Abstract
The processes of supramolecular aggregation occurring on carbon surfaces in aqueous solutions of bovine serum albumin (BSA) during drying were studied using modern scanning electron microscopy (SEM). The carbon materials studied were highly oriented pyrolytic graphite (HOPG) and glassy carbon (GC). Based on the analysis of SEM images and EDX-scanning element distribution maps, a possible mechanism for the formation of the observed intricate structures on the surface was proposed. The formation of fuzzy lacy structures resembling shadow replicas was explained by relatively strong hydrophobic–hydrophobic interactions of albumin molecules with carbon surfaces.
1. Introduction
The advanced scanning electron microscopy (SEM) equipped with energy-dispersive X-ray (EDX) spectroscopy instrument is one of the most versatile instruments available for examining and analyzing solid inorganic and organic materials, including heterogeneous catalysts and carbon dots, on a nanometer (nm) and micrometer (μm) scale [1,2,3,4,5]. In addition, this method opens up new opportunities for visualization of the interaction and aggregation of various biological nano-objects (proteins, antibodies, bacteria), which provides new insights into how biomolecules can form amazing supramolecular structures [1,6]. The SEM study of nanoparticle aggregates and visualization of the amazing fractal structures that formed on various metallic surfaces such as aluminum and gold has been described in [7]. The authors continued to study the supramolecular aggregation processes occurring during the drying of microdroplets of BSA aqueous solutions supported on surfaces of various chemical nature, including carbon.
Highly oriented pyrolytic graphite (HOPG) and glassy carbon (GC) have been developed for the fabrication of functionalized electrodes used in electrochemical and electrocatalytic research studies [8,9,10,11,12]. Numerous studies are devoted to the R&D of GC electrodes as a sensitive element of sensors and biosensors capable of specifically detecting analytes in aqueous solutions, such as treated/untreated waters [13,14,15], multicomponent biological fluids (milk, blood) [16,17,18].
Precise control of self-assembling molecule aggregates to create highly ordered microstructures is challenging. In [19], the authors described the supramolecular structures with a strictly controlled arrangement of methylated derivatives of α-cyclodextrin (2-Me-α-CD) on the HOPG surface: self-assembly of 2-Me-α-CD occurred, and vertically oriented hexagonal microrods were formed. The self-assembly of peptide nanoribbons on mica and HOPG interfaces and mechanism of these processes were discussed in [20]. The authors believed that their findings would inspire the design of motif-specific peptides with high binding affinity and would mediate the green synthesis of peptide-based bionanomaterials with unique function and application potential [20]. At present, the synthesis of inherently chiral materials and the study of self-assembly into molecular wires or two-dimensional crystals are hot topics in nanotechnology. In [21], the authors described the characteristic splitting of the orientational symmetry of molecular wires 10–500 nm long, assembled from 3 nm helicene-based chiral macrocycles, on an atomically flat HOPG surface, which opens up new possibilities for modeling large molecular systems.
Bovine serum albumin, its molecular structure (DOI database: 10.2210/pdb4F5S/pdb, https://www.rcsb.org/structure/4F5S, accessed on 6 December 2022), biological functions and capability to form supramolecular aggregates are well studied [22,23]. At a neutral pH, hydrophobic–hydrophobic interactions predominate between the BSA globules, leading to the formation of disordered aggregates. At a high protein concentration, partially folded globules–conformers aggregate into oligomers (dimers, tetramers, etc.), which at elevated temperatures, turn into ordered amyloid-like fibrils [24].
Comprehensive studies of albumin adsorption on supports based on carbonaceous materials have been carried out. In [25], the authors studied the physical adsorption of BSA on hydrophobic carbon aerogel (C1) with basic surface pH and specific surface area (SspBET) 847 m2 g–1, and hydrophilic microporous carbon (C2) with neutral surface pH and SspBET = 1248 m2 g–1. For C1 and C2 adsorbents, the main parameters of BSA adsorption isotherms, approximated by the hyperbolic Langmuir equation, were estimated as the following values, respectively: monolayer capacity 880 and 90 mg·g–1; equilibrium affinity constants 0.13 and 3.30 [25]. This meant that albumin interacted with carbon surfaces with different strengths, viz. weaker with hydrophobic C1 than with hydrophilic C2. A large amount of albumin adsorbed on C1 was due to the presence of suitable-by-size mesopores. In [6], the authors studied the adsorption of BSA on graphite (G), SspBET = 8 m2 g–1 and bulk catalytic filamentous carbon (CFC), Ssp = 100 m2 g–1. For G and CFC adsorbents, the main parameters of BSA adsorption isotherms were estimated as the following values, respectively, monolayer capacity 52 and 86 mg·g–1, equilibrium affinity constants 0.03 and 0.11 [6]. This meant that albumin interacted with the graphite surface weaker than with the carbon nanofilaments. Analyzing and comparing all obtained experimental data, the authors concluded that BSA adsorption on the smooth hydrophobic surface of graphite was accompanied by the aggregation of albumin molecules due to stronger intermolecular hydrophobic interactions and the relatively low affinity toward the surface of the G adsorbent [6]. Thus, the above data confirmed that albumin molecules interacted with carbon surfaces and were able to form aggregates.
