3.1. New Channel Materials
In our previous reports [
2,
5], a high-viscosity acrylic resin monomer supplied by Tokyo Ohka Kogyo Co., Ltd. (Kawasaki, Japan) was applied without including an initiator for the channel material. However, channel deformities have been frequently observed, resulting in quite low reproducibility. From the screening results of other channel materials, we reach the conclusion that the large polarity difference between the channel material and the PDMS monomer makes it difficult for the channel material to maintain a cylindrical shape for a long interim time, particularly when the viscosity of channel materials is not high enough. Therefore, it is necessary to find materials that have a good compatibility with the PDMS monomer.
Figure 4 shows top- and cross-sectional views of a fabricated microchannel in which we apply a new channel material, silicone oil (dimethyl siloxane base: KF-96, 60,000 cP, and methyl phenyl siloxane base: KF-50, 3000 cP from Shin-Etsu silicone). The polarity of silicone oiled should be close to that of PDMS monomer since they have the same siloxane backbone structure. In particular, dimethyl siloxane has the same structure as PDMS, except for its molecular weight. However, the cross-section of the channels fabricated with KF-96 in
Figure 4 is not circular but a vertically long oval. Meanwhile, when KF-50 is used, almost perfect circular cross-sections are formed. Since KF-96 has sufficiently high viscosity and since its polarity is very close to the PDMS monomer’s, the cross-section is less likely to be circular. In contrast, an appropriate polarity difference between KF-50 and the PDMS monomer allows for the self-formation of a circular cross-section due to surface tension. In addition, an appropriate viscosity (not too high or too low) preserves its cylindrical shape in the PDMS monomer until it is cured. Then, it becomes possible to stabilize the channel pattern without disconnection or distortion. In addition, the channel diameter variation is also small enough (ca. 5 μm). Hence, the polarity difference between the channel material and PDMS monomer is a key to make the channel cross-section circular: the channel material whose polarity moderately differs from PDMS monomer’s is likely to form circular channels.
