery: A comparative study of formulation methods. Int. J. Pharm. X 2023, 5, 100173. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Barajas, A.Y.; Olvera, M.d.l.L.; Romero-Paredes, G.; Altuzar, V.; Garrido-Guerrero, E.; Mendoza-Barrera, C. Chitosan, Chitosan/IgG-Loaded, and N-Trimethyl Chitosan Chloride Nanoparticles as Potential Adjuvant and Carrier-Delivery Systems. Molecules 2023, 28, 4107. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Preparation of size-tunable sub-200 nm PLGA-based nanoparticles with a wide size range using a microfluidic platform. PLoS ONE 2022, 17, e0271050. [Google Scholar] [CrossRef] [PubMed]
- Roffo, F.; Ponsiglione, A.M.; Netti, P.A.; Torino, E. coupled Hydrodynamic Flow Focusing (cHFF) to Engineer Lipid–Polymer Nanoparticles (LiPoNs) for Multimodal Imaging and Theranostic Applications. Biomedicines 2022, 10, 438. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Wang, J.; Qi, J.; Li, J.; Ma, J.; Chen, L. A rotary multi-positioned cloth/paper hybrid microfluidic device for simultaneous fluorescence sensing of mercury and lead ions by using ion imprinted technologies. J. Hazard. Mater. 2022, 428, 128165. [Google Scholar] [CrossRef]
- Herranz-Blanco, B.; Ginestar, E.; Zhang, H.; Hirvonen, J.; Santos, H.A. Microfluidics platform for glass capillaries and its application in droplet and nanoparticle fabrication. Int. J. Pharm. 2017, 516, 100–105. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, T.; Li, Q.; Wang, F.; Li, J. A microfluidic approach for rapid and continuous synthesis of glycoprotein-imprinted nanospheres. Talanta 2022, 239, 123084. [Google Scholar] [CrossRef]
- Abad, M.; Mendoza, G.; Usón, L.; Arruebo, M.; Piñol, M.; Sebastián, V.; Oriol, L. Microfluidic Synthesis of Block Copolymer Micelles: Application as Drug Nanocarriers and as Photothermal Transductors When Loading Pd Nanosheets. Macromol. Biosci. 2022, 22, 2100528. [Google Scholar] [CrossRef]
- Gimondi, S.; Reis, R.L.; Ferreira, H.; Neves, N.M. Microfluidic-driven mixing of high molecular weight polymeric complexes for precise nanoparticle downsizing. Nanomed. Nanotechnol. Biol. Med. 2022, 43, 102560. [Google Scholar] [CrossRef]
- Tiboni, M.; Tiboni, M.; Pierro, A.; Del Papa, M.; Sparaventi, S.; Cespi, M.; Casettari, L. Microfluidics for nanomedicines manufacturing: An affordable and low-cost 3D printing approach. Int. J. Pharm. 2021, 599, 120464. [Google Scholar] [CrossRef]
- Cesur, S.; Cam, M.E.; Sayın, F.S.; Su, S.; Harker, A.; Edirisinghe, M.; Gunduz, O. Metformin-loaded polymer-based microbubbles/nanoparticles generated for the treatment of type 2 diabetes mellitus. Langmuir 2021, 38, 5040–5051. [Google Scholar] [CrossRef] [PubMed]
- Lari, A.S.; Zahedi, P.; Ghourchian, H.; Khatibi, A. Microfluidic-based synthesized carboxymethyl chitosan nanoparticles containing metformin for diabetes therapy: In vitro and in vivo assessments. Carbohydr. Polym. 2021, 261, 117889. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, E.; Greco, A.; Dorati, R.; Conti, B.; Bruni, G.; Lamprou, D.; Genta, I. Microfluidic-assisted synthesis of multifunctional iodinated contrast agent polymeric nanoplatforms. Int. J. Pharm. 2021, 599, 120447. [Google Scholar] [CrossRef] [PubMed]