In this communication, the authors describe the results of ongoing research in an interesting and educational area of modern SEM study on supramolecular aggregation processes occurring during the drying of microdroplets of aqueous solutions of bovine serum albumin (BSA). Highly oriented pyrolytic graphite (HOPG) and glassy carbon (GS) were used as the substrates (supports) for applying microdroplets. Processes of supramolecular aggregation occurring on the carbon surfaces were visualized using advanced scanning electron microscopy, equipped with an EDX analysis instrument. The phenomena and possible mechanism of aggregation on carbons and the formation of specific shadow and lacy-like replicas were discussed.
2. Materials and Methods
An ultra-high-resolution field emission scanning electron microscope (SEM) Regulus 8230 (Hitachi, Tokyo, Japan) was used for the investigations of biomaterials such as proteins at low energy, E0 = 5 keV, in secondary electrons (SE) mode. Such low-voltage operation provided an effective route to minimizing changes in the sample [1].
Bovine serum albumin produced by SIGMA Co. (Sigma-Aldrich, Moscow, Russia) was dissolved in distilled water. Solutions with different protein concentrations 0.2 and 1.0 mg mL–1 were used for sample preparation for microscopy. Microdroplets of 1–2 µL in volume were applied very carefully onto a surface of solid substrate (support), followed by drying for several hours in a desiccator under ambient conditions (20 ± 2 °C, 1 bar).
Highly oriented pyrolytic graphite (HOPG) with freshly prepared surface and glassy carbon (GC) disk were donated.
3. Results and Discussion
During drying of the microdroplets of aqueous colloidal dispersions, the suspended particles were deposited in a ring at the droplet periphery due to capillary forces ensuring the flow of the solvent to a fixed contact line [26]. As was observed in [7], the fixed lines of the outer periphery of microdroplets of BSA solutions dried on the surface of Al and Au retained a perfectly rounded black edge, and EDX scanning analysis showed that almost all of the amount of albumin (for Al, more than 95%) was in the area of this edge. In this research, SEM images of the dried microdroplets of BSA solutions of different concentrations on HOPG (Figure 1a,b) demonstrated that their periphery lines, as in [7], were fixed and relatively round but were white in color and partially cut by planar graphene defects. The thickness of the microdroplet edges depended on the concentration of albumin in the solution: the greater the concentration, the thicker and brighter the edge (Figure 1a,b). The microdroplets dried on glassy carbon had a black jagged edge (Figure 1c). The uneven and crooked boundary of the edge may be due to the more pronounced defectiveness of GC surface compared to HOPG as described below.

Figure 1.
SEM images of dried microdroplet of BSA solution on HOPG (a,b) and GC (c): (a,c) BSA concentration 0.2 mg·mL–1; (b) BSA concentration 1.0 mg·mL–1.
EDX analysis was applied to determine the chemical composition of the edge of the microdroplet, presented in Figure 1b. As found in [7], the lyophilized BSA sample contained 17.5 wt% nitrogen, 19.9 wt% oxygen and 1.9 wt% sulfur. Nitrogen and sulfur were chosen as labels, indicating the presence of protein. Element distribution maps are presented in Figure 2. Since HOPG was composed entirely of carbon (C), the edge of the droplet looked black on the map due to less contrast (Figure 2a). Considering Figure 2, the conclusion was made that the protein molecules were concentrated along the edge of the microdroplet, similar to that observed on aluminum in [7]. When droplets of BSA solutions were dried on smooth surfaces, capillary forces pulled the molecules to the edge. Almost all of the amount of albumin (for HOPG, more than 80%) was in the area of this edge. This rough semi-quantitative evaluation was made by calculations, assuming that the edge had the shape of a torus cut in half, and at the same scaling of microscopic images, its volume could be estimated by measurement with a ruler. The ratio between the volumes of the tori of the rings presented in Figure 1a,b, amounted to 4.3, although it should be 5.5 according to a fivefold difference in BSA concentrations. This meant that ca. 20% of albumin amount remained inside the ring due to the fact that the interaction between BSA and the carbon surface exceeded the capillary forces that moved the solution to the edge of the droplet. This undoubtedly rough estimate allowed the authors to conclude that, in contrast to the metallic surfaces described in [7], the carbon surface and albumin molecules were not indifferent to each other, and BSA molecules could be relatively strongly adsorbed and fixed on carbon supports as described in [6,25].
