Geochemistry and Microbiology of Atacamite-Paratacamite Biofilms Floating on Underground Brine and Petroleum Pools in the White Pine Copper Mine, Michigan (USA)
Abstract
:1. Introduction
2. Materials and Methods
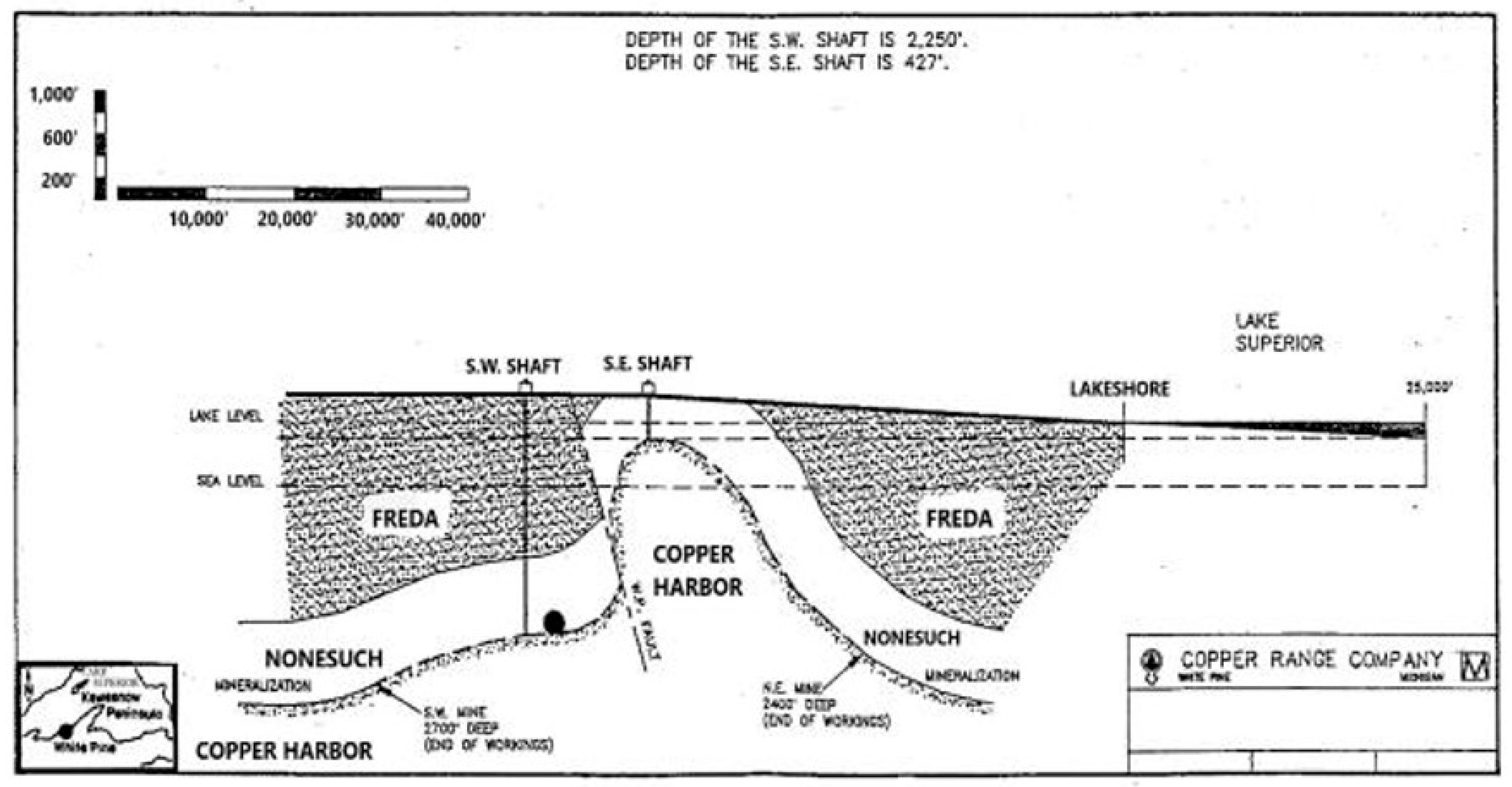
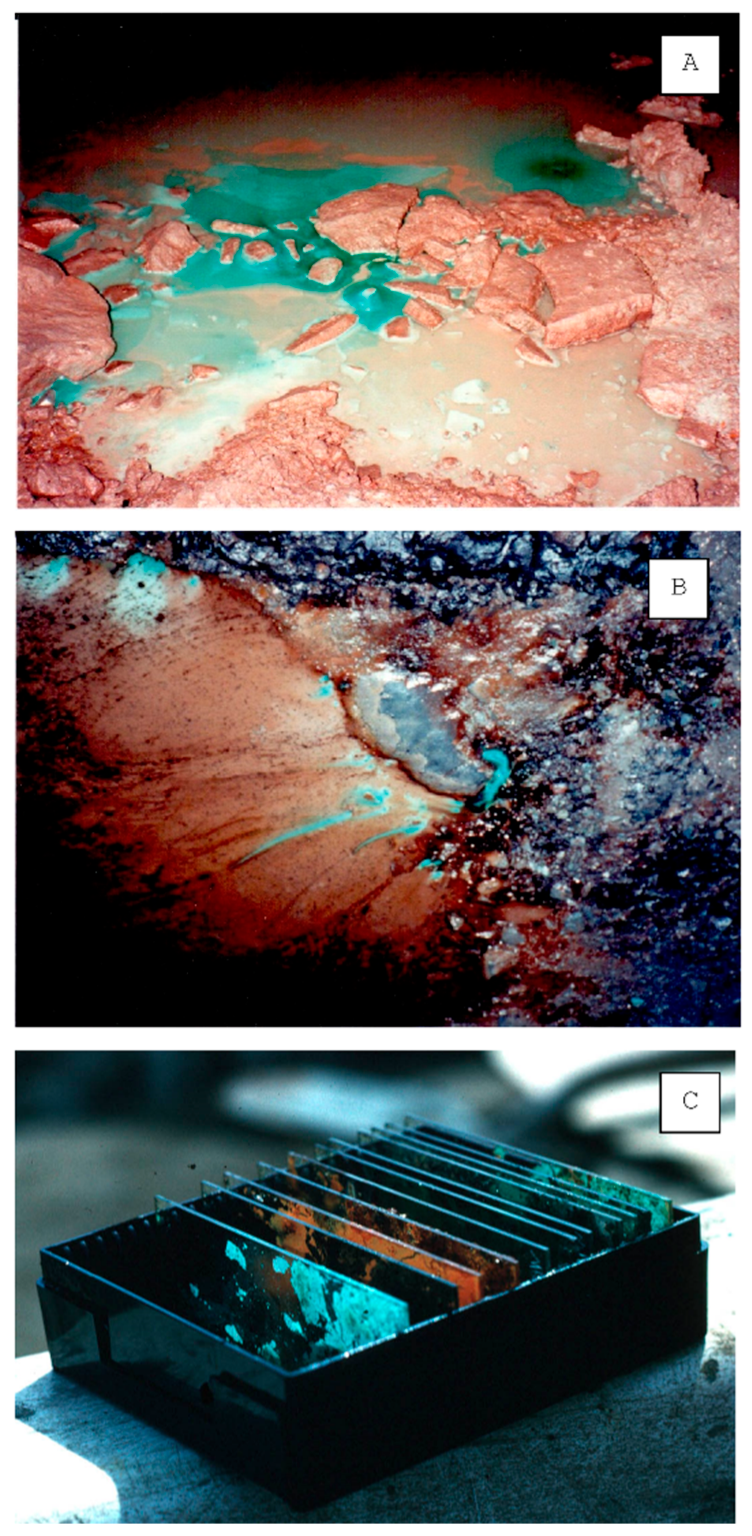
2.1. Geological Setting and Biofilm Sampling
2.2. Analytical Methods
2.2.1. X-ray Powder Diffraction and Energy Dispersive X-ray Spectroscopy
2.2.2. Microscopy
2.2.3. Microbiology
2.2.4. Water Chemistry
2.2.5. Isotope Geochemistry
2.2.6. Thermodynamic Analysis
3. Results
3.1. Mineralogy
3.2. Water Chemistry
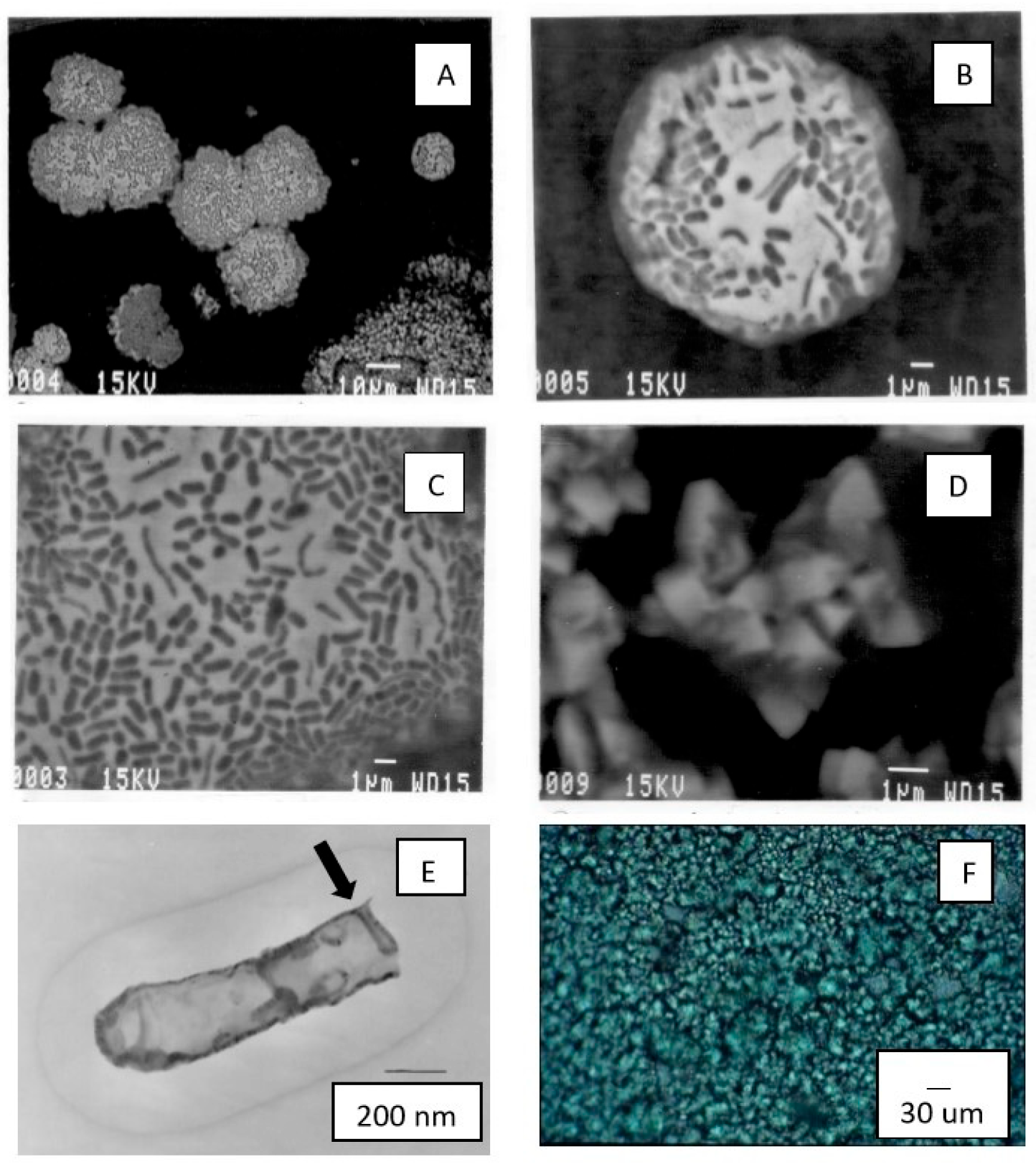
3.3. Microscopy
3.4. Microbiology
3.5. Isotope Geochemistry
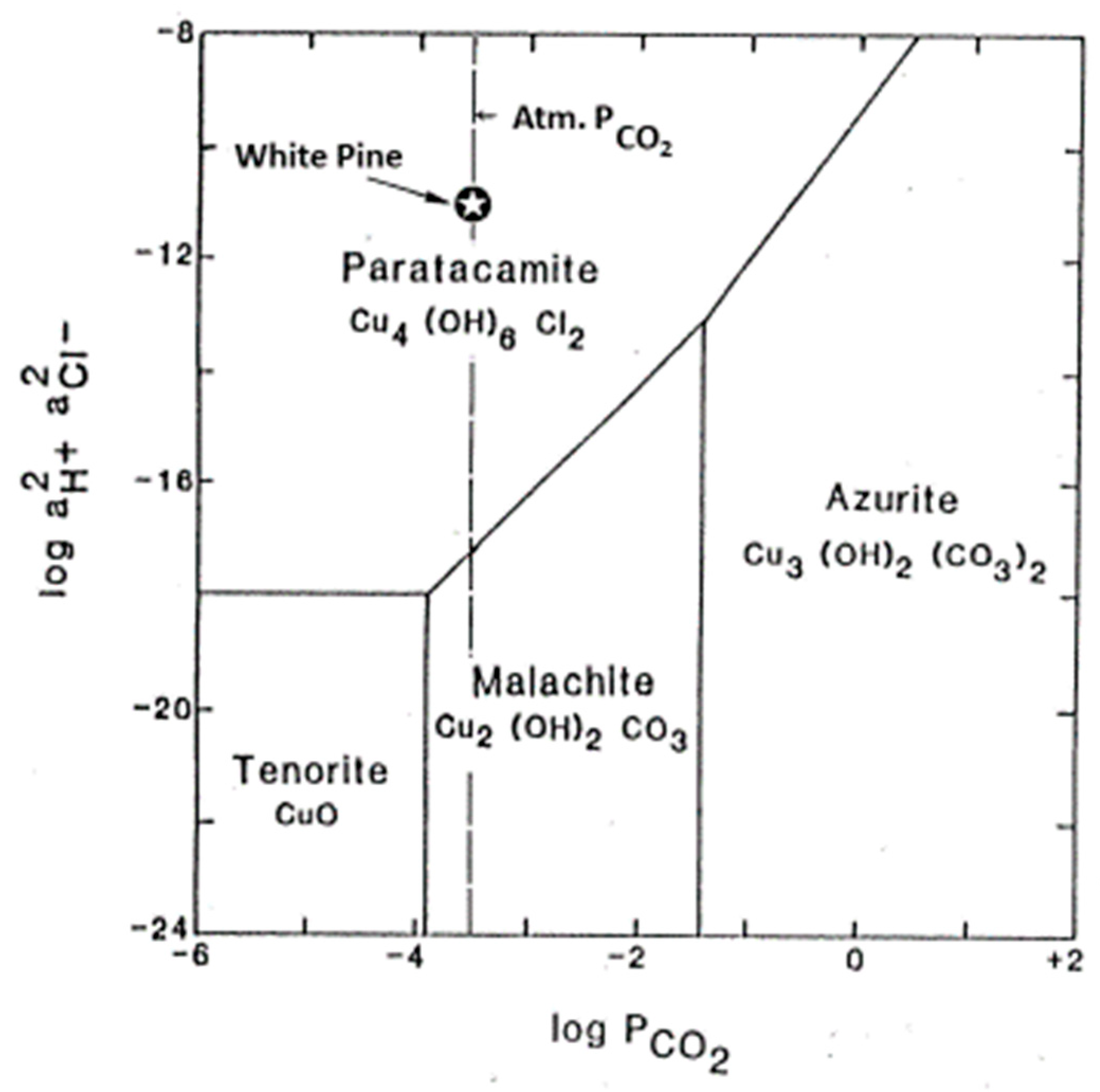
3.6. Thermodynamics
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phelps, R.W. Copper Range, Last All-Underground Copper Mine in the United States is a Game Survivor--Now with a More Assured Future. Eng. Min. J. 1990, 191, C47–C57. [Google Scholar]
- Robbins, E.I.; Stanton, M.R.; Tilk, J.E.; Congdon, R.D.; Evans, H.T., Jr.; Gullett, C.D.; Sanders, M.B.; Sato, M.; Schaef, H.T.; Seal, R.R. Association of Microbes with Authigenic Copper Chloride Mineral Films (Atacamite and Paratacamite) and Petroleum Residues at Depth in the White Pine Copper Mine, Michigan. In Proceedings of the Waterloo ’94, Geol. Assoc. Canada and Mineral. Assoc. Canada, Waterloo, ON, Canada, Abs. with Program. 13–18 May 1994; p. A94. [Google Scholar]
- Copper Range Company. Cross Section of the White Pine Mine; Copper Range Company: Houghton, MI, USA, 1993. [Google Scholar]