- Baby, T.; Liu, Y.; Yang, G.; Chen, D.; Zhao, C.-X. Microfluidic synthesis of curcumin loaded polymer nanoparticles with tunable drug loading and pH-triggered release. J. Colloid Interface Sci. 2021, 594, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Solomun, J.I.; Totten, J.D.; Wongpinyochit, T.; Florence, A.J.; Seib, F.P. Manual versus microfluidic-assisted nanoparticle manufacture: Impact of silk fibroin stock on nanoparticle characteristics. ACS Biomater. Sci. Eng. 2020, 6, 2796–2804. [Google Scholar] [CrossRef]
- Wan, F.; Bohr, S.S.-R.; Kłodzińska, S.N.; Jumaa, H.; Huang, Z.; Nylander, T.; Thygesen, M.B.; Sørensen, K.K.; Jensen, K.J.; Sternberg, C. Ultrasmall TPGS–PLGA hybrid nanoparticles for site-specific delivery of antibiotics into Pseudomonas aeruginosa biofilms in lungs. ACS Appl. Mater. Interfaces 2019, 12, 380–389. [Google Scholar] [CrossRef]
- Huang, Y.; Moini Jazani, A.; Howell, E.P.; Oh, J.K.; Moffitt, M.G. Controlled microfluidic synthesis of biological stimuli-responsive polymer nanoparticles. ACS Appl. Mater. Interfaces 2019, 12, 177–190. [Google Scholar] [CrossRef]
- Kim, S.; Choi, B.; Kim, Y.; Shim, G. Immune-Modulating Lipid Nanomaterials for the Delivery of Biopharmaceuticals. Pharmaceutics 2023, 15, 1760. [Google Scholar] [CrossRef]
- Vogelaar, A.; Marcotte, S.; Cheng, J.; Oluoch, B.; Zaro, J. Use of Microfluidics to Prepare Lipid-Based Nanocarriers. Pharmaceutics 2023, 15, 1053. [Google Scholar] [CrossRef]
- Lin, Z.; Zou, Z.; Pu, Z.; Wu, M.; Zhang, Y. Application of microfluidic technologies on COVID-19 diagnosis and drug discovery. Acta Pharm. Sin. B 2023, 13, 2877–2896. [Google Scholar] [CrossRef]
- Prakash, G.; Shokr, A.; Willemen, N.; Bashir, S.M.; Shin, S.R.; Hassan, S. Microfluidic fabrication of lipid nanoparticles for the delivery of nucleic acids. Adv. Drug Deliv. Rev. 2022, 184, 114197. [Google Scholar] [CrossRef]
- Carvalho, B.G.; Ceccato, B.T.; Michelon, M.; Han, S.W.; de la Torre, L.G. Advanced microfluidic technologies for lipid nano-microsystems from synthesis to biological application. Pharmaceutics 2022, 14, 141. [Google Scholar] [CrossRef]
- van Der Meel, R.; Chen, S.; Zaifman, J.; Kulkarni, J.A.; Zhang, X.R.S.; Tam, Y.K.; Bally, M.B.; Schiffelers, R.M.; Ciufolini, M.A.; Cullis, P.R. Modular lipid nanoparticle platform technology for siRNA and lipophilic prodrug delivery. Small 2021, 17, 2103025. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, W.; Li, Q.; He, C.; Liu, Y.; Liu, J.; Wang, R.; Wu, J.; Xiang, D.; Chen, C. Coaxial electrostatic spray-based preparation of localization missile liposomes on a microfluidic chip for targeted treatment of triple-negative breast cancer. Int. J. Pharm. 2023, 643, 123220. [Google Scholar] [CrossRef] [PubMed]
- Fathordoobady, F.; Sannikova, N.; Guo, Y.; Singh, A.; Kitts, D.D.; Pratap-Singh, A. Comparing microfluidics and ultrasonication as formulation methods for developing hempseed oil nanoemulsions for oral delivery applications. Sci. Rep. 2021, 11, 72. [Google Scholar] [CrossRef]
- Li, Y.; Lee, R.J.; Huang, X.; Li, Y.; Lv, B.; Wang, T.; Qi, Y.; Hao, F.; Lu, J.; Meng, Q. Single-step microfluidic synthesis of transferrin-conjugated lipid nanoparticles for siRNA delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Wang, H.; Liu, Y.; Hui, Y.; Sun, Q.; Seth, A.; Wibowo, D.; Chen, D.; Zhao, C.-X. Microfluidic self-assembly of a combinatorial library of single-and dual-ligand liposomes for in vitro and in vivo tumor targeting. Eur. J. Pharm. Biopharm. 2018, 130, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Han, X.; Mukalel, A.J.; El-Mayta, R.; Thatte, A.S.; Wu, J.; Padilla, M.S.; Alameh, M.-G.; Srikumar, N.; Lee, D. Throughput-scalable manufacturing of SARS-CoV-2 mRNA lipid nanoparticle vaccines. Proc. Natl. Acad. Sci. USA 2023, 120, e2303567120. [Google Scholar] [CrossRef]