The channel size as well as the viscosity of the channel material could influence the channel cross-sectional shape. Just after the channel material is dispensed into the PDMS monomer, the channel cross-section is reshaped by the PDMS monomer pressure from the periphery and the material’s surface tension. When the channel material with a high viscosity is dispensed to have a larger diameter, it could take longer to transform to its final shape (circular).
Since the diffusion of silicone oil into the PDMS monomer could strongly affect the channel diameter, which could lead to a smaller channel diameter, we increase the PDMS monomer viscosity before dispensing the silicone oil by a semi-curing step (a short-time UV exposure). Finally, the channels with a circularity as high as 0.96 are successfully formed, with quite a high reproducibility.
3.2. Pressurisation Experiment
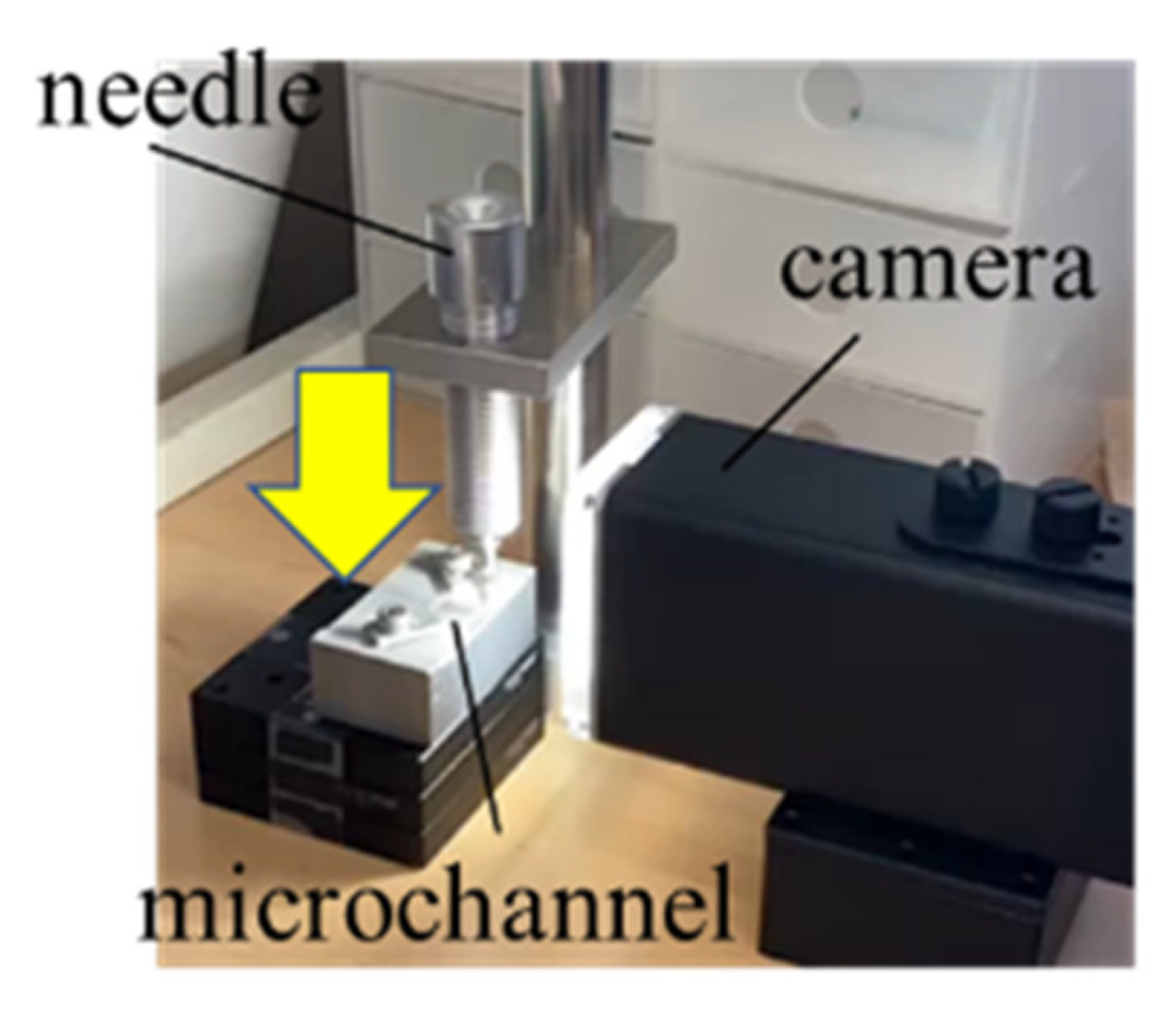
In order to investigate the on-off valve functionality in the fabricated microchannels, we visually observe the channel cross-sectional collapse when applying a load. Here, we find it necessary for the cured PDMS to exhibit low elastic modulus in order to completely close the flow lines. For a lower elastic modulus, we reduce the amount of initiator involved in the base monomer to 5:1 and 10:1 from the specified feed ratio of 1:1. The relationship between the on-off valve functionality and the elastic modulus (initiator feed ratio) is experimentally investigated using the apparatus shown in
Figure 5.
Figure 6 shows photos of the cross-sectional shape of the pressurized microchannels. We confirm the that the channels are completely closed when pressure is uniformly applied across the cross section. The channels when monomer feed ratios are A:B = 5:1 and 10:1 require less load (only about 20% of the load required for 1:1) compared to that of 1:1, from which we find that the modulus of elasticity of the cured PDMS is lowered by reducing the amount of initiator included in KER-4690-B, and the channels can close with a lower pressure, as shown in
Figure 6. However, by decreasing the initiator ratio, the monomer viscosity increases (KER-4690-A has higher viscosity than KER-4690-B), and the cross-sectional shape of the formed microchannels tends to deviate from a perfect circle. Furthermore, it takes longer to cure the PDMS monomer with a decrease of the initiator ratio. Therefore, a ratio between 3:1 and 5:1 is found to be optimal for fabrication.