- Daniels, P.A., Jr. Upper Precambrian Sedimentary Rocks; Oronto Group, Michigan-Wisconsin. In Geology and Tectonics of the Lake Superior Basin; Wold, R.J., Hinze, W.J., Eds.; The Geological Society of America, Inc.: Boulder, CO, USA, 1982; Chapter 7C; Volume 156, pp. 107–133. [Google Scholar] [CrossRef]
- White, W.S.; Wright, J.C. The White Pine Copper Deposit, Ontonagon County, Michigan. Econ. Geol. 1954, 49, 675–716. [Google Scholar] [CrossRef]
- Mauk, J.L.; Hieshima, G.B. Organic Matter and Copper Mineralization at White Pine, Michigan. Chem. Geol. 1992, 99, 189–211. [Google Scholar] [CrossRef]
- White, W.S.; Wright, J.C. Sulfide Mineral Zoning in the Basal Nonesuch Shale, Northern Michigan. Econ. Geol. 1966, 61, 1171–1190. [Google Scholar] [CrossRef]
- Brown, A.C. Mineral Assemblages in the White Pine Copper Deposit, Northern Michigan (USA). In Freiberger Forschungshefte, Reihe C, Geowissenschafte, Mineralogie, und Geochemie; Akademie-Verlag: Freiberg, Germany, 1973; Volume 293, pp. 65–83. [Google Scholar]
- Alyanak, N.; Vogel, T.A. Framboidal Chalcocite from White Pine, Michigan. Econ. Geol. 1974, 69, 697–703. [Google Scholar] [CrossRef]
- Ho, E.S.; Mauk, J.L. Relationship between Organic Matter and Copper Mineralization in the Proterozoic Nonesuch Formation, Northern Michigan. Ore Geol. Rev. 1996, 11, 71–87. [Google Scholar] [CrossRef]
- Robbins, E.I. Accumulation of Fossil Fuels and Metallic Minerals in Active and Ancient Rifts. Tectonophysics 1983, 94, 633–658. [Google Scholar] [CrossRef]
- Eglinton, G.; Scott, P.M.; Belsky, T.; Burlingame, A.L.; Calvin, M. Hydrocarbons of Biological Origin from a One-Billion-Year-Old Sediment. Science 1964, 145, 263–264. [Google Scholar] [CrossRef]
