Probing Italy: A Scanning Probe Microscopy Storyline
Abstract
1. Early History of SPM in Italy
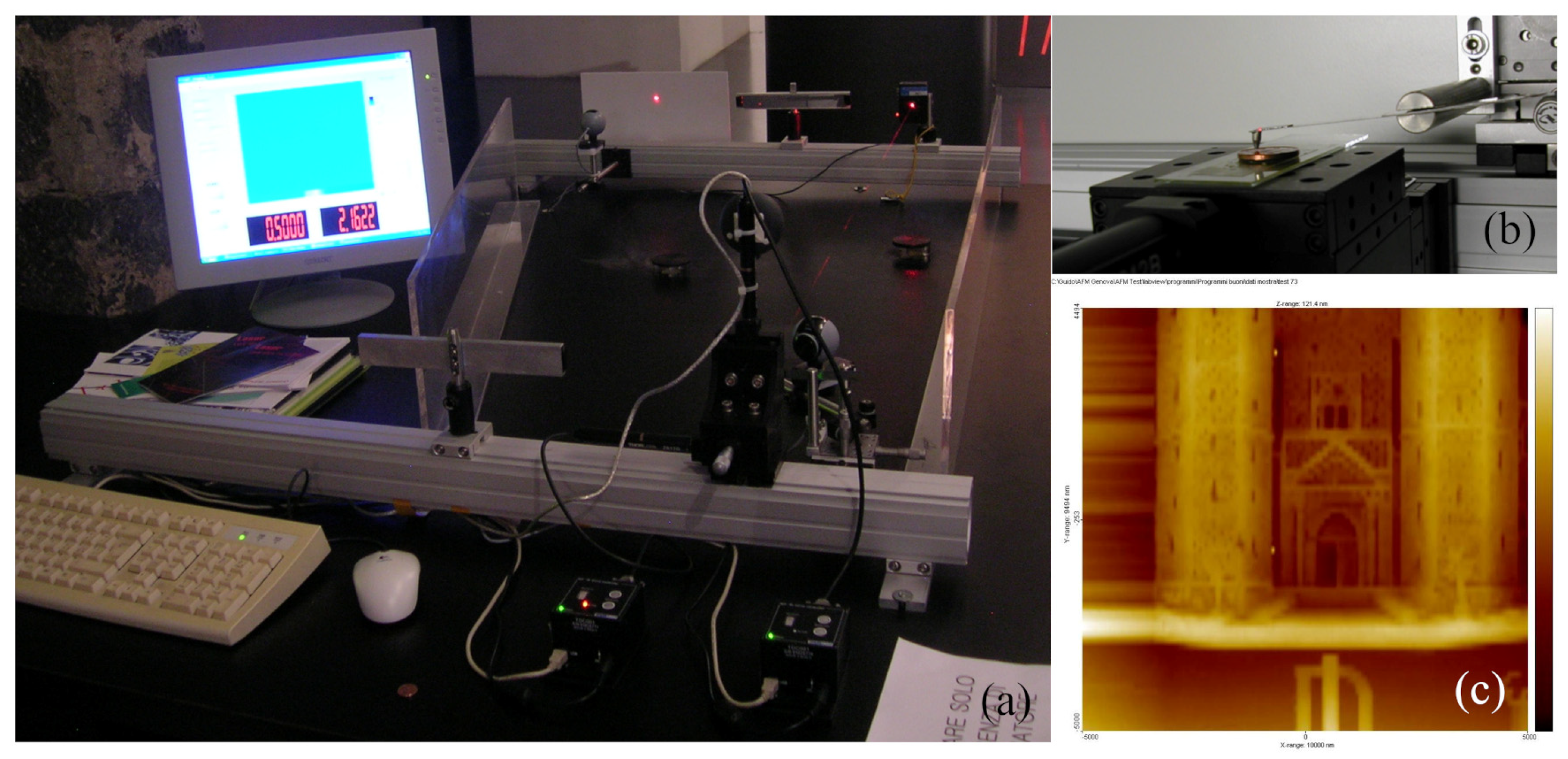
1.1. AFM and STM First Italian Prototypes
1.2. Earlier Experiments
1.3. Other Working Modes
2. Following Generations of Scanning Probe Microscopists
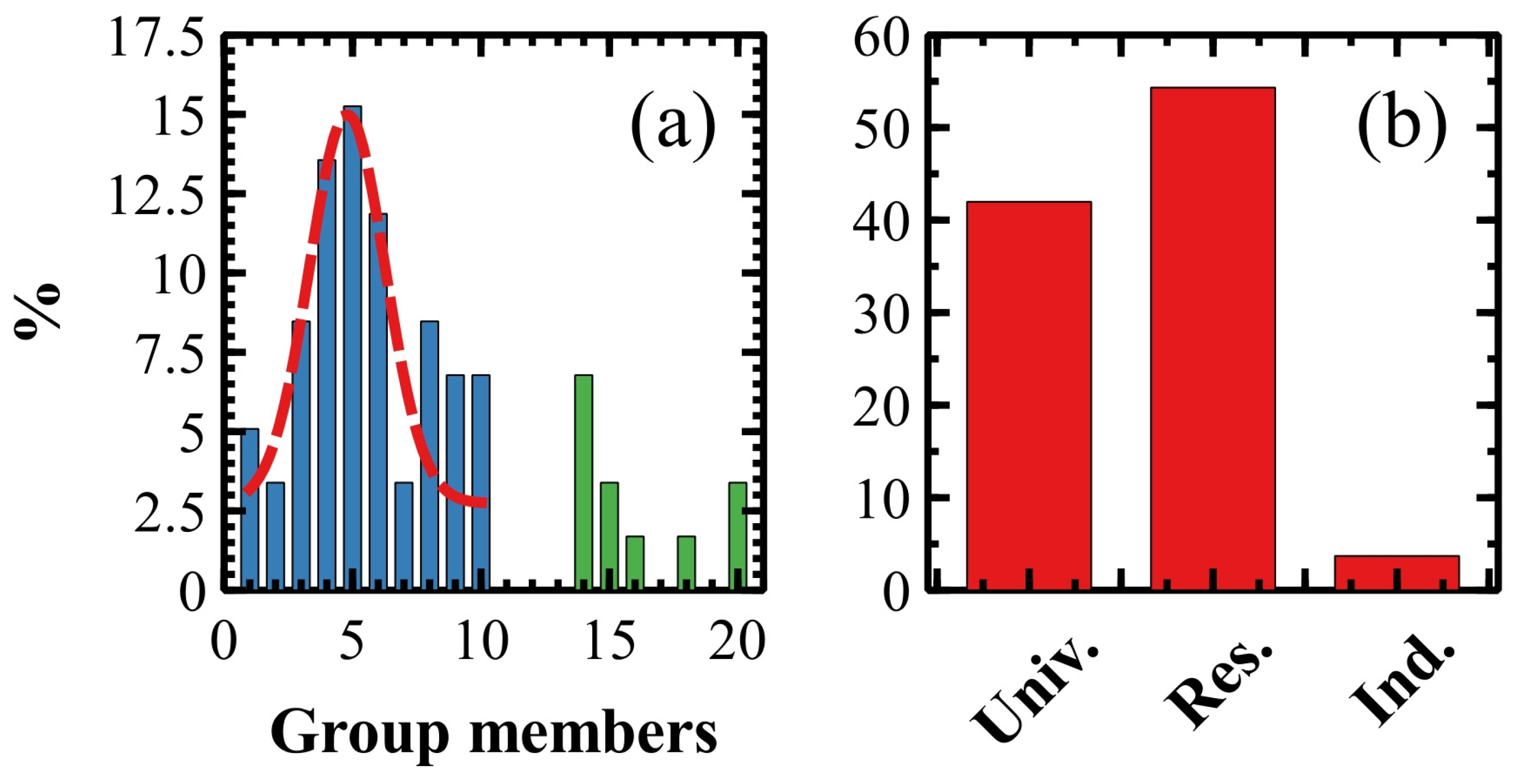
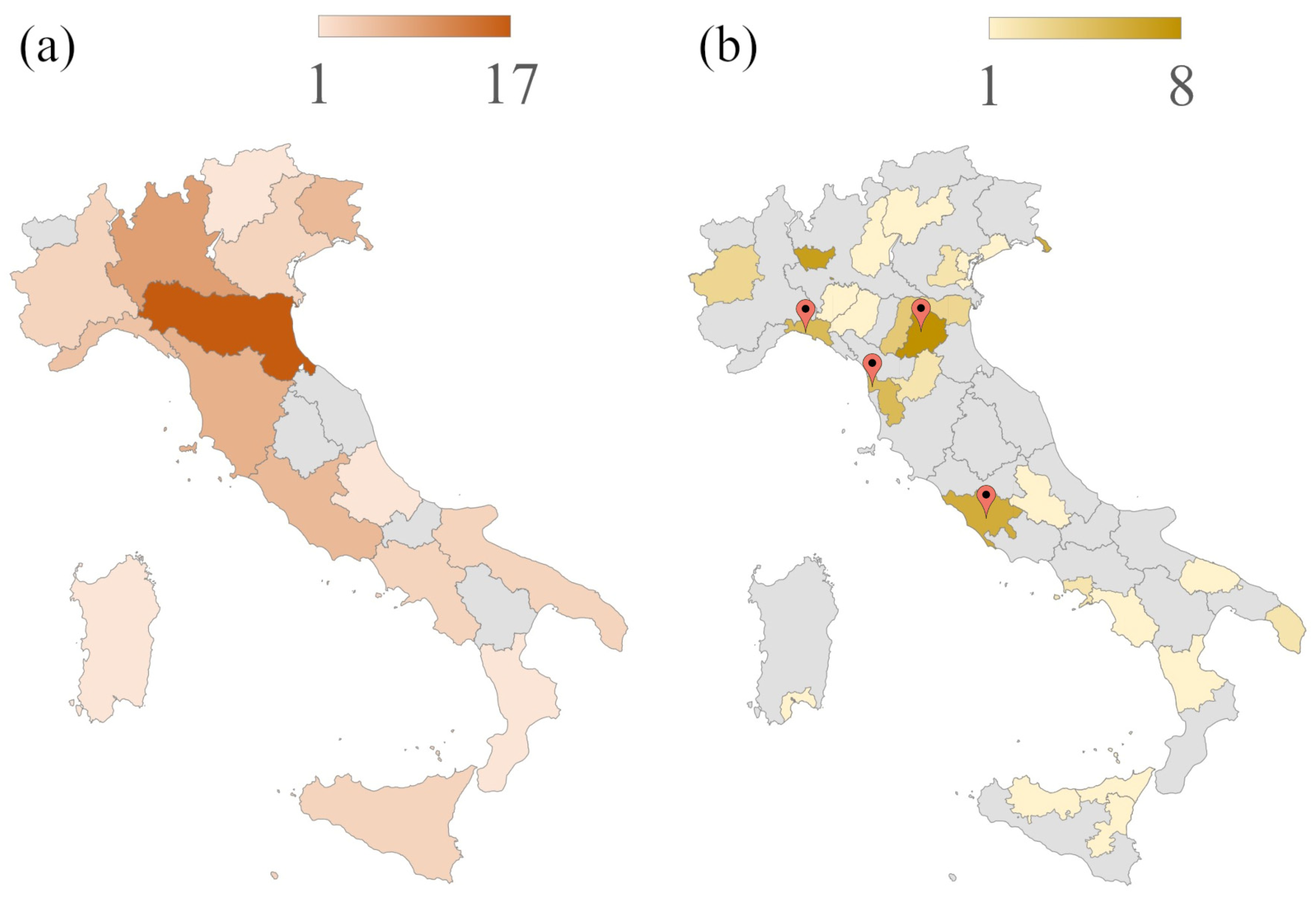
2.1. Anatomy of an Italian SPM Research Group
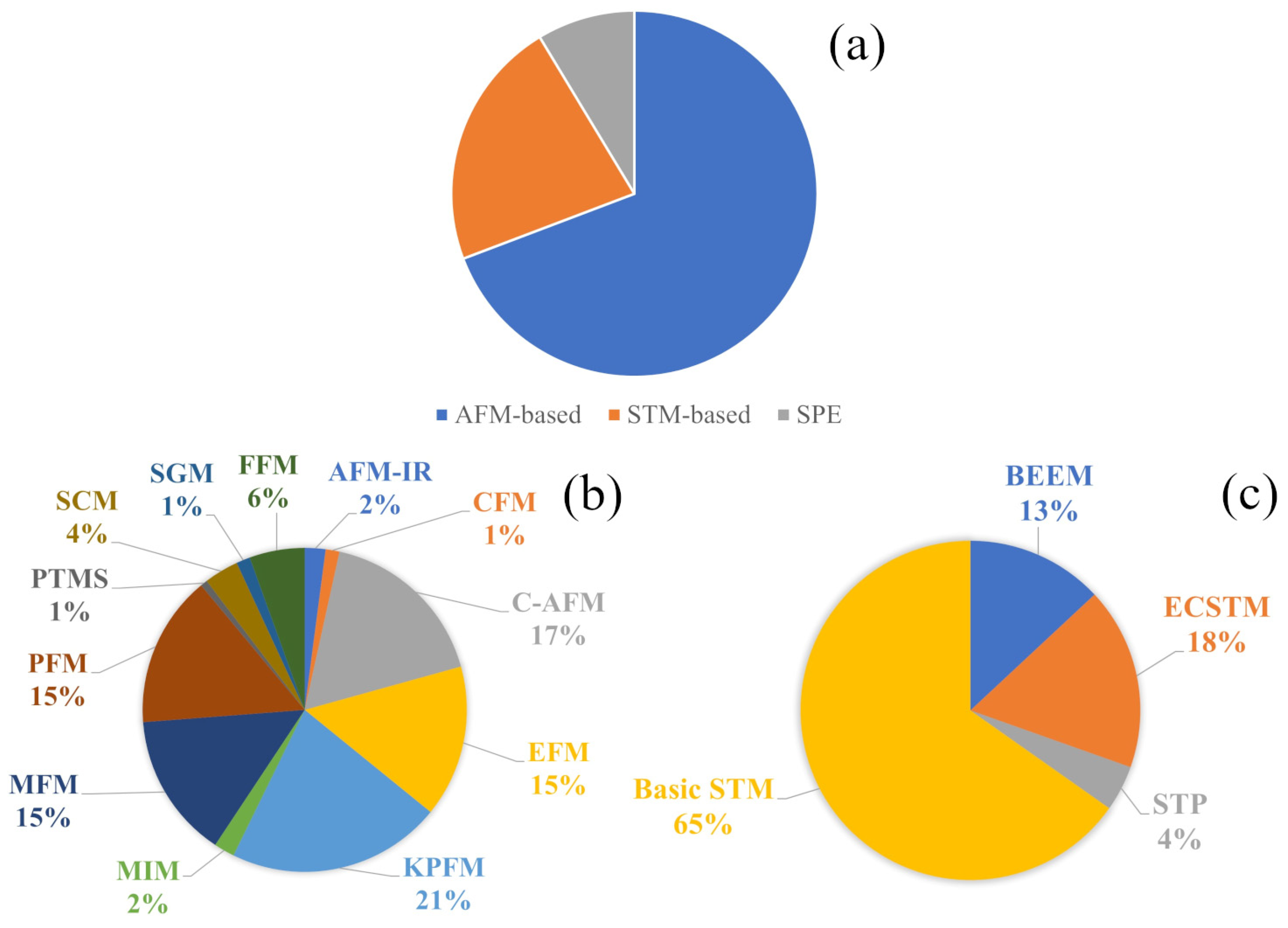
2.2. Microscopes and SPM Modes
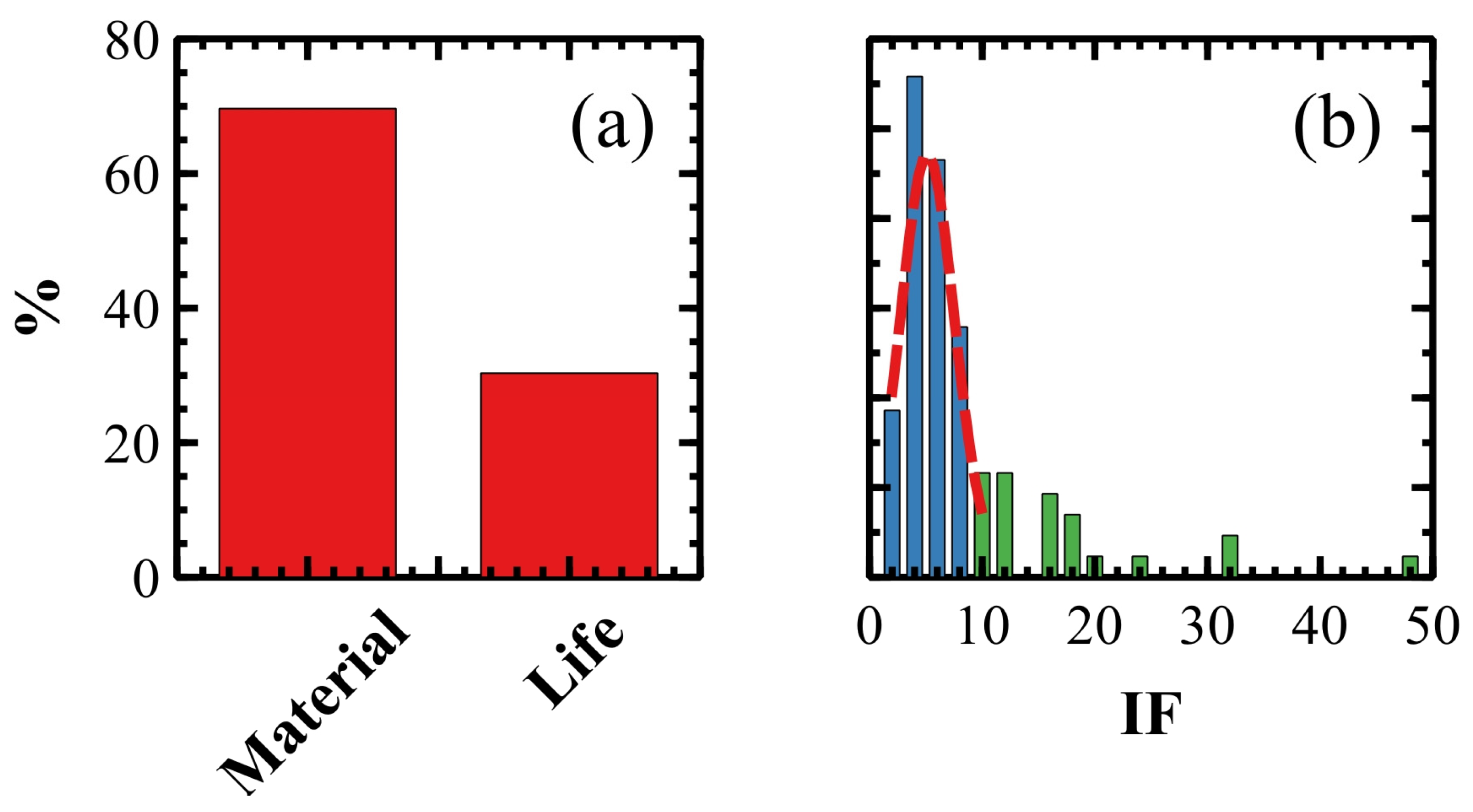
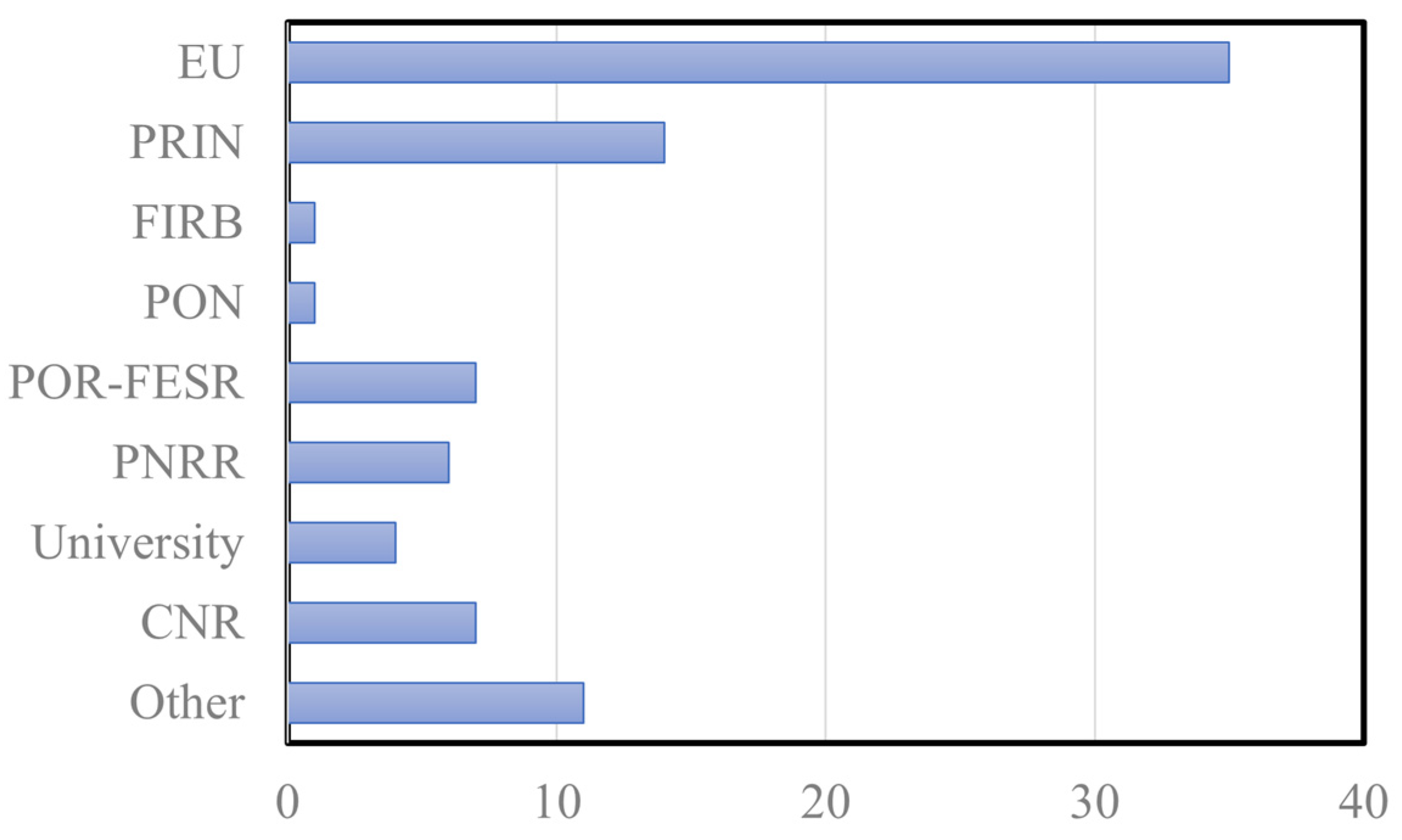
2.3. Scientific Research, Publications and Fundings of Italian SPM Groups
2.4. Education and Dissemination
3. History and Present of SPM SME in Italy
3.1. Technobiochip and Elbatech
3.2. A.P.E. Research
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binnig, G.; Rohrer, H.; Gerber, C.; Weibel, E. Tunneling through a Controllable Vacuum Gap. Appl. Phys. Lett. 1982, 40, 178–180. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Casalboni, M.; Stella, A. Gianfranco Chiarotti (1928–2017). Available online: https://www.sif.it/riviste/sif/sag/ricordo/chiarotti (accessed on 17 April 2023).
- Cricenti, A.; Selci, S.; Generosi, R.; Gori, E.; Chiarotti, G. A Graphite Study with a New Air Operating Scanning Tunnelling Microscope. J. Microsc. 1988, 152, 789–794. [Google Scholar] [CrossRef]
- Cricenti, A.; Selci, S.; Felici, A.C.; Generosi, R.; Gori, E.; Djaczenko, W.; Chiarotti, G. Molecular Structure of DNA by Scanning Tunneling Microscopy. Science 1989, 245, 1226–1227. [Google Scholar] [CrossRef]
- Allegrini, M.; Arpa, E.; Ascoli, C.; Baschieri, P.; Dinelli, F.; Frediani, C.; Labardi, M.; Lio, A.; Mariani, T.; Vanni, L. Scanning Probe Microscope with Interchangeable AFM-FFM and STM Heads. Nuovo Cim. D 1993, 15, 279–292. [Google Scholar] [CrossRef]
- Allegrini, M.; Ascoli, C.; Baschieri, P.; Dinelli, F.; Frediani, C.; Lio, A.; Mariani, T. Laser Thermal Effects on Atomic Force Microscope Cantilevers. Ultramicroscopy 1992, 42–44, 371–378. [Google Scholar] [CrossRef]