Next, we investigate the minimum load required for complete closure of the channel with circular and non-circular cross-sections using the same apparatus shown in
Figure 5. Then, it is confirmed that the channels with the circular and vertically long oval cross-sections require loads of 14.0 g and 52.8 g, respectively. In the case of oval cross-sections, the applied pressure is likely to disperse, and the effective load to close the channel tends to be lower. On the other hand, the load is uniformly applied to the channel with a circular cross-section, and therefore the channel can completely close even with a lower pressure. We also confirm that circular cross-sectional microchannels are closed with approximately one fourth of the pressure required for the channel with vertically long oval cross-section. Therefore, circular channels are more easily closed, which are experimentally verified by comparing with oval channels as it was theoretically expected in Ref. [
1], although circular cross-section channels might not necessarily be the solitary candidate for on-off valves.
3.3. Redesigned Microchannels with On-Off Valves
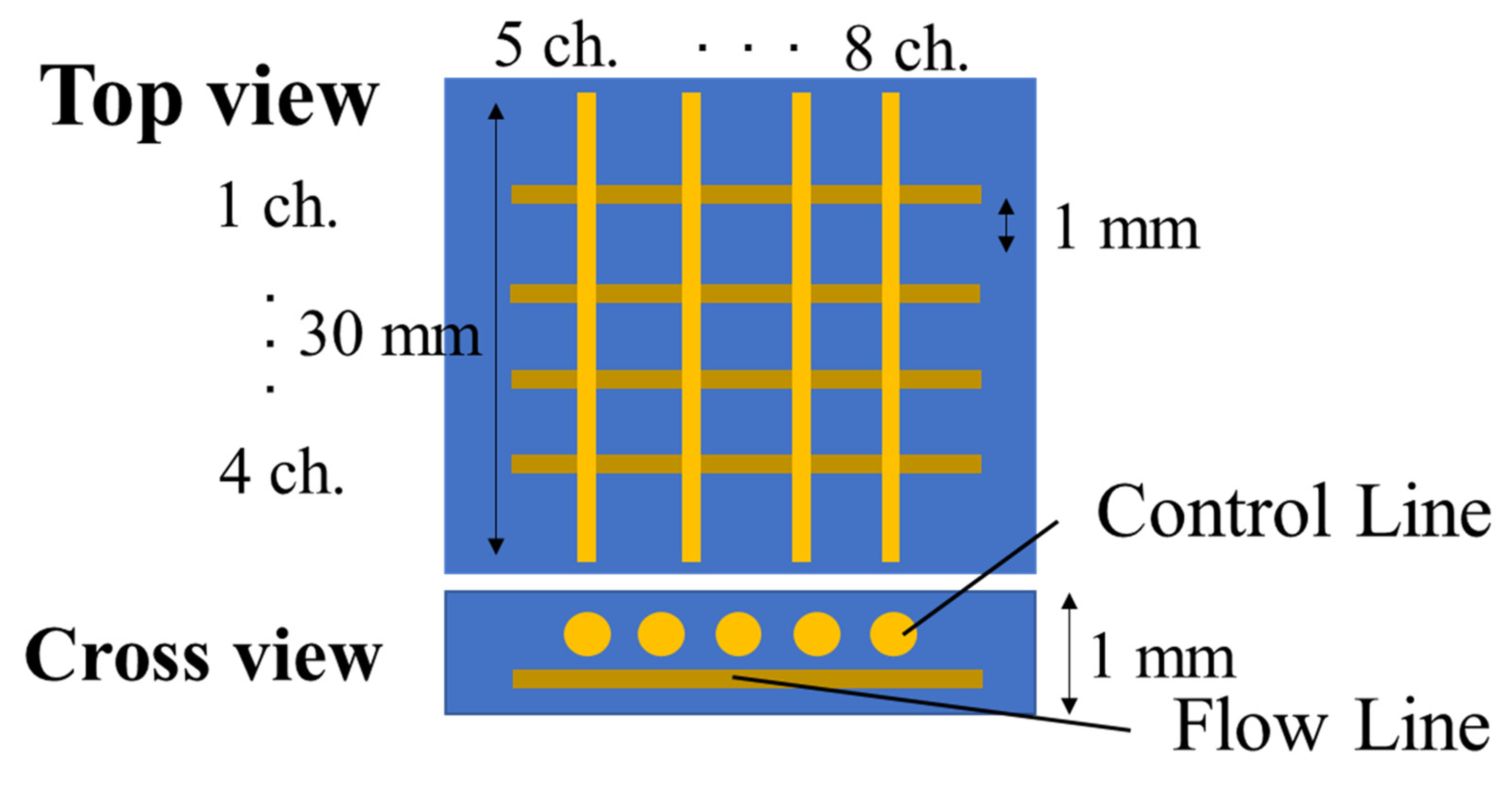
The ability to arrange channels three-dimensionally is one of the greatest advantages of the Mosquito method. Here, microchannels with a “3-D lattice” structure are designed and fabricated using a PDMS monomer with a low initiator feed ratio. In normal lattice structures, microchannels that are aligned on one plane must cross perpendicularly. In this microchannel, we would not be able to separate the flow and control lines. Therefore, the channels are formed on two separated layers: the lower layer channels are aligned in parallel to the