- Eglinton, G.; Scott, P.M.; Belsky, T.; Burlingame, A.L.; Richter, W.; Calvin, M. Occurrence of isoprenoid alkanes in a precambrian sediment. In Advances in Organic Geochemistry; Hobson, G.D., Louis, M.C., Eds.; Pergamon Press: Oxford, UK, 1964; pp. 41–74. [Google Scholar] [CrossRef]
- Kelly, W.C.; Rye, R.O.; Livnat, A. Saline Minewaters of the Keweenaw Peninsula, Northern Michigan: Their Nature, Origin, and Relation to Similar Deep Waters in Precambrian Crystalline Rocks of the Canadian Shield. Am. J. Sci. 1986, 286, 281–308. [Google Scholar] [CrossRef]
- Andrei, A.; Öztürk, Y.; Khalfaoui-Hassani, B.; Rauch, J.; Marckmann, D.; Trasnea, P.-J.; Daldal, F.; Koch, H.-G. Cu Homeostasis in Bacteria: The Ins and Outs. Membranes 2020, 10, 242. [Google Scholar] [CrossRef]
- Beale, D.J.; Morrison, P.D.; Key, C.; Palombo, E.A. Metabolic Profiling of Biofilm Bacteria Known to Cause Microbial Influenced Corrosion. Water Sci. Technol. 2014, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vargas, I.T.; Alsina, M.A.; Pavissich, J.P.; Jeria, G.A.; Pasten, P.A.; Walczak, M.; Pizarro, G.E. Multi-Technique Approach to Assess the Effects of Microbial Biofilms Involved in Copper Plumbing Corrosion. Bioelectrochemistry 2014, 97, 15–22. [Google Scholar] [CrossRef]
- Narenkumar, J.; Devanesan, S.; AlSalhi, M.S.; Kokilaramani, S.; Ting, Y.-P.; Rahman, P.K.S.M.; Rajasekar, A. Biofilm Formation on Copper and its Control by Inhibitor/Biocide in Cooling Water Environment. Saudi J. Biol. Sci. 2021, 28, 7588–7594. [Google Scholar] [CrossRef] [PubMed]
- Pschedt, J.W. Copper-Based Bactericides and Fungicides, Pacific Northwest Pest Management Handbook, Oregon State University. Available online: https://pnwhandbooks.org/plantdisease/pesticide-articles/copper-based-bactericides-fungicides (accessed on 28 July 2023).