- Newton, I. Opticks, or, a Treatise of the Reflections, Refractions, Inflections, and Colours of Light; Landmarks of Science; W. and J. Innys: London, UK, 1718. [Google Scholar]
- Cluzel, B.; De Fornel, F. Frustrated Total Internal Reflection: The Newton Experiment Revisited. Photoniques 2022, 116, 32–37. [Google Scholar] [CrossRef]
- Allegrini, M.; Ascoli, C.; Gozzini, A. Measurements of Changes in Length by an Inhomogeneous Wave Device. Opt. Commun. 1971, 2, 435–437. [Google Scholar] [CrossRef]
- Dürig, U.; Pohl, D.W.; Rohner, F. Near-field Optical-scanning Microscopy. J. Appl. Phys. 1986, 59, 3318–3327. [Google Scholar] [CrossRef]
- Mate, C.M.; McClelland, G.M.; Erlandsson, R.; Chiang, S. Atomic-Scale Friction of a Tungsten Tip on a Graphite Surface. Phys. Rev. Lett. 1987, 59, 1942–1945. [Google Scholar] [CrossRef]
- Ascoli, C.; Dinelli, F.; Frediani, C.; Petracchi, D.; Salerno, M.; Labardi, M.; Allegrini, M.; Fuso, F. Normal and Lateral Forces in Scanning Force Microscopy. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. Process Meas. Phenom. 1994, 12, 1642–1645. [Google Scholar] [CrossRef]
- Alzetta, G.; Arimondo, E.; Ascoli, C.; Gozzini, A. Paramagnetic Resonance Experiments at Low Fields with Angular-Momentum Detection. Nuovo Cim. B 1967, 52, 392–402. [Google Scholar] [CrossRef]
- Ascoli, C.; Baschieri, P.; Frediani, C.; Lenci, L.; Martinelli, M.; Alzetta, G.; Celli, R.M.; Pardi, L. Micromechanical Detection of Magnetic Resonance by Angular Momentum Absorption. Appl. Phys. Lett. 1996, 69, 3920–3922. [Google Scholar] [CrossRef]
- B Cappella; P Baschieri; C Frediani; P Miccoli; C Ascoli Improvements in AFM Imaging of the Spatial Variation of Force—Distance Curves: On-Line Images. Nanotechnology 1997, 8, 82. [CrossRef]
- Samorí, B.; Siligardi, G.; Quagliariello, C.; Weisenhorn, A.L.; Vesenka, J.; Bustamante, C.J. Chirality of DNA Supercoiling Assigned by Scanning Force Microscopy. Proc. Natl. Acad. Sci. USA 1993, 90, 3598–3601. [Google Scholar] [CrossRef]
- Samorì, B. Stretching Single Molecules Along Unbinding and Unfolding Pathways with the Scanning Force Microscope. Chem.-A Eur. J. 2000, 6, 4249–4255. [Google Scholar] [CrossRef]