x-axis, while the upper layer channels are in parallel to the
y-axis. The upper and lower channels are utilized for the control and flow lines, respectively, as shown in
Figure 7. They are designed such that the flow lines are nearby the inflated control lines.
Although we confirmed in
Section 3.2 that the monomer feed ratio (A:B) should be 5:1, it influenced the monomer viscosity as well. When the viscosity of the PDMS monomer increased with less initiator, the channels’ cross-sections tended to be less circular. We also found in
Section 3.1 that the polarity and viscosity of the silicone oil were also key for the cross-sectional shape. The designed 3-D lattice structure is formed by scanning the needle in the
x- and
y-directions with different needle tip heights. When a monomer feed-ratio of A and B is set to 5:1 whilst KF-50 is dispensed, a top view of the fabricated 3-D lattice channels is shown in
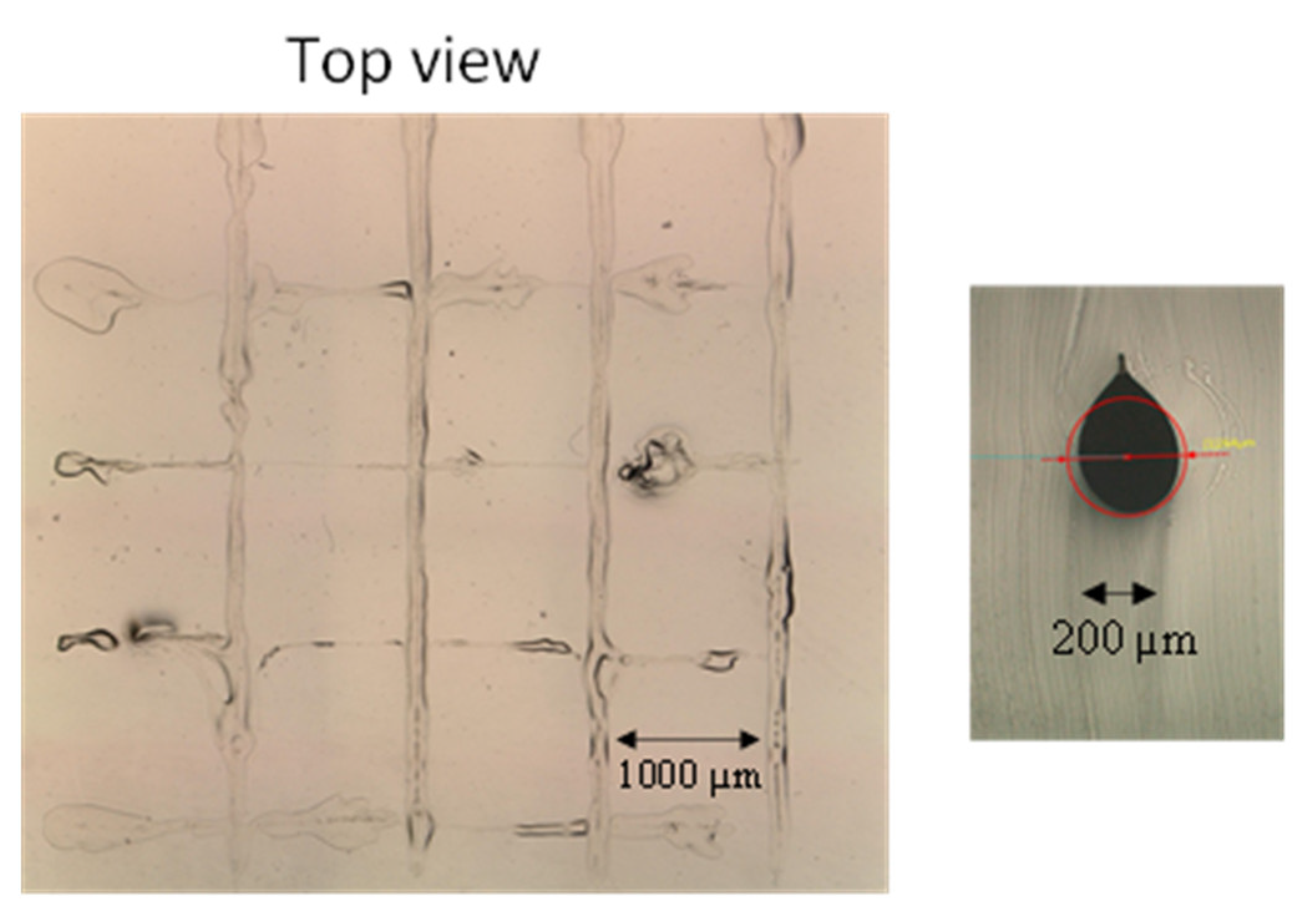
Figure 8. Although it is formed via the combination of A:B = 5:1 and KF-50, the structure is obviously distorted. To fabricate the 3-D lattice structure, the needle needs to scan in perpendicular directions for a longer time compared to the parallel monolayer structure. The viscosity of KF-50 is not high enough to maintain its cylindrical shape until the PDMS monomer is cured completely. In order to increase the curing speed, a feed ratio of A:B is set to 1:1 and 3:1. Top and cross-sectional views of the fabricated 3-D lattice channels are shown in