- O’Brien Laramy, M.N.; Costa, A.P.; Cebrero, Y.M.; Joseph, J.; Sarode, A.; Zang, N.; Kim, L.J.; Hofmann, K.; Wang, S.; Goyon, A. Process Robustness in Lipid Nanoparticle Production: A Comparison of Microfluidic and Turbulent Jet Mixing. Mol. Pharm. 2023, 20, 4285–4296. [Google Scholar] [CrossRef]
- Di Francesco, V.; Boso, D.P.; Moore, T.L.; Schrefler, B.A.; Decuzzi, P. Machine Learning Instructed Microfluidic Synthesis of Curcumin-loaded Liposomes. Biomed. Microdevices 2023, 25, 29. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, W.; Ren, Y.; Li, S.; Liu, M.; Xing, J.; Han, Y.; Chen, Y.; Tao, R.; Guo, L. Lipid nanoparticle-encapsulated VEGFa siRNA facilitates cartilage formation by suppressing angiogenesis. Int. J. Biol. Macromol. 2022, 221, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Renzi, S.; Quagliarini, E.; Digiacomo, L.; Amenitsch, H.; Masuelli, L.; Bei, R.; Ferri, G.; Cardarelli, F.; Wang, J. Efficient delivery of DNA using lipid nanoparticles. Pharmaceutics 2022, 14, 1698. [Google Scholar] [CrossRef] [PubMed]
- Yathindranath, V.; Safa, N.; Sajesh, B.V.; Schwinghamer, K.; Vanan, M.I.; Bux, R.; Sitar, D.S.; Pitz, M.; Siahaan, T.J.; Miller, D.W. Spermidine/Spermine N1-Acetyltransferase 1 (SAT1)—A Potential Gene Target for Selective Sensitization of Glioblastoma Cells Using an Ionizable Lipid Nanoparticle to Deliver SiRNA. Cancers 2022, 14, 5179. [Google Scholar] [CrossRef]
- Okuda, K.; Sato, Y.; Iwakawa, K.; Sasaki, K.; Okabe, N.; Maeki, M.; Tokeshi, M.; Harashima, H. On the size-regulation of RNA-loaded lipid nanoparticles synthesized by microfluidic device. J. Control Release 2022, 348, 648–659. [Google Scholar] [CrossRef]
- Han, J.P.; Kim, M.; Choi, B.S.; Lee, J.H.; Lee, G.S.; Jeong, M.; Lee, Y.; Kim, E.-A.; Oh, H.-K.; Go, N. In vivo delivery of CRISPR-Cas9 using lipid nanoparticles enables antithrombin gene editing for sustainable hemophilia A and B therapy. Sci. Adv. 2022, 8, eabj6901. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Chen, S.; Tam, Y.Y.C. Scalable production of lipid nanoparticles containing Amphotericin B. Langmuir 2021, 37, 7312–7319. [Google Scholar] [CrossRef]
- Terada, T.; Kulkarni, J.A.; Huynh, A.; Chen, S.; van der Meel, R.; Tam, Y.Y.C.; Cullis, P.R. Characterization of lipid nanoparticles containing ionizable cationic lipids using design-of-experiments approach. Langmuir 2021, 37, 1120–1128. [Google Scholar] [CrossRef]
- Younis, M.A.; Khalil, I.A.; Elewa, Y.H.; Kon, Y.; Harashima, H. Ultra-small lipid nanoparticles encapsulating sorafenib and midkine-siRNA selectively-eradicate sorafenib-resistant hepatocellular carcinoma in vivo. J. Control Release 2021, 331, 335–349. [Google Scholar] [CrossRef]
- Forbes, N.; Hussain, M.T.; Briuglia, M.L.; Edwards, D.P.; Ter Horst, J.H.; Szita, N.; Perrie, Y. Rapid and scale-independent microfluidic manufacture of liposomes entrapping protein incorporating in-line purification and at-line size monitoring. Int. J. Pharm. 2019, 556, 68–81. [Google Scholar] [CrossRef]
- Ayad, C.; Libeau, P.; Lacroix-Gimon, C.; Ladavière, C.; Verrier, B. Lipoparticles: Lipid-coated PLA nanoparticles enhanced in vitro mRNA transfection compared to liposomes. Pharmaceutics 2021, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- Quagliarini, E.; Renzi, S.; Digiacomo, L.; Giulimondi, F.; Sartori, B.; Amenitsch, H.; Tassinari, V.; Masuelli, L.; Bei, R.; Cui, L. Microfluidic formulation of DNA-loaded multicomponent lipid nanoparticles for gene delivery. Pharmaceutics 2021, 13, 1292. [Google Scholar] [CrossRef] [PubMed]