- Wisconsin Department of Natural Resources. Copper Compounds Chemical Fact Sheet, DNR PUB-WT-968. 2012; 2p. Available online: https://dnr.wi.gov/lakes/plants/factsheets/CopperFactsheet.pdf (accessed on 28 July 2023).
- Barranguet, C.; van den Ende, P.F.; Rutgers, J.; Breure, A.M.; Greijdanus, M.; Sinke, J.S.; Admiraal, W. Copper-Induced Modifications of the Trophic Relations in Riverine Algal-Bacterial Biofilms. Environ. Toxicol. Chem. 2003, 22, 1340–1349. [Google Scholar] [PubMed]
- Woods, T.L.; Garrels, R.M. Phase Relations of Some Cupric Hydroxyl Minerals. Econ. Geol. 1986, 81, 1989–2007. [Google Scholar] [CrossRef]
- Pollard, A.M.; Thomas, R.G.; Williams, P.A. Synthesis and Stabilities of the Basic Copper (II) Chlorides Atacamite, Paratacamite and Botallackite. Mineral. Mag. 1989, 53, 557–563. [Google Scholar] [CrossRef]
- Crisman, D.P. Groundwater Geochemistry in the Portage Lake Volcanics, Hancock, Michigan and Implications for Native Copper Stability. Master’s Thesis, Michigan Technical University, Houghton, MI, USA, 1982. [Google Scholar]
- Mindat, Atacamite, Paratacamite. Available online: https://www.mindat.org/min (accessed on 28 July 2023).
- Palacios, C.; Rouxel, O.; Reich, M.; Cameron, E.M.; Meybourne, M.L. Pleistocene Recycling of Copper in a Porphyry System, Atacama Desert, Chile: Cu Isotope Evidence. Mineral. Deposita 2011, 46, 1–7. [Google Scholar] [CrossRef]
- Reich, M.; Palacios, C.; Parada, M.A.; Fehn, U.; Cameron, E.M.; Leybourne, M.L.; Zuniga, A. Atacamite Formation by Deep Saline Waters in Copper Deposits from the Atacama Desert, Chile: Evidence from Fluid Inclusions, Groundwater Geochemistry, TEM, and 36Cl Data. Miner. Deposita 2008, 43, 663. [Google Scholar] [CrossRef]