- Samorì, B.; Zuccheri, G.; Baschieri, P. Protein Unfolding and Refolding under Force: Methodologies for Nanomechanics. ChemPhysChem 2005, 6, 29–34. [Google Scholar] [CrossRef]
- Hansma, P.K.; Drake, B.; Marti, O.; Gould, S.A.C.; Prater, C.B. The Scanning Ion-Conductance Microscope. Science 1989, 243, 641–643. [Google Scholar] [CrossRef]
- Pellegrino, M.; Orsini, P.; Pellegrini, M.; Baschieri, P.; Dinelli, F.; Petracchi, D.; Tognoni, E.; Ascoli, C. Integrated SICM-AFM-Optical Microscope to Measure Forces Due to Hydrostatic Pressure Applied to a Pipette. Micro Nano Lett. 2012, 7, 317–320. [Google Scholar] [CrossRef]
- Pellegrino, M.; Pellegrini, M.; Orsini, P.; Tognoni, E.; Ascoli, C.; Baschieri, P.; Dinelli, F. Measuring the Elastic Properties of Living Cells through the Analysis of Current–Displacement Curves in Scanning Ion Conductance Microscopy. Pflügers Arch.-Eur. J. Physiol. 2012, 464, 307–316. [Google Scholar] [CrossRef]
- Pellegrino, M.; Orsini, P.; Pellegrini, M.; Tognoni, E.; Ascoli, C.; Baschieri, P.; Dinelli, F. Scanning Ion Conductance Microscopy (SICM): From Measuring Cell Mechanical Properties to Guiding Neuron Growth. Opt. Methods Inspection Charact. Imaging Biomater. 2013, 8792, 200–207. [Google Scholar]
- Hu, Y.; Das, A.; Hecht, M.H.; Scoles, G. Nanografting De Novo Proteins onto Gold Surfaces. Langmuir 2005, 21, 9103–9109. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Sun, Q.; Selloni, A.; Scoles, G. Side-by-Side Characterization of Electron Tunneling through Monolayers of Isomeric Molecules: A Combined Experimental and Theoretical Study. J. Phys. Chem. B 2006, 110, 24797–24801. [Google Scholar] [CrossRef]
- Hulla, J.E.; Sahu, S.C.; Hayes, A.W. Nanotechnology: History and Future. Hum. Exp. Toxicol. 2015, 34, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, C.; LimeSurvey Project Team. LimeSurvey: An Open Source Survey Tool; LimeSurvey Project Team: Hamburg, Germany, 2012. [Google Scholar]
- Casinelli, M. New Technique of Sample Preparation for the Morphological Investigation of Ziegler-Natta Supports and Catalysts. Available online: https://analyticalscience.wiley.com/do/10.1002/micro.2685 (accessed on 17 April 2023).