Figure 9a,b, respectively. By increasing the feed ratio of the initiator, the structure tends to be stable, and the designed lattice structure is visually confirmed. Hence, a monomer feed ratio of 3:1 should be selected, although the modulus of elasticity slightly increases compared to that of 5:1. However, the cross-section of the channels shown in
Figure 9b is not circular. The viscosity of KF-50 (3000 cP) is still low to maintain its circular cross-section under multi-directional monomer flow due to the two-layered needle scan. Therefore, in order to increase the viscosity of silicone oil, KF-96 (60,000 cP) is mixed with KF-50. Top and cross-sectional views of the fabricated 3-D lattice structure are in
Figure 10a,b, respectively, using the mixed silicone oils with weight ratios of KF-50 and KF-96 are 1:2 and 1:1. Here, a monomer feed ratio (A:B) of 3:1 is fixed for PDMS. In both mixed ratios, 3-D structures without disconnection and distortion are visually confirmed, whilst a cross-section in
Figure 10b is closer to a perfect circle than in
Figure 10a. Hence, a mixed ratio of 1:1 should be selected for the silicone oil.
Finally, the different channel diameters are designed for the channels on the lower and upper layers: the upper layer for the control line should be larger than the lower layer in order to pressurize the flow line completely.
Figure 11a,b are top and cross-sectional views of fabricated microchannels when the needle scan velocities for the lines of (flow, control) are set to (1, 2 mm/s) and (2, 4 mm/s), respectively. The channel diameter is theoretically estimated by Equation (1) derived from the Hagen–Poiseuille flow, based on the Naiver–Stokes Equation (2).
where
U,
p,
d,
L are the scan velocity, the dispensing pressure, the needle inner diameter, and the needle length, respectively.