- Shaerkey, J.B.; Lewin, S.Z. Conditions Governing the Formation of Atacamite and Paratacamite. Am. Mineral. 1971, 56, 179–192. [Google Scholar]
- Calagari, A.A. Stable Isotope (S, O, H and C) Studies of the Phyllic and Potassic-Phyllic Alteration Zones of the Porphyry Copper Deposit at Sungun, East Azarbaidjan, Iran. J. Asian Earth Sci. 2003, 21, 767–780. [Google Scholar] [CrossRef]
- Kojima, S.; Astudillo, J.; Rojo, J.; Tristá, D.; Hayashi, K. Ore Mineralogy, Fluid Inclusion, and Stable Isotopic Characteristics of Stratiform Copper Deposits in the Coastal Cordillera of Northern Chile. Miner. Depos. 2003, 38, 208–216. [Google Scholar] [CrossRef]
- Bodden, T.J.; Bornhorst, T.J.; Bégué, F.; Deering, C. Sources of Hydrothermal Fluids Inferred from Oxygen and Carbon Isotope Composition of Calcite, Keweenaw Peninsula Native Copper District, Michigan, USA. Minerals 2022, 12, 474. [Google Scholar] [CrossRef]
- Kendall, C.; Sklash, M.G.; Bullen, T.D. Isotope Tracers in Water and Solute Sources in Catchments. In Solute Modelling in Catchments Systems; Trudgill, S.T., Ed.; John Wiley & Sons: New York, NY, USA, 1995; pp. 261–303. [Google Scholar]
- Enders, M.S.; Knickerbocker, C.; Titley, S.R.; Southam, G. The Role of Bacteria in the Supergene Environment of the Morenci Porphyry Copper Deposit, Greenlee County, Arizona. Econ. Geol. 2006, 101, 59–70. [Google Scholar] [CrossRef]