- Pergolini, S.; Valdrè, U. Study of Image Contrast Effects and Field Trends in Magnetic Recording Media by Static Magnetic Force Microscopy. Microsc. Microanal. Microstruct. 1995, 6, 665–672. [Google Scholar] [CrossRef]
- Biscarini, F.; Zamboni, R.; Samorí, P.; Ostoja, P.; Taliani, C. Growth of Conjugated Oligomer Thin Films Studied by Atomic-Force Microscopy. Phys. Rev. B 1995, 52, 14868–14877. [Google Scholar] [CrossRef]
- Conti, R.; Rusponi, S.; Pagnotta, D.; Boragno, C.; Valbusa, U. A New UHV Variable Temperature STM for Gas Adsorption Studies. Vacuum 1997, 48, 639–641. [Google Scholar] [CrossRef]
- Kelly, K.F.; Sarkar, D.; Prato, S.; Resh, J.S.; Hale, G.D.; Halas, N.J. Direct Observation of Fullerene-adsorbed Tips by Scanning Tunneling Microscopy. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. Process Meas. Phenom. 1996, 14, 593–596. [Google Scholar] [CrossRef]
- Prato, S.; Floreano, L.; Cvetko, D.; De Renzi, V.; Morgante, A.; Modesti, S.; Biscarini, F.; Zamboni, R.; Taliani, C. Anisotropic Ordered Planar Growth of α-Sexithienyl Thin Films. J. Phys. Chem. B 1999, 103, 7788–7795. [Google Scholar] [CrossRef]
- Wikipedia Contributors Scanning Probe Microscopy—Wikipedia, The Free Encyclopedia 2023. Available online: https://en.wikipedia.org/wiki/Scanning_probe_microscopy (accessed on 17 April 2023).
- Battistella, A.; Andolfi, L.; Stebel, M.; Ciubotaru, C.; Lazzarino, M. Investigation on the Change of Spermatozoa Flagellar Beating Forces before and after Capacitation. Biomater. Adv. 2023, 145, 213242. [Google Scholar] [CrossRef]
- Ingham, J.; Craig, T.; Smith, C.I.; Varro, A.; Pritchard, D.M.; Barrett, S.D.; Martin, D.S.; Harrison, P.; Unsworth, P.; Kumar, J.D.; et al. Submicron Infrared Imaging of an Oesophageal Cancer Cell with Chemical Specificity Using an IR-FEL. Biomed. Phys. Eng. Express 2019, 5, 15009. [Google Scholar] [CrossRef]
- Abiedh, K.; Dhanabalan, B.; Kutkan, S.; Lauciello, S.; Pasquale, L.; Toma, A.; Salerno, M.; Arciniegas, M.P.; Hassen, F.; Krahne, R. Surface-Dependent Properties and Tunable Photodetection of CsPbBr3 Microcrystals Grown on Functional Substrates. Adv. Opt. Mater. 2022, 10, 2101807. [Google Scholar] [CrossRef]
- Vecchi, P.; Armaroli, G.; Di Sabatino, M.; Cavalcoli, D. Iron Related Precipitates in Multicrystalline Silicon by Conductive Atomic Force Microscopy. Mater. Sci. Semicond. Process 2021, 129, 105789. [Google Scholar] [CrossRef]
- Albonetti, C.; Chiodini, S.; Annibale, P.; Stoliar, P.; Martinez, R.V.; Garcia, R.; Biscarini, F. Quantitative Phase-Mode Electrostatic Force Microscopy on Silicon Oxide Nanostructures. J. Microsc. 2020, 280, 252–269. [Google Scholar] [CrossRef] [PubMed]
- Scaini, D.; Biscarini, F.; Casalis, L.; Albonetti, C. Substrate Roughness Influence on the Order of Nanografted Self-Assembled Monolayers. Chem. Phys. Lett. 2022, 803, 139819. [Google Scholar] [CrossRef]
- De Bastiani, M.; Armaroli, G.; Jalmood, R.; Ferlauto, L.; Li, X.; Tao, R.; Harrison, G.T.; Eswaran, M.K.; Azmi, R.; Babics, M.; et al. Mechanical Reliability of Fullerene/Tin Oxide Interfaces in Monolithic Perovskite/Silicon Tandem Cells. ACS Energy Lett. 2022, 7, 827–833. [Google Scholar] [CrossRef]
- Chianese, F.; Fusco, S.; Barra, M.; Chiarella, F.; Carella, A.; Cassinese, A. Space-Charge Accumulation and Band Bending at Conductive P3HT/PDIF-CN2 Interfaces Investigated by Scanning-Kelvin Probe Microscopy. J. Mater. Chem. C 2021, 9, 17143–17151. [Google Scholar] [CrossRef]
- Bours, L.; Guiducci, S.; Mreńca-Kolasińska, A.; Szafran, B.; Maan, J.C.; Heun, S. Manipulating Quantum Hall Edge Channels in Graphene through Scanning Gate Microscopy. Phys. Rev. B 2017, 96, 195423. [Google Scholar] [CrossRef]
- Calavalle, F.; Zaccaria, M.; Selleri, G.; Cramer, T.; Fabiani, D.; Fraboni, B. Piezoelectric and Electrostatic Properties of Electrospun PVDF-TrFE Nanofibers and Their Role in Electromechanical Transduction in Nanogenerators and Strain Sensors. Macromol. Mater. Eng. 2020, 305, 2000162. [Google Scholar] [CrossRef]
- Serri, M.; Cucinotta, G.; Poggini, L.; Serrano, G.; Sainctavit, P.; Strychalska-Nowak, J.; Politano, A.; Bonaccorso, F.; Caneschi, A.; Cava, R.J.; et al. Enhancement of the Magnetic Coupling in Exfoliated CrCl3 Crystals Observed by Low-Temperature Magnetic Force Microscopy and X-Ray Magnetic Circular Dichroism. Adv. Mater. 2020, 32, 2000566. [Google Scholar] [CrossRef]
- Spizzo, F.; Greco, G.; Del Bianco, L.; Coïsson, M.; Pugno, N.M. Magnetostrictive and Electroconductive Stress-Sensitive Functional Spider Silk. Adv. Funct. Mater. 2022, 32, 2207382. [Google Scholar] [CrossRef]
- Veronesi, S.; Commodo, M.; Basta, L.; De Falco, G.; Minutolo, P.; Kateris, N.; Wang, H.; D’Anna, A.; Heun, S. Morphology and Electronic Properties of Incipient Soot by Scanning Tunneling Microscopy and Spectroscopy. Combust. Flame 2022, 243, 111980. [Google Scholar] [CrossRef]
- Serrano, G.; Poggini, L.; Briganti, M.; Sorrentino, A.L.; Cucinotta, G.; Malavolti, L.; Cortigiani, B.; Otero, E.; Sainctavit, P.; Loth, S.; et al. Quantum Dynamics of a Single Molecule Magnet on Superconducting Pb(111). Nat. Mater. 2020, 19, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Turco, E.; Stredansky, M.; Costantini, R.; Martinez, J.A.; Dell’Angela, M.; Zerbato, E.; Toffoli, D.; Fronzoni, G.; Morgante, A.; Floreano, L.; et al. On-Surface Synthesis of Boroxine-Based Molecules. Chemistry 2021, 3, 1401–1410. [Google Scholar] [CrossRef]
- Buzio, R.; Gerbi, A.; He, Q.; Qin, Y.; Mu, W.; Jia, Z.; Tao, X.; Xu, G.; Long, S. Benchmarking β-Ga2O3 Schottky Diodes by Nanoscale Ballistic Electron Emission Microscopy. Adv. Electron. Mater. 2020, 6, 1901151. [Google Scholar] [CrossRef]
- Bussetti, G.; Filoni, C.; Li Bassi, A.; Bossi, A.; Campione, M.; Orbelli Biroli, A.; Castiglioni, C.; Trabattoni, S.; De Rosa, S.; Tortora, L.; et al. Driving Organic Nanocrystals Dissolution through Electrochemistry. ChemistryOpen 2021, 10, 748–755. [Google Scholar] [CrossRef]
- Bartolini, L.; Malferrari, M.; Lugli, F.; Zerbetto, F.; Paolucci, F.; Pelicci, P.G.; Albonetti, C.; Rapino, S. Interaction of Single Cells with 2D Organic Monolayers: A Scanning Electrochemical Microscopy Study. ChemElectroChem 2018, 5, 2975–2981. [Google Scholar] [CrossRef]
- Micheletti, C.; Dini, V.A.; Carlotti, M.; Fuso, F.; Genovese, D.; Zaccheroni, N.; Gualandi, C.; Pucci, A. Blending or Bonding? Mechanochromism of an Aggregachromic Mechanophore in a Thermoplastic Elastomer. ACS Appl. Polym. Mater. 2023, 5, 1545–1555. [Google Scholar] [CrossRef]
- D’Andrea, C.; Foti, A.; Cottat, M.; Banchelli, M.; Capitini, C.; Barreca, F.; Canale, C.; de Angelis, M.; Relini, A.; Maragò, O.M.; et al. Nanoscale Discrimination between Toxic and Nontoxic Protein Misfolded Oligomers with Tip-Enhanced Raman Spectroscopy. Small 2018, 14, 1800890. [Google Scholar] [CrossRef]
- Foti, A.; Barreca, F.; Fazio, E.; D’Andrea, C.; Matteini, P.; Maragò, O.M.; Gucciardi, P.G. Low Cost Tips for Tip-Enhanced Raman Spectroscopy Fabricated by Two-Step Electrochemical Etching of 125 Μm Diameter Gold Wires. Beilstein J. Nanotechnol. 2018, 9, 2718–2729. [Google Scholar] [CrossRef]
- Dinelli, F.; Fabbri, F.; Forti, S.; Coletti, C.; Kolosov, O.V.; Pingue, P. Scanning Probe Spectroscopy of Ws2/Graphene van Der Waals Heterostructures. Nanomaterials 2020, 10, 2494. [Google Scholar] [CrossRef] [PubMed]
- Dinelli, F.; Pingue, P.; Kay, N.D.; Kolosov, O. V Subsurface Imaging of Two-Dimensional Materials at the Nanoscale. Nanotechnology 2017, 28, 85706. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, S.; Dinelli, F.; Martinez, N.F.; Donati, S.; Albonetti, C. Identification of Ultra-Thin Molecular Layers atop Monolayer Terraces in Sub-Monolayer Organic Films with Scanning Probe Microscopy. Ultramicroscopy 2022, 240, 113598. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, A.; Devlin, R.C.; Capasso, F.; Wilson, W.L. Observation of Nanoscale Refractive Index Contrast via Photoinduced Force Microscopy. ACS Photonics 2017, 4, 846–851. [Google Scholar] [CrossRef]
- Di Giorgio, C.; Blundo, E.; Pettinari, G.; Felici, M.; Bobba, F.; Polimeni, A. Mechanical, Elastic, and Adhesive Properties of Two-Dimensional Materials: From Straining Techniques to State-of-the-Art Local Probe Measurements. Adv. Mater. Interfaces 2022, 9, 2102220. [Google Scholar] [CrossRef]
- Angeloni, L.; Reggente, M.; Passeri, D.; Natali, M.; Rossi, M. Identification of Nanoparticles and Nanosystems in Biological Matrices with Scanning Probe Microscopy. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1521. [Google Scholar] [CrossRef]
- W., J. What’s a Good Impact Factor (Ranking in 27 Categories). 2022. Available online: https://www.scijournal.org/articles/good-impact-factor (accessed on 17 April 2023).