- Connerty, P.; Moles, E.; de Bock, C.E.; Jayatilleke, N.; Smith, J.L.; Meshinchi, S.; Mayoh, C.; Kavallaris, M.; Lock, R.B. Development of siRNA-loaded lipid nanoparticles targeting long non-coding RNA LINC01257 as a novel and safe therapeutic approach for t (8; 21) pediatric acute myeloid leukemia. Pharmaceutics 2021, 13, 1681. [Google Scholar] [CrossRef]
- Khadke, S.; Roces, C.B.; Donaghey, R.; Giacobbo, V.; Su, Y.; Perrie, Y. Scalable solvent-free production of liposomes. J. Pharm. Pharmacol. 2020, 72, 1328–1340. [Google Scholar] [CrossRef]
- Gkionis, L.; Campbell, R.A.; Aojula, H.; Harris, L.K.; Tirella, A. Manufacturing drug co-loaded liposomal formulations targeting breast cancer: Influence of preparative method on liposomes characteristics and in vitro toxicity. Int. J. Pharm. 2020, 590, 119926. [Google Scholar] [CrossRef]
- Billingsley, M.M.; Singh, N.; Ravikumar, P.; Zhang, R.; June, C.H.; Mitchell, M.J. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020, 20, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Okabe, N.; Note, Y.; Hashiba, K.; Maeki, M.; Tokeshi, M.; Harashima, H. Hydrophobic scaffolds of pH-sensitive cationic lipids contribute to miscibility with phospholipids and improve the efficiency of delivering short interfering RNA by small-sized lipid nanoparticles. Acta Biomater. 2020, 102, 341–350. [Google Scholar] [CrossRef]
- Zukancic, D.; Suys, E.J.; Pilkington, E.H.; Algarni, A.; Al-Wassiti, H.; Truong, N.P. The importance of poly (Ethylene glycol) and lipid structure in targeted gene delivery to lymph nodes by lipid nanoparticles. Pharmaceutics 2020, 12, 1068. [Google Scholar] [CrossRef]
- Hashiba, A.; Toyooka, M.; Sato, Y.; Maeki, M.; Tokeshi, M.; Harashima, H. The use of design of experiments with multiple responses to determine optimal formulations for in vivo hepatic mRNA delivery. J. Control Release 2020, 327, 467–476. [Google Scholar] [CrossRef]
- Roces, C.B.; Lou, G.; Jain, N.; Abraham, S.; Thomas, A.; Halbert, G.W.; Perrie, Y. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics 2020, 12, 1095. [Google Scholar] [CrossRef]
- Hamano, N.; Böttger, R.; Lee, S.E.; Yang, Y.; Kulkarni, J.A.; Ip, S.; Cullis, P.R.; Li, S.-D. Robust microfluidic technology and new lipid composition for fabrication of curcumin-loaded liposomes: Effect on the anticancer activity and safety of cisplatin. Mol. Pharm. 2019, 16, 3957–3967. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Tchung, E.; Nowell, C.; Kaga, S.; Leong, N.; Mehta, D.; Kaminskas, L.M.; Boyd, B.J. Microfluidic preparation of drug-loaded PEGylated liposomes, and the impact of liposome size on tumour retention and penetration. J. Liposome Res. 2019, 29, 1–9. [Google Scholar] [CrossRef]
- Kim, H.; Sung, J.; Chang, Y.; Alfeche, A.; Leal, C. Microfluidics synthesis of gene silencing cubosomes. ACS Nano 2018, 12, 9196–9205. [Google Scholar] [CrossRef]
- Kimura, N.; Maeki, M.; Sato, Y.; Note, Y.; Ishida, A.; Tani, H.; Harashima, H.; Tokeshi, M. Development of the iLiNP device: Fine tuning the lipid nanoparticle size within 10 nm for drug delivery. ACS Omega 2018, 3, 5044–5051. [Google Scholar] [CrossRef]