- Liu, J.; Hua, Z.-S.; Chen, L.-X.; Kuang, J.-L.; Li, S.-J.; Shu, W.-S.; Huang, L.-N. Correlating Microbial Diversity Patterns with Geochemistry in an Extreme and Heterogeneous Environment of Mine Tailings. Appl. Environ. Microbiol. 2014, 80, 3677–3686. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.C.; Ray, M.K.; Koch, C.; Bhattacharyya, S.; Shivaji, S.; Stackebrandt, E. Molecular Characterization of Two Acidophilic Heterotrophic Bacteria Isolated from a Copper Mine of India, System. Appl. Microbiol. 1996, 19, 78–82. [Google Scholar] [CrossRef]
- Galleguillos, P.A.; Hallberg, K.B.; Johnson, D.B. Microbial Diversity and Genetic Response to Stress Conditions of Extremophilic Bacteria Isolated from the Escondida Copper Mine. Adv. Mater. Res. 2009, 71–73, 55–58. [Google Scholar] [CrossRef]
- Pusz, W.; Kita, W.; Weber, R. Microhabitat Influences the Occurrence of Airborne Fungi in Copper Mine in Poland. J. Cave Karst Stud. 2014, 76, 14–19. [Google Scholar] [CrossRef]
- Cervantes, C.; Gutierrez-Corona, F. Copper Resistance Mechanisms in Bacteria and Fungi. FEMS Microbiol. Rev. 1994, 14, 121–138. [Google Scholar] [CrossRef]
- Ladomersky, E.; Petris, M.J. Copper Tolerance and Virulence in Bacteria. Metallomics 2016, 7, 957–964. [Google Scholar] [CrossRef]
- Glauert, A.M.; Lewis, P.R. Biological Specimen Preparation for Transmission Electron Microscopy. In Practical Methods in Electron Microscopy; Glauert, A.M., Ed.; Portland Press: London, UK, 1998; Volume 17. [Google Scholar] [CrossRef]
- Tornos, F.; Oggerin, M.; de los Rios, A.; Rodriguez, N.; Amils, R.; Sanz, J.L.; Rojas, P.; Velasco, F.; Escobar, J.M.; Gomez, C.; et al. Do Microbes Control the Formation of Giant Copper Deposits? Geology 2019, 47, 143–146. [Google Scholar] [CrossRef]