From Equation (1), we find that the channel diameter is inversely proportional to the square-root of scan velocity. Since the scan velocity is twice as high for the lower layer channels, the channel diameter on the upper layer should be 1.4 times larger than that on the lower layer. Meanwhile, there is a concern that a higher scan velocity could disturb the channel arrangement due to the large monomer flow. Contrastingly, a higher scan velocity contributes to a shorter dispensing time for all the channels, which helps to maintain a cylindrical channel shape. Since silicone oil with a mixing ratio of 1:1 has both well-adjusted viscosity and polarity, higher scan velocities (2 and 4) mm/s can be selected for keeping the 3-D lattice structure with perfect circular cross-sections without deformation. From
Figure 11a, the diameters of the upper and lower channels formed are measured as 299 μm and 199 μm, respectively. The control line on the upper layer has a 1.5 times larger diameter than that of the flow line on the lower layer, as expected. The heights of the upper and lower channels that are dispensed are 526 μm and 290 μm, respectively. Although the vertical channel spacing between them is designed to be larger than 200 μm, it is narrowed to 39 μm due to the different scanning velocities, as shown in
Figure 11a. This vertical channel spacing is small enough to make them work as on-off valves.
3.4. Demonstration of Liquid-Flow
Finally, the channel closure experiment is conducted by actually flowing liquid into the fabricated microchannels with the “3-D lattice” structure to evaluate the functionality of the valve. Although several methods such as a micro vertically allocated SU-8 check valve or a microfabricated piston-less syringe pump to evaluate the valve performance have been reported, the method employed here utilizing the fluorescein solution is just an affordable method with our available equipment. For broader ranging analysis of the valve, we are willing to employ the other methods listed in the reviewer’s comment. In this setup, fluorescein water solution (1.0 × 10
−2 g/L) was injected into one of the flow lines, and the closure of the flow line was observed by detecting the fluorescence intensity from the fluorescein. The fluorescein solution liquid was injected into the flow line using a needle. Here, the needle outer diameter is 20 µm smaller than the channel diameter to provide a gap between them for the overflown solution when the flow line is closed. Meanwhile, nitrogen (N
2) gas was injected into one control line, and the pressure varied from 0 to 300 kPa to inflate it and thus close the flow line. Top views of the intersections of the flow and control lines under different N
2 gas flowing pressures are shown in
Figure 12. In
Figure 12, the flow line is aligned horizontally, as illustrated in
Figure 7. Under a 0-kPa pressure in
Figure 12, a high fluorescence intensity is observed from the intersection area marked with a red-coloured rectangle. However, the fluorescence intensity from the intersection decreases with the increase in N
2 gas pressure. Hence, the liquid flow rate was reduced by the inflated control line.
Subsequently, we analysed these top-view images to quantitatively evaluate the change in fluorescence intensity.
Figure 13 shows the number of pixels with respect to their brightness counted at the intersections in the red rectangle. When the fluorescent solution keeps flowing in the flow line under 0-kPa N
2 gas, the number of bright pixels is high due to the large amount of flowing fluorescein. On the other hand, if the control line is inflated with a 100-kPa N
2 pressure, the bright pixels decrease slightly, which is attributed to the fluorescein flow decrease. Then, with the increase in N
2 pressure in the control line, dark pixels increased, as shown in the top view under 300 kPa in
Figure 13, indicating a successful off-valve state. For comparison, two liquid flow rates, 1 and 5 µL/min were set to confirm that the valve could work under low flow rates. Even under higher flow rate (5 µL/min), the flow line can effectively be closed by inflating the control line under N
2 gas pressure.
From
Figure 14, the average number of bright pixels was statistically analysed, where the bright pixel number under no flow of N
2 (0 kPa) was defined as a 100% intensity. Contrastingly, it decreased to 53.5% under 300-kPa N
2 pressure as the minimum value. Meanwhile, if the peak number of bright pixels was analysed in the red rectangle, the intensity was found to decrease to 35.8% under 300-kPa N
2 pressure. Regarding the fluorescence intensity as the volume of liquid flowing in the flow line, 300-kPa N
2 pressure to the control can block approximately 40–50% of the flow volume independent of the liquid flow rate, confirming the capability of the 3-D lattice microchannels as an on-off valve.