- Balbino, T.A.; Serafin, J.M.; Radaic, A.; de Jesus, M.B.; de la Torre, L.G. Integrated microfluidic devices for the synthesis of nanoscale liposomes and lipoplexes. Colloids Surf. B Biointerfaces 2017, 152, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Krzysztoń, R.; Salem, B.; Lee, D.; Schwake, G.; Wagner, E.; Rädler, J. Microfluidic self-assembly of folate-targeted monomolecular siRNA-lipid nanoparticles. Nanoscale 2017, 9, 7442–7453. [Google Scholar] [CrossRef]
- Maeki, M.; Fujishima, Y.; Sato, Y.; Yasui, T.; Kaji, N.; Ishida, A.; Tani, H.; Baba, Y.; Harashima, H.; Tokeshi, M. Understanding the formation mechanism of lipid nanoparticles in microfluidic devices with chaotic micromixers. PLoS ONE 2017, 12, e0187962. [Google Scholar] [CrossRef]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Note, Y.; Maeki, M.; Kaji, N.; Baba, Y.; Tokeshi, M.; Harashima, H. Elucidation of the physicochemical properties and potency of siRNA-loaded small-sized lipid nanoparticles for siRNA delivery. J. Control Release 2016, 229, 48–57. [Google Scholar] [CrossRef]
- Kastner, E.; Verma, V.; Lowry, D.; Perrie, Y. Microfluidic-controlled manufacture of liposomes for the solubilisation of a poorly water soluble drug. Int. J. Pharm. 2015, 485, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yu, B.; Zhu, J.; Huang, X.; Xie, J.; Xu, S.; Yang, X.; Wang, X.; Yung, B.C.; Lee, L.J. A microfluidic method to synthesize transferrin-lipid nanoparticles loaded with siRNA LOR-1284 for therapy of acute myeloid leukemia. Nanoscale 2014, 6, 9742–9751. [Google Scholar] [CrossRef]
- Balbino, T.A.; Azzoni, A.R.; de La Torre, L.G. Microfluidic devices for continuous production of pDNA/cationic liposome complexes for gene delivery and vaccine therapy. Colloids Surf. B Biointerfaces 2013, 111, 203–210. [Google Scholar] [CrossRef]
- Belliveau, N.M.; Huft, J.; Lin, P.J.; Chen, S.; Leung, A.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K. Microfluidic synthesis of highly potent limit-size lipid nanoparticles for in vivo delivery of siRNA. Mol. Ther.-Nucleic Acids 2012, 1, E37. [Google Scholar] [CrossRef] [PubMed]
- Aftab, A.; Ahmad, B.; Bashir, S.; Rafique, S.; Bashir, M.; Ghani, T.; Gul, A.; Shah, A.U.; Khan, R.; Sajini, A.A. Comparative study of microscale and macroscale technique for encapsulation of Calotropis gigantea extract in metal-conjugated nanomatrices for invasive ductal carcinoma. Sci. Rep. 2023, 13, 13474. [Google Scholar] [CrossRef] [PubMed]
- Alhalili, Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef]
- Acharya, G.; Mitra, A.K.; Cholkar, K. Nanosystems for diagnostic imaging, biodetectors, and biosensors. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 217–248. [Google Scholar]
- Wacker, J.B.; Lignos, I.; Parashar, V.K.; Gijs, M.A. Controlled synthesis of fluorescent silica nanoparticles inside microfluidic droplets. Lab Chip 2012, 12, 3111–3116. [Google Scholar] [CrossRef]
- Maxwell, T.; Campos, M.G.N.; Smith, S.; Doomra, M.; Thwin, Z.; Santra, S. Quantum dots. In Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–265. [Google Scholar]
- Shmeis, R.M.A. Nanotechnology in wastewater treatment. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 99, pp. 105–134. [Google Scholar]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Khizar, S.; Zine, N.; Errachid, A.; Jaffrezic-Renault, N.; Elaissari, A. Microfluidic-based nanoparticle synthesis and their potential applications. Electrophoresis 2022, 43, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Imanparast, A.; Shaegh, S.A.M.; Attaran, N.; Ameri, A.R.; Sazgarnia, A. Opto-microfluidic assisted synthesis of photo-protoporphyrin (pPP) conjugated to hollow gold-albumin hybrid nanoshells to enhance the efficiency of photodynamic therapy of triple negative breast cancer cells. Photodiagn. Photodyn. Ther. 2023, 2023, 103632. [Google Scholar] [CrossRef] [PubMed]