- Arif, S.; Nacke, H.; Schliekmann, E.; Reimer, A.; Arp, G.; Hoppert, M. Composition and Niche-Specific Characteristics of Microbial Consortia Colonizing Marsberg Copper Mine in the Rhenish Massif. Biogeosciences 2022, 19, 4883–4902. [Google Scholar] [CrossRef]
- Kendall, C.; Caldwell, E.A. Fundamentals of isotope geochemistry. In Isotope Tracers in Catchment Hydrology; Kendall, C., McDonnell, J.J., Eds.; Elsevier Science: Amsterdam, The Netherlands; New York, NY, USA, 1998; Chapter 2; pp. 51–86. [Google Scholar]
- Walter-Levy, L.; Goreaud, M. Sur la Formation des Chlorures Basiques Cuivriques en Solution Aqueuse de 25° à 200 °C; Bulletin de la Societe Chimique de France: Paris, France, 1969; pp. 2623–2634. [Google Scholar]
- Nagakubo, T.; Nomura, N.; Toyofuku, M. Cracking Open Bacterial Membrane Vesicles. Front. Microbiol. 2020, 10, 3026. [Google Scholar] [CrossRef]
- Hieshima, G.B. Organic and Isotopic Geochemical Study of the Middle Proterozoic Nonesuch Formation, North American Midcontinent Rift. Ph.D. Thesis, Indiana University, Bloomington, IN, USA, 1992. [Google Scholar]
- Mauk, J.L. Geologic and Geochemical Investigations of the White Pine Sediment Hosted Stratiform Copper Deposit, Ontonagon County, Michigan. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 1993. [Google Scholar]
- Cameron, E.M.; Leybourne, M.L.; Palacios, C. Atacamite in the Oxide Zone of Copper Deposits in Northern Chile: Involvement of Deep Formation Waters? Miner. Depos. 2007, 42, 205–218. [Google Scholar] [CrossRef]

| Crisman | This Study | This Study 1 | |
|---|---|---|---|
| (1982) [24] | (10/93 raw water) | (8/94 collected; 12/95 analyzed) | |
| Ca | 91,780 | 69,190 | 13,264 |
| Na | 17,640 | 16,400 | 2156 |
| Mg | 1373 | 940 | 256 |
| Sr | 1261 | nd | 125 |
| K | 234 | nd | 200 |
| Al | nd | nd | 2.6 |