- Cui, X. Journal Impact Factor, Trend and Distribution. Available online: https://www.biz-genius.com/journal-impact-factor-trend-and-distribution/ (accessed on 17 April 2023).
- Ferbel, L.; Veronesi, S.; Heun, S. Rb-Induced (3 × 1) and (6 × 1) Reconstructions on Si(111)-(7 × 7): A LEED and STM Study. Surf. Sci. 2022, 718, 122011. [Google Scholar] [CrossRef]
- Trainer, D.J.; Nieminen, J.; Bobba, F.; Wang, B.; Xi, X.; Bansil, A.; Iavarone, M. Visualization of Defect Induced In-Gap States in Monolayer MoS2. Npj 2D Mater. Appl. 2022, 6, 13. [Google Scholar] [CrossRef]
- Grazianetti, C.; Cinquanta, E.; Tao, L.; De Padova, P.; Quaresima, C.; Ottaviani, C.; Akinwande, D.; Molle, A. Silicon Nanosheets: Crossover between Multilayer Silicene and Diamond-like Growth Regime. ACS Nano 2017, 11, 3376–3382. [Google Scholar] [CrossRef]
- Schio, L.; Bavdek, G.; Grazioli, C.; Gutiérrez Bolaños, C.; Goldoni, A.; Vittadini, A.; Tormen, M.; Floreano, L. Role of Axial Coordination in the Adsorption Configuration of M(II)-Tetraphenylporphyrins (M = Co, Ni, Cu, Zn) on r-TiO2 (110). Appl. Surf. Sci. 2023, 616, 156548. [Google Scholar] [CrossRef]
- Buzio, R.; Gerbi, A.; Bernini, C.; Repetto, L.; Vanossi, A. Graphite Superlubricity Enabled by Triboinduced Nanocontacts. Carbon 2021, 184, 875–890. [Google Scholar] [CrossRef]
- Raimondo, L.; Trabattoni, S.; Sassella, A. Control of Post-Growth Processes for the Selection of Metallo-Tetraphenylporphyrin Nanowires. Phys. Chem. Chem. Phys. 2019, 21, 8482–8488. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Chiarella, F.; Barra, M.; Chianese, F.; Kubozono, Y.; Cassinese, A. Balanced Ambipolar Charge Transport in Phenacene/Perylene Heterojunction-Based Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2021, 13, 8631–8642. [Google Scholar] [CrossRef]
- Ciambriello, L.; Cavaliere, E.; Vassalini, I.; Alessandri, I.; Ferroni, M.; Leoncino, L.; Brescia, R.; Gavioli, L. Role of Electrode Thickness in NiFe Nanogranular Films for Oxygen Evolution Reaction. J. Phys. Chem. C 2022, 126, 21759–21770. [Google Scholar] [CrossRef]
- Beltrami, M.; Zilio, S.D.; Kapun, G.; Ciubotaru, C.D.; Rigoni, F.; Lazzarino, M.; Sbaizero, O. Surface Roughness Control in Nanolaminate Coatings of Chromium and Tungsten Nitrides. Micro Nano Eng. 2022, 14, 100107. [Google Scholar] [CrossRef]
- Bystrenova, E.; Bednarikova, Z.; Barbalinardo, M.; Albonetti, C.; Valle, F.; Gazova, Z. Amyloid Fragments and Their Toxicity on Neural Cells. Regen. Biomater. 2019, 6, 121–127. [Google Scholar] [CrossRef]
- Perissinotto, F.; Rondelli, V.; Senigagliesi, B.; Brocca, P.; Almásy, L.; Bottyán, L.; Merkel, D.G.; Amenitsch, H.; Sartori, B.; Pachler, K.; et al. Structural Insights into Fusion Mechanisms of Small Extracellular Vesicles with Model Plasma Membranes. Nanoscale 2021, 13, 5224–5233. [Google Scholar] [CrossRef]
- Balleza, D.; Mescola, A.; Marín–Medina, N.; Ragazzini, G.; Pieruccini, M.; Facci, P.; Alessandrini, A. Complex Phase Behavior of GUVs Containing Different Sphingomyelins. Biophys. J. 2019, 116, 503–517. [Google Scholar] [CrossRef]
- Ulfo, L.; Cantelli, A.; Petrosino, A.; Costantini, P.E.; Nigro, M.; Starinieri, F.; Turrini, E.; Zadran, S.K.; Zuccheri, G.; Saporetti, R.; et al. Orthogonal Nanoarchitectonics of M13 Phage for Receptor Targeted Anticancer Photodynamic Therapy. Nanoscale 2022, 14, 632–641. [Google Scholar] [CrossRef]
- Figuereido, I.; Paiotta, A.; Magro, R.D.; Tinelli, F.; Corti, R.; Re, F.; Cassina, V.; Caneva, E.; Nicotra, F.; Russo, L. A New Approach for Glyco-Functionalization of Collagen-Based Biomaterials. Int. J. Mol. Sci. 2019, 20, 1747. [Google Scholar] [CrossRef]
- Gagni, P.; Romanato, A.; Bergamaschi, G.; Bettotti, P.; Vanna, R.; Piotto, C.; Morasso, C.F.; Chiari, M.; Cretich, M.; Gori, A. A Self-Assembling Peptide Hydrogel for Ultrarapid 3D Bioassays. Nanoscale Adv. 2019, 1, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Cabada, T.; Ruben, M.; El Merhie, A.; Proietti Zaccaria, R.; Alabastri, A.; Petrini, E.M.; Barberis, A.; Salerno, M.; Crepaldi, M.; Davis, A.; et al. Electrostatic Polarization Fields Trigger Glioblastoma Stem Cell Differentiation. Nanoscale Horiz. 2023, 8, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Raccosta, S.; Librizzi, F.; Jagger, A.M.; Noto, R.; Martorana, V.; Lomas, D.A.; Irving, J.A.; Manno, M. Scaling Concepts in Serpin Polymer Physics. Materials 2021, 14, 2577. [Google Scholar] [CrossRef]
- Chiodini, S.; Straub, A.; Donati, S.; Albonetti, C.; Borgatti, F.; Stoliar, P.; Murgia, M.; Biscarini, F. Morphological Transitions in Organic Ultrathin Film Growth Imaged by In Situ Step-by-Step Atomic Force Microscopy. J. Phys. Chem. C 2020, 124, 14030–14042. [Google Scholar] [CrossRef]
- Chiodini, S.; Stoliar, P.; Garrido, P.F.; Albonetti, C. Differential Entropy: An Appropriate Analysis to Interpret the Shape Complexity of Self-Similar Organic Islands. Materials 2021, 14, 6529. [Google Scholar] [CrossRef] [PubMed]
- Barbalinardo, M.; Antosova, A.; Gambucci, M.; Bednarikova, Z.; Albonetti, C.; Valle, F.; Sassi, P.; Latterini, L.; Gazova, Z.; Bystrenova, E. Effect of Metallic Nanoparticles on Amyloid Fibrils and Their Influence to Neural Cell Toxicity. Nano Res. 2020, 13, 1081–1089. [Google Scholar] [CrossRef]
- Bondžić, A.M.; Leskovac, A.R.; Petrović, S.Ž.; Vasić Anićijević, D.D.; Luce, M.; Massai, L.; Generosi, A.; Paci, B.; Cricenti, A.; Messori, L.; et al. Conjugates of Gold Nanoparticles and Antitumor Gold(III) Complexes as a Tool for Their AFM and SERS Detection in Biological Tissue. Int. J. Mol. Sci. 2019, 20, 6306. [Google Scholar] [CrossRef]
- Alderighi, M.; Carrai, P.; Nobili, C.; Lopez, F.; Cuomo, F.; Ambrosone, L. Nanoparticles from Paper Mills: A Seasonal, Numerical and Morphological Analysis. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 102–107. [Google Scholar] [CrossRef]
- Calisi, N.; Giuliani, A.; Alderighi, M.; Schnorr, J.M.; Swager, T.M.; Di Francesco, F.; Pucci, A. Factors Affecting the Dispersion of MWCNTs in Electrically Conducting SEBS Nanocomposites. Eur. Polym. J. 2013, 49, 1471–1478. [Google Scholar] [CrossRef]
- Adel, A.M.; Al-Shemy, M.T.; Diab, M.A.; El-Sakhawy, M.; Toro, R.G.; Cerri, L.; Caschera, D. Immobilization of TiO2NP@ Oxidized Cellulose Nanocrystals for Paper-Based Active Packaging Materials. Int. J. Biol. Macromol. 2023, 231, 123270. [Google Scholar] [CrossRef]
- Antal, T.K.; Volgusheva, A.A.; Kukarskikh, G.P.; Lukashev, E.P.; Bulychev, A.A.; Margonelli, A.; Orlanducci, S.; Leo, G.; Cerri, L.; Tyystjärvi, E.; et al. Single-Walled Carbon Nanotubes Protect Photosynthetic Reactions in Chlamydomonas Reinhardtii against Photoinhibition. Plant Physiol. Biochem. 2022, 192, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Cialone, M.; Celegato, F.; Scaglione, F.; Barrera, G.; Raj, D.; Coïsson, M.; Tiberto, P.; Rizzi, P. Nanoporous FePd Alloy as Multifunctional Ferromagnetic SERS-Active Substrate. Appl. Surf. Sci. 2021, 543, 148759. [Google Scholar] [CrossRef]
- Fazi, L.; Raimondo, L.; Bonanni, B.; Fanfoni, M.; Paolesse, R.; Sgarlata, A.; Sassella, A.; Goletti, C. Unveiling the Robustness of Porphyrin Crystalline Nanowires toward Aggressive Chemicals. Eur. Phys. J. Plus 2022, 137, 300. [Google Scholar] [CrossRef]
- Guo, X.; Luo, S.; Amidani, D.; Rivetti, C.; Pieraccini, G.; Pioselli, B.; Catinella, S.; Murgia, X.; Salomone, F.; Xu, Y.; et al. In Vitro Characterization and in Vivo Comparison of the Pulmonary Outcomes of Poractant Alfa and Calsurf in Ventilated Preterm Rabbits. PLoS ONE 2020, 15, e0230229. [Google Scholar] [CrossRef]