- Bîrcă, A.C.; Gherasim, O.; Niculescu, A.-G.; Grumezescu, A.M.; Neacșu, I.A.; Chircov, C.; Vasile, B.Ș.; Oprea, O.C.; Andronescu, E.; Stan, M.S. A Microfluidic Approach for Synthesis of Silver Nanoparticles as a Potential Antimicrobial Agent in Alginate–Hyaluronic Acid-Based Wound Dressings. Int. J. Mol. Sci. 2023, 24, 11466. [Google Scholar] [CrossRef]
- Podlesnaia, E.; Gerald Inangha, P.; Vesenka, J.; Seyring, M.; Hempel, H.J.; Rettenmayr, M.; Csáki, A.; Fritzsche, W. Microfluidic-Generated Seeds for Gold Nanotriangle Synthesis in Three or Two Steps. Small 2023, 2023, 2204810. [Google Scholar] [CrossRef]
- Ochoa-Vazquez, G.; Kharisov, B.; Arizmendi-Morquecho, A.; Cario, A.; Aymonier, C.; Marre, S.; López, I. Continuous segmented-flow synthesis of Ag and Au nanoparticles using a low cost microfluidic PTFE tubing reactor. IEEE Trans. NanoBiosci. 2021, 21, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Kafali, M.; Şahinoğlu, O.B.; Tufan, Y.; Orsel, Z.C.; Aygun, E.; Alyuz, B.; Saritas, E.U.; Erdem, E.Y.; Ercan, B. Antibacterial properties and osteoblast interactions of microfluidically synthesized chitosan–SPION composite nanoparticles. J. Biomed. Mater. Res. Part A 2023, 111, 1662–1677. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Leem, J.; Yoon, S.Y.; Sung, H.J. Continuous synthesis of zinc oxide nanoparticles in a microfluidic system for photovoltaic application. Nanoscale 2014, 6, 2840–2846. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Liu, X.; Cabeza, V.S.; Jensen, K.F. Synthesis, assembly and reaction of a nanocatalyst in microfluidic systems: A general platform. Lab Chip 2012, 12, 4080–4084. [Google Scholar] [CrossRef]
- Lee, W.-B.; Weng, C.-H.; Cheng, F.-Y.; Yeh, C.-S.; Lei, H.-Y.; Lee, G.-B. Biomedical microdevices synthesis of iron oxide nanoparticles using a microfluidic system. Biomed. Microdevices 2009, 11, 161–171. [Google Scholar] [CrossRef]
- Frenz, L.; El Harrak, A.; Pauly, M.; Bégin-Colin, S.; Griffiths, A.D.; Baret, J.C. Droplet-based microreactors for the synthesis of magnetic iron oxide nanoparticles. Angew. Chem. Int. Ed. 2008, 47, 6817–6820. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, M.; Jin, Y.; Lu, Y.; Xiong, S.; Wang, M.; Xu, J.; Wei, C.; Li, J. Microfluidic synthesis of pH-responsive molecularly imprinted silica nanospheres for fluorescence sensing target glycoprotein. Food Chem. 2023, 2023, 136570. [Google Scholar] [CrossRef]
- Lisina, S.; Inam, W.; Huhtala, M.; Howaili, F.; Zhang, H.; Rosenholm, J.M. Nano Differential Scanning Fluorimetry as a Rapid Stability Assessment Tool in the Nanoformulation of Proteins. Pharmaceutics 2023, 15, 1473. [Google Scholar] [CrossRef]
- Küçüktürkmen, B.; Inam, W.; Howaili, F.; Gouda, M.; Prabhakar, N.; Zhang, H.; Rosenholm, J.M. Microfluidic-assisted fabrication of dual-coated pH-sensitive mesoporous silica nanoparticles for protein delivery. Biosensors 2022, 12, 181. [Google Scholar] [CrossRef]
- He, P.; Greenway, G.; Haswell, S.J. The on-line synthesis of enzyme functionalized silica nanoparticles in a microfluidic reactor using polyethylenimine polymer and R5 peptide. Nanotechnology 2008, 19, 315603. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).