| Cd | nd | nd | 0.122 |
| Cu | nd | 7 | 0.35 |
| Fe | nd | 7 | 1.59 |
| Mn | 40 | nd | 6.97 |
| Ni | nd | nd | 0.33 |
| Pb | nd | nd | 1.31 |
| Zn | nd | nd | 2.34 |
| Cl | 156,500 | 105,152 | 41,000 |
| Br | 1520 | nd | 0.1 |
| HCO3− | 15 | nd | 21.8 |
| SO4− | nd | 0.49 | 132 |
| NO3− | nd | nd | 946 |
| TDS | 270,363 | nd | 58,117 |
| pH | nd | 6.0 + 0.2 | 5.9 |
| T(C) | 17° | 17° | nd |
| Eh (mV) ORP (Pt) | nd | +488.6 | nd |
| Eh (mV) Ag/AgCl | nd | 199.6 | nd |
| Eh (mV) Cu | nd | 64.6 | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robbins, E.I.; Stanton, M.R.; Young, C.D. Geochemistry and Microbiology of Atacamite-Paratacamite Biofilms Floating on Underground Brine and Petroleum Pools in the White Pine Copper Mine, Michigan (USA). Micro 2023, 3, 728-738. https://doi.org/10.3390/micro3030051
Robbins EI, Stanton MR, Young CD. Geochemistry and Microbiology of Atacamite-Paratacamite Biofilms Floating on Underground Brine and Petroleum Pools in the White Pine Copper Mine, Michigan (USA). Micro. 2023; 3(3):728-738. https://doi.org/10.3390/micro3030051
Chicago/Turabian StyleRobbins, Eleanora I., Mark R. Stanton, and Cheryl D. Young. 2023. "Geochemistry and Microbiology of Atacamite-Paratacamite Biofilms Floating on Underground Brine and Petroleum Pools in the White Pine Copper Mine, Michigan (USA)" Micro 3, no. 3: 728-738. https://doi.org/10.3390/micro3030051
APA StyleRobbins, E. I., Stanton, M. R., & Young, C. D. (2023). Geochemistry and Microbiology of Atacamite-Paratacamite Biofilms Floating on Underground Brine and Petroleum Pools in the White Pine Copper Mine, Michigan (USA). Micro, 3(3), 728-738. https://doi.org/10.3390/micro3030051