- Filoni, C.; Wandelt, K.; Marfori, L.; Leone, M.; Duò, L.; Ciccacci, F.; Bussetti, G. A Combined EC-STM and EC-AFM Investigation of the Sulfate Adsorption on a Cu(111) Electrode Surface up to the Anodic Corrosion Potential. Appl. Surf. Sci. 2023, 611, 155542. [Google Scholar] [CrossRef]
- Carcione, R.; Politi, S.; Iacob, E.; Potrich, C.; Lunelli, L.; Vanzetti, L.E.; Bartali, R.; Micheli, V.; Pepponi, G.; Terranova, M.L.; et al. Exploring a New Approach for Regenerative Medicine: Ti-Doped Polycrystalline Diamond Layers as Bioactive Platforms for Osteoblast-like Cells Growth. Appl. Surf. Sci. 2021, 540, 148334. [Google Scholar] [CrossRef]
- Dell’anna, R.; Iacob, E.; Tripathi, M.; Dalton, A.; Böttger, R.; Pepponi, G. AFM and Raman Study of Graphene Deposited on Silicon Surfaces Nanostructured by Ion Beam Irradiation. J. Microsc. 2020, 280, 183–193. [Google Scholar] [CrossRef]
- Marchiori, G.; Gambardella, A.; Berni, M.; Bellucci, D.; Cassiolas, G.; Cannillo, V. Impact of Surface Functionalization by Nanostructured Silver Thin Films on Thermoplastic Central Venous Catheters: Mechanical, Microscopical and Thermal Analyses. Coatings 2020, 10, 1034. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Vigliotta, G.; Picariello, E.; Banfi, F.; Cavaliere, E.; Ciambriello, L.; Gavioli, L. Ag Functionalization of Al-Doped ZnO Nanostructured Coatings on PLA Substrate for Antibacterial Applications. Coatings 2020, 10, 1238. [Google Scholar] [CrossRef]
- Temperini, M.E.; Di Giacinto, F.; Romanò, S.; Di Santo, R.; Augello, A.; Polito, R.; Baldassarre, L.; Giliberti, V.; Papi, M.; Basile, U.; et al. Antenna-Enhanced Mid-Infrared Detection of Extracellular Vesicles Derived from Human Cancer Cell Cultures. J. Nanobiotechnol. 2022, 20, 530. [Google Scholar] [CrossRef]
- Di Russo, E.; Sgarbossa, F.; Ranieri, P.; Maggioni, G.; Ndiaye, S.; Duguay, S.; Vurpillot, F.; Rigutti, L.; Rouvière, J.L.; Morandi, V.; et al. Synthesis of Relaxed Ge0.9Sn0.1/Ge by Nanosecond Pulsed Laser Melting. Appl. Surf. Sci. 2023, 612, 155817. [Google Scholar] [CrossRef]
- Palleschi, S.; D’Olimpio, G.; Benassi, P.; Nardone, M.; Alfonsetti, R.; Moccia, G.; Renzelli, M.; Cacioppo, O.A.; Hichri, A.; Jaziri, S.; et al. On the Role of Nano-Confined Water at the 2D/SiO2 Interface in Layer Number Engineering of Exfoliated MoS2 via Thermal Annealing. 2D Mater. 2020, 7, 25001. [Google Scholar] [CrossRef]
- Mazzetta, I.; Viti, L.; Rigoni, F.; Quaranta, S.; Gasparotto, A.; Barucca, G.; Palma, F.; Riello, P.; Cattaruzza, E.; Asgari, M.; et al. Microwave Driven Synthesis of Narrow Bandgap Alpha-Tin Nanoparticles on Silicon. Mater. Des. 2022, 217, 110632. [Google Scholar] [CrossRef]
- Cesano, F.; Uddin, M.J.; Damin, A.; Scarano, D. Multifunctional Conductive Paths Obtained by Laser Processing of Non-Conductive Carbon Nanotube/Polypropylene Composites. Nanomaterials 2021, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Marinello, F.; La Storia, A.; Mauriello, G.; Passeri, D. Atomic Force Microscopy Techniques to Investigate Activated Food Packaging Materials. Trends Food Sci. Technol. 2019, 87, 84–93. [Google Scholar] [CrossRef]
- Maggi, S.; Yabre, K.; Ferrari, A.; Lazzi, C.; Kawano, M.; Rivetti, C.; Folli, C. Functional Characterization of the Type I Toxin Lpt from Lactobacillus Rhamnosus by Fluorescence and Atomic Force Microscopy. Sci. Rep. 2019, 9, 15208. [Google Scholar] [CrossRef] [PubMed]
- Dinarelli, S.; Longo, G.; Germanova-Taneva, S.; Todinova, S.; Krumova, S.; Girasole, M. Surprising Structural and Functional Properties of Favism Erythrocytes Are Linked to Special Metabolic Regulation: A Cell Aging Study. Int. J. Mol. Sci. 2023, 24, 637. [Google Scholar] [CrossRef]
- Dinarelli, S.; Longo, G.; Cannata, S.; Bernardini, S.; Gomiero, A.; Fabi, G.; Marco, G. Metal-Based Micro and Nanosized Pollutant in Marine Organisms: What Can We Learn from a Combined Atomic Force Microscopy-Scanning Electron Microscopy Study. J. Mol. Recognit. 2020, 33, e2851. [Google Scholar] [CrossRef]
- Martini, L.; Chen, Z.; Mishra, N.; Barin, G.B.; Fantuzzi, P.; Ruffieux, P.; Fasel, R.; Feng, X.; Narita, A.; Coletti, C.; et al. Structure-Dependent Electrical Properties of Graphene Nanoribbon Devices with Graphene Electrodes. Carbon 2019, 146, 36–43. [Google Scholar] [CrossRef]
- Brunella, V.; Rossatto, B.G.; Scarano, D.; Cesano, F. Thermal, Morphological, Electrical Properties and Touch-Sensor Application of Conductive Carbon Black-Filled Polyamide Composites. Nanomaterials 2021, 11, 3103. [Google Scholar] [CrossRef]
- Nappini, S.; Boukhvalov, D.W.; D’Olimpio, G.; Zhang, L.; Ghosh, B.; Kuo, C.N.; Zhu, H.; Cheng, J.; Nardone, M.; Ottaviano, L.; et al. Transition-Metal Dichalcogenide NiTe2: An Ambient-Stable Material for Catalysis and Nanoelectronics. Adv. Funct. Mater. 2020, 30, 2000915. [Google Scholar] [CrossRef]
- Giannazzo, F.; Panasci, S.E.; Schilirò, E.; Roccaforte, F.; Koos, A.; Nemeth, M.; Pécz, B. Esaki Diode Behavior in Highly Uniform MoS2/Silicon Carbide Heterojunctions. Adv. Mater. Interfaces 2022, 9, 2200915. [Google Scholar] [CrossRef]
- Boschi, A.; Cinili, S.; Bystrenova, E.; Ruani, G.; Groppi, J.; Credi, A.; Baroncini, M.; Candini, A.; Gentili, D.; Cavallini, M. Multimodal Sensing in Rewritable, Data Matrix Azobenzene-Based Devices. J. Mater. Chem. C 2022, 10, 10132–10138. [Google Scholar] [CrossRef]
- Colangelo, F.; Pingue, P.; Mišeikis, V.; Coletti, C.; Beltram, F.; Roddaro, S. Mapping the Mechanical Properties of a Graphene Drum at the Nanoscale. 2D Mater. 2019, 6, 25005. [Google Scholar] [CrossRef]
- Cascione, M.; De Matteis, V.; Persano, F.; Leporatti, S. AFM Characterization of Halloysite Clay Nanocomposites’ Superficial Properties: Current State-of-the-Art and Perspectives. Materials 2022, 15, 3441. [Google Scholar] [CrossRef]
- Tognoni, E.; Orsini, P.; Pellegrino, M. Nonlinear Indentation of Single Human Erythrocytes under Application of a Localized Mechanical Force. Micron 2019, 127, 102760. [Google Scholar] [CrossRef]
- Oropesa-Nuñez, R.; Mescola, A.; Vassalli, M.; Canale, C. Impact of Experimental Parameters on Cell–Cell Force Spectroscopy Signature. Sensors 2021, 21, 1069. [Google Scholar] [CrossRef]
- Iturri, J.; Weber, A.; Moreno-Cencerrado, A.; Vivanco, M.D.; Benítez, R.; Leporatti, S.; Toca-Herrera, J.L. Resveratrol-Induced Temporal Variation in the Mechanical Properties of MCF-7 Breast Cancer Cells Investigated by Atomic Force Microscopy. Int. J. Mol. Sci. 2019, 20, 3275. [Google Scholar] [CrossRef]
- Bontempi, M.; Salamanna, F.; Capozza, R.; Visani, A.; Fini, M.; Gambardella, A. Nanomechanical Mapping of Hard Tissues by Atomic Force Microscopy: An Application to Cortical Bone. Materials 2022, 15, 7521. [Google Scholar] [CrossRef]
- Senigagliesi, B.; Samperi, G.; Cefarin, N.; Gneo, L.; Petrosino, S.; Apollonio, M.; Caponnetto, F.; Sgarra, R.; Collavin, L.; Cesselli, D.; et al. Triple Negative Breast Cancer-Derived Small Extracellular Vesicles as Modulator of Biomechanics in Target Cells. Nanomed. Nanotechnol. Biol. Med. 2022, 44, 102582. [Google Scholar] [CrossRef]
- Ridolfi, A.; Brucale, M.; Montis, C.; Caselli, L.; Paolini, L.; Borup, A.; Boysen, A.T.; Loria, F.; van Herwijnen, M.J.C.; Kleinjan, M.; et al. AFM-Based High-Throughput Nanomechanical Screening of Single Extracellular Vesicles. Anal. Chem. 2020, 92, 10274–10282. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, R.; Musicò, A.; Brucale, M.; Ridolfi, A.; Galbiati, S.; Vago, R.; Bergamaschi, G.; Ferretti, A.M.; Chiari, M.; Valle, F.; et al. Extracellular Vesicles Analysis in the COVID-19 Era: Insights on Serum Inactivation Protocols towards Downstream Isolation and Analysis. Cells 2021, 10, 544. [Google Scholar] [CrossRef]
- Adamo, G.; Fierli, D.; Romancino, D.P.; Picciotto, S.; Barone, M.E.; Aranyos, A.; Božič, D.; Morsbach, S.; Raccosta, S.; Stanly, C.; et al. Nanoalgosomes: Introducing Extracellular Vesicles Produced by Microalgae. J. Extracell. Vesicles 2021, 10, e12081. [Google Scholar] [CrossRef] [PubMed]
- Mescola, A.; Ragazzini, G.; Alessandrini, A. Daptomycin Strongly Affects the Phase Behavior of Model Lipid Bilayers. J. Phys. Chem. B 2020, 124, 8562–8571. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, M.; Marfori, L.; Asperti, S.; De Vita, A.; Giannangeli, M.; Caselli, A.; Milani, P.; Podestà, A. Interaction of Imidazolium-Based Ionic Liquids with Supported Phospholipid Bilayers as Model Biomembranes. Phys. Chem. Chem. Phys. 2022, 24, 27328–27342. [Google Scholar] [CrossRef] [PubMed]
- Corti, R.; Marrano, C.A.; Salerno, D.; Brocca, S.; Natalello, A.; Santambrogio, C.; Legname, G.; Mantegazza, F.; Grandori, R.; Cassina, V. Depicting Conformational Ensembles of α-Synuclein by Single Molecule Force Spectroscopy and Native Mass Spectroscopy. Int. J. Mol. Sci. 2019, 20, 5181. [Google Scholar] [CrossRef]
- Raspadori, A.; Vignali, V.; Murello, A.; Giachin, G.; Samorì, B.; Tanaka, M.; Bustamante, C.; Zuccheri, G.; Legname, G. Evidence of Orientation-Dependent Early States of Prion Protein Misfolded Structures from Single Molecule Force Spectroscopy. Biology 2022, 11, 1358. [Google Scholar] [CrossRef]
- Chen, Y.; D’Antuono, M.; Brookes, N.B.; De Luca, G.M.; Di Capua, R.; Di Gennaro, E.; Ghiringhelli, G.; Piamonteze, C.; Preziosi, D.; Jouault, B.; et al. Ferromagnetic Quasi-Two-Dimensional Electron Gas with Trigonal Crystal Field Splitting. ACS Appl. Electron. Mater. 2022, 4, 3226–3231. [Google Scholar] [CrossRef]
- Gutiérrez, Y.; Ovvyan, A.P.; Santos, G.; Juan, D.; Rosales, S.A.; Junquera, J.; García-Fernández, P.; Dicorato, S.; Giangregorio, M.M.; Dilonardo, E.; et al. Interlaboratory Study on Sb2S3 Interplay between Structure, Dielectric Function, and Amorphous-to-Crystalline Phase Change for Photonics. iScience 2022, 25, 104377. [Google Scholar] [CrossRef]
- Mescola, A.; Paolicelli, G.; Ogilvie, S.P.; Guarino, R.; McHugh, J.G.; Rota, A.; Iacob, E.; Gnecco, E.; Valeri, S.; Pugno, N.M.; et al. Graphene Confers Ultralow Friction on Nanogear Cogs. Small 2021, 17, 2104487. [Google Scholar] [CrossRef]
- Coïsson, M.; Barrera, G.; Celegato, F.; Tiberto, P. Rotatable Magnetic Anisotropy in Fe78Si9B13 Thin Films Displaying Stripe Domains. Appl. Surf. Sci. 2019, 476, 402–411. [Google Scholar] [CrossRef]
- Fin, S.; Silvani, R.; Tacchi, S.; Marangolo, M.; Garnier, L.-C.; Eddrief, M.; Hepburn, C.; Fortuna, F.; Rettori, A.; Pini, M.G.; et al. Straight Motion of Half-Integer Topological Defects in Thin Fe-N Magnetic Films with Stripe Domains. Sci. Rep. 2018, 8, 9339. [Google Scholar] [CrossRef] [PubMed]
- Garnier, L.-C.; Marangolo, M.; Eddrief, M.; Bisero, D.; Fin, S.; Casoli, F.; Pini, M.G.; Rettori, A.; Tacchi, S. Stripe Domains Reorientation in Ferromagnetic Films with Perpendicular Magnetic Anisotropy. J. Phys. Mater. 2020, 3, 24001. [Google Scholar] [CrossRef]
- Gutiérrez, Y.; Giangregorio, M.M.; Dicorato, S.; Palumbo, F.; Losurdo, M. Exploring the Thickness-Dependence of the Properties of Layered Gallium Sulfide. Front. Chem. 2021, 9, 781467. [Google Scholar] [CrossRef] [PubMed]
- Pécz, B.; Nicotra, G.; Giannazzo, F.; Yakimova, R.; Koos, A.; Kakanakova-Georgieva, A. Indium Nitride at the 2D Limit. Adv. Mater. 2021, 33, 2006660. [Google Scholar] [CrossRef] [PubMed]
- Bettotti, P.; Visone, V.; Lunelli, L.; Perugino, G.; Ciaramella, M.; Valenti, A. Structure and Properties of DNA Molecules over the Full Range of Biologically Relevant Supercoiling States. Sci. Rep. 2018, 8, 6163. [Google Scholar] [CrossRef] [PubMed]
- Greenfeld, I.; Camposeo, A.; Portone, A.; Romano, L.; Allegrini, M.; Fuso, F.; Pisignano, D.; Wagner, H.D. WO3 Nanowires Enhance Molecular Alignment and Optical Anisotropy in Electrospun Nanocomposite Fibers: Implications for Hybrid Light-Emitting Systems. ACS Appl. Nano Mater. 2022, 5, 3654–3666. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, S.; Kerfoot, J.; Venturi, G.; Mignuzzi, S.; Alexeev, E.M.; Teixeira Rosa, B.; Tongay, S.; Taniguchi, T.; Watanabe, K.; Ferrari, A.C.; et al. Moiré Modulation of Van Der Waals Potential in Twisted Hexagonal Boron Nitride. ACS Nano 2022, 16, 7589–7604. [Google Scholar] [CrossRef]
- Picotto, G.B.; Vallino, M.; Ribotta, L. Tip–Sample Characterization in the AFM Study of a Rod-Shaped Nanostructure. Meas. Sci. Technol. 2020, 31, 84001. [Google Scholar] [CrossRef]
- Bellotti, R.; Picotto, G.B.; Ribotta, L. AFM Measurements and Tip Characterization of Nanoparticles with Different Shapes. Nanomanuf. Metrol. 2022, 5, 127–138. [Google Scholar] [CrossRef]
- Albonetti, C.; Kshirsagar, R.; Cavallini, M.; Biscarini, F. Patterning Organic Nanostructures by Scanning Probe Nanolithography; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 9783527312696. [Google Scholar]
- Marchetto, D.; Rota, A.; Calabri, L.; Gazzadi, G.C.; Menozzi, C.; Valeri, S. AFM Investigation of Tribological Properties of Nano-Patterned Silicon Surface. Wear 2008, 265, 577–582. [Google Scholar] [CrossRef]
- D’Antuono, M.; Kalaboukhov, A.; Caruso, R.; Wissberg, S.; Weitz Sobelman, S.; Kalisky, B.; Ausanio, G.; Salluzzo, M.; Stornaiuolo, D. Nanopatterning of Oxide 2-Dimensional Electron Systems Using Low-Temperature Ion Milling. Nanotechnology 2022, 33, 85301. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, M.; Mercuri, F.; Cavallini, M. A Molecular Drone for Atomic-Scale Fabrication Working under Ambient Conditions. Adv. Mater. 2021, 33, 2007150. [Google Scholar] [CrossRef]
- Foti, A.; Venkatesan, S.; Lebental, B.; Zucchi, G.; Ossikovski, R. Comparing Commercial Metal-Coated AFM Tips and Home-Made Bulk Gold Tips for Tip-Enhanced Raman Spectroscopy of Polymer Functionalized Multiwalled Carbon Nanotubes. Nanomaterials 2022, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Chighizola, M.; Puricelli, L.; Bellon, L.; Podestà, A. Large Colloidal Probes for Atomic Force Microscopy: Fabrication and Calibration Issues. J. Mol. Recognit. 2021, 34, e2879. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, L.; Greco, S.L.M.; Tierno, D.; Chignola, R.; Martinelli, M.; Giolo, E.; Luppi, S.; Delfino, I.; Zanetti, M.; Battistella, A.; et al. Planar AFM Macro-Probes to Study the Biomechanical Properties of Large Cells and 3D Cell Spheroids. Acta Biomater. 2019, 94, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Bonafè, F.; Decataldo, F.; Zironi, I.; Remondini, D.; Cramer, T.; Fraboni, B. AC Amplification Gain in Organic Electrochemical Transistors for Impedance-Based Single Cell Sensors. Nat. Commun. 2022, 13, 5423. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, L.; Poletti, A.; Marks, R.; Verlato, E.; Paolucci, F.; Rapino, S.; Albonetti, C. Revised Electrochemical Etching System for a Reproducible Fabrication of Ultra-Sharp Tungsten Tips. J. Appl. Electrochem. 2021, 51, 551–566. [Google Scholar] [CrossRef]
- Becerra, N.; Salis, B.; Tedesco, M.; Moreno Flores, S.; Vena, P.; Raiteri, R. AFM and Fluorescence Microscopy of Single Cells with Simultaneous Mechanical Stimulation via Electrically Stretchable Substrates. Materials 2021, 14, 4131. [Google Scholar] [CrossRef]
- Caluori, G.; Pribyl, J.; Pesl, M.; Jelinkova, S.; Rotrekl, V.; Skladal, P.; Raiteri, R. Non-Invasive Electromechanical Cell-Based Biosensors for Improved Investigation of 3D Cardiac Models. Biosens. Bioelectron. 2019, 124, 129–135. [Google Scholar] [CrossRef]
- CNR. Il Linguaggio Della Ricerca. Available online: https://ldr-network.bo.cnr.it/ (accessed on 17 April 2023).
- CNR. SperimeEstate. Available online: http://sperimestate.bo.imm.cnr.it/index.html (accessed on 17 April 2023).
- Albonetti, C. Art at the Nanoscale. Available online: https://www.youtube.com/watch?v=5gSiBuuhgiY (accessed on 17 April 2023).
- Scientaomicron Nobel Prize Technologies. Available online: https://scientaomicron.com/en/about-us (accessed on 17 April 2023).
- Harris, C.M. Product Review: The Saga of AFM. Anal. Chem. 2001, 73, 627A–635A. [Google Scholar] [CrossRef] [PubMed]
- Materassi, D.; Baschieri, P.; Tiribilli, B.; Zuccheri, G.; Samorì, B. An Open Source/Real-Time Atomic Force Microscope Architecture to Perform Customizable Force Spectroscopy Experiments. Rev. Sci. Instrum. 2009, 80, 84301. [Google Scholar] [CrossRef] [PubMed]
- Sartore, M.; Pace, R.; Faraci, P.; Nardelli, D.; Adami, M.; Ram, M.K.; Nicolini, C. Controlled-Atmosphere Chamber for Atomic Force Microscopy Investigations. Rev. Sci. Instrum. 2000, 71, 2409–2413. [Google Scholar] [CrossRef]
- Nevernov, I.; Sartore, M.; Galletti, R. Object-Oriented Data Model for Scanning Probe Microscopy Image Processing. Image Vis. Comput. 1996, 14, 435–443. [Google Scholar] [CrossRef]
- Pechkova, E.; Sartore, M.; Giacomelli, L.; Nicolini, C. Atomic Force Microscopy of Protein Films and Crystals. Rev. Sci. Instrum. 2007, 78, 93704. [Google Scholar] [CrossRef] [PubMed]
- Sartore, M.; Eggenhöffner, R.; Terencio, T.B.C.; Stura, E.; Hainsworth, E.; LaBaer, J.; Nicolini, C. Label Free Detection of NAPPA via Atomic Force Microscopy. In Functional Proteomics & Nanotechnology-Based Microarrays; Jenny Stanford Publishing: Singapore, 2019; pp. 109–120. ISBN 0429111592. [Google Scholar]
- Ram, M.K.; Adami, M.; Sartore, M.; Salerno, M.; Paddeu, S.; Nicolini, C. Comparative Studies on Langmuir-Schaefer Films of Polyanilines. Synth. Met. 1999, 100, 249–259. [Google Scholar] [CrossRef]
- Nicolini, C.; Adami, M.; Sartore, M.; Bragazzi, N.L.; Bavastrello, V.; Spera, R.; Pechkova, E. Prototypes of Newly Conceived Inorganic and Biological Sensors for Health and Environmental Applications. Sensors 2012, 12, 17112–17127. [Google Scholar] [CrossRef] [PubMed]
- Mouro, J.; Paoletti, P.; Sartore, M.; Vassalli, M.; Tiribilli, B. Photothermal Self-Excitation of a Phase-Controlled Microcantilever for Viscosity or Viscoelasticity Sensing. Sensors 2022, 22, 8421. [Google Scholar] [CrossRef]
- Mouro, J.; Paoletti, P.; Sartore, M.; Tiribilli, B. Dynamical Response and Noise Limit of a Parametrically Pumped Microcantilever Sensor in a Phase-Locked Loop. Sci. Rep. 2023, 13, 2157. [Google Scholar] [CrossRef]
- Sartore, M.; Vassalli, M. Extending a Raspberry Pi® Mini PC with real-time capa-bilities for advanced Atomic Force Microscopy applications. Microsolutions 2016, 25–26. Available online: http://ww1.microchip.com/downloads/en/DeviceDoc/MicroSolutions-JanFeb-2016.pdf#page=25 (accessed on 17 April 2023).
- Trevisan, E.; Fabbretti, E.; Medic, N.; Troian, B.; Prato, S.; Vita, F.; Zabucchi, G.; Zweyer, M. Novel Approaches for Scanning Near-Field Optical Microscopy Imaging of Oligodendrocytes in Culture. Neuroimage 2010, 49, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Andolfi, L.; Trevisan, E.; Zweyer, M.; Prato, S.; Troian, B.; Vita, F.; Borelli, V.; Soranzo, M.R.; Melato, M.; Zabucchi, G. The Crocidolite Fibres Interaction with Human Mesothelial Cells as Investigated by Combining Electron Microscopy, Atomic Force and Scanning near-Field Optical Microscopy. J. Microsc. 2013, 249, 173–183. [Google Scholar] [CrossRef]
- Andolfi, L.; Battistella, A.; Zanetti, M.; Lazzarino, M.; Pascolo, L.; Romano, F.; Ricci, G. Scanning Probe Microscopies: Imaging and Biomechanics in Reproductive Medicine Research. Int. J. Mol. Sci. 2021, 22, 3823. [Google Scholar] [CrossRef]
- Troian, B.; Boscolo, R.; Ricci, G.; Lazzarino, M.; Zito, G.; Prato, S.; Andolfi, L. Ultra-Structural Analysis of Human Spermatozoa by Aperture Scanning near-Field Optical Microscopy. J. Biophotonics 2020, 13, e2418. [Google Scholar] [CrossRef] [PubMed]
- Malenica, M.; Vukomanović, M.; Kurtjak, M.; Masciotti, V.; dal Zilio, S.; Greco, S.; Lazzarino, M.; Krušić, V.; Perčić, M.; Jelovica Badovinac, I.; et al. Perspectives of Microscopy Methods for Morphology Characterisation of Extracellular Vesicles from Human Biofluids. Biomedicines 2021, 9, 603. [Google Scholar] [CrossRef]
- Vinai, G.; Motti, F.; Bonanni, V.; Petrov, A.Y.; Benedetti, S.; Rinaldi, C.; Stella, M.; Cassese, D.; Prato, S.; Cantoni, M.; et al. Reversible Modification of Ferromagnetism through Electrically Controlled Morphology. Adv. Electron. Mater. 2019, 5, 1900150. [Google Scholar] [CrossRef]
- Polewczyk, V.; Magrin Maffei, R.; Vinai, G.; Lo Cicero, M.; Prato, S.; Capaldo, P.; Dal Zilio, S.; di Bona, A.; Paolicelli, G.; Mescola, A.; et al. ZnO Thin Films Growth Optimization for Piezoelectric Application. Sensors 2021, 21, 6114. [Google Scholar] [CrossRef]
- Motti, F.; Vinai, G.; Bonanni, V.; Polewczyk, V.; Mantegazza, P.; Forrest, T.; Maccherozzi, F.; Benedetti, S.; Rinaldi, C.; Cantoni, M.; et al. Interplay between Morphology and Magnetoelectric Coupling in Fe/PMN-PT Multiferroic Heterostructures Studied by Microscopy Techniques. Phys. Rev. Mater. 2020, 4, 114418. [Google Scholar] [CrossRef]
- Pedio, M.; Magnano, E.; Moras, P.; Borgatti, F.; Felici, R.; Troian, B.; Prato, S.; Soncini, C.; Cepek, C. Tuning 3C-SiC(100)/Si(100) Heterostructure Interface Quality. Cryst. Growth Des. 2022, 22, 5182–5188. [Google Scholar] [CrossRef]
| Review | IF | Q |
|---|---|---|
| Microscopy and Microanalysis | 4.099 | Q1 |
| Ultramicroscopy | 2.994 | Q1 |
| Microscopy Research and Technique | 2.893 | Q2 |
| Micron | 2.381 | Q3 |
| Microscopy (Oxford, England) | 2.072 | Q3 |
| Journal of Microscopy | 1.952 | Q4 |
| Scanning | 1.75 | Q4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinelli, F.; Brucale, M.; Valle, F.; Ascoli, C.; Samorì, B.; Sartore, M.; Adami, M.; Galletti, R.; Prato, S.; Troian, B.; et al. Probing Italy: A Scanning Probe Microscopy Storyline. Micro 2023, 3, 549-565. https://doi.org/10.3390/micro3020037
Dinelli F, Brucale M, Valle F, Ascoli C, Samorì B, Sartore M, Adami M, Galletti R, Prato S, Troian B, et al. Probing Italy: A Scanning Probe Microscopy Storyline. Micro. 2023; 3(2):549-565. https://doi.org/10.3390/micro3020037
Chicago/Turabian StyleDinelli, Franco, Marco Brucale, Francesco Valle, Cesare Ascoli, Bruno Samorì, Marco Sartore, Manuela Adami, Riccardo Galletti, Stefano Prato, Barbara Troian, and et al. 2023. "Probing Italy: A Scanning Probe Microscopy Storyline" Micro 3, no. 2: 549-565. https://doi.org/10.3390/micro3020037
APA StyleDinelli, F., Brucale, M., Valle, F., Ascoli, C., Samorì, B., Sartore, M., Adami, M., Galletti, R., Prato, S., Troian, B., & Albonetti, C. (2023). Probing Italy: A Scanning Probe Microscopy Storyline. Micro, 3(2), 549-565. https://doi.org/10.3390/micro3020037