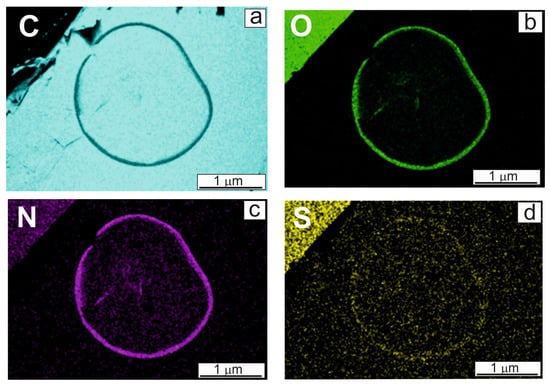
Figure 2.
(a–d) Element distribution maps corresponding to microdroplet SEM image (Figure 1b): (a) carbon (C), (b) oxygen (O), (c) nitrogen (N), (d) sulfur (S).
For highly oriented pyrolytic graphite, an SEM study was carried out to elucidate the aggregation features on the HOPG surface, which possessed planar step-like defects formed by graphene planes. Inside the dried microdroplet shown in Figure 1a, at a BSA concentration of 0.2 mg·mL–1, large lace-like structures that look like replicas formed by “bubbles” were observed (Figure 3). Inside the microdroplet shown in Figure 1b, at a high BSA concentration of 1.0 mg·mL–1, an openwork structure such as an intricate lace-like replica covered, quite densely, the entire HOPG surface, not concentrating along the planar defects (Figure 3(a2)). These observations may be explained by the ability of BSA molecules to form ordered amyloid-like fibrils as described in [22,23,24]. The SEM images in Figure 3 indicate that the BSA globules–conformers and the protein fibrils were fixed on the HOPG surface due to their ability to interact with carbon supports and to be adsorbed as noted above.
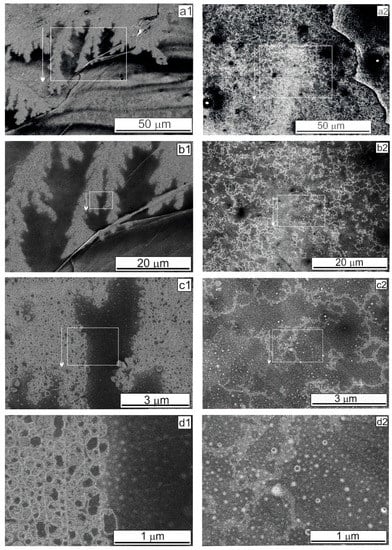
Figure 3.
(a1–d2) SEM images of structures formed on the HOPG with different magnifications, obtained in the SE mode. Rectangles and arrows indicate areas with a higher magnification. (a1–d1) droplet as presented in Figure 1a (BSA concentration 0.2 mg·mL–1). (a2–d2) droplet as presented in Figure 1b (BSA concentration 1.0 mg·mL–1).
Analyzing the SEM images in Figure 3 and comparing them with the images for Al and Au presented in [7], we can conclude that the observed differences between carbon and metallic supports were due exclusively to the interaction of albumin molecules with carbon adsorbents, followed by their fixation on their surface. This property of BSA also affects the quality of the obtained SEM images: the images have weak contrast and turbidity. Faint, barely noticeable replicas were visible in high magnification (Figure 3(d2)). In addition, fixation of BSA on the carbon surface can explain the formation of visible imaginary “air bubbles”. As is known, one NH2 group can coordinate around two-three-H2O, and one hydrated BSA molecule, consisting of 583 amino acid residues, can bind several thousand water molecules. The electron beam in vacuum heated up the carbon surface, leading to “boiling” water associated with the fixed hydrated protein globules; consequently, “bubbles” were formed (Figure 3(c1–d2)).
For glassy carbon, as seen on the SEM images (Figure 4), lace-like replicas were formed on the GC surface similar to those described for HOPG above. Comparing Figure 3(a1,b1) and Figure 4a,b, we noticed that under the same BSA concentration (0.2 mg·mL–1), the lace-like structures covered, very densely, the entire surface of glassy carbon perhaps due to a multipoint interaction of the protein globule with a rougher surface of GC. Black “nanoholes” visible in Figure 4a,b, apparently, ensured a greater roughness and defectiveness of the GC surface than that of the HOPG. “Bubbles” on the surface of the glassy carbon were several times larger than those on graphite (comparing Figure 3(d1,d2) and Figure 4c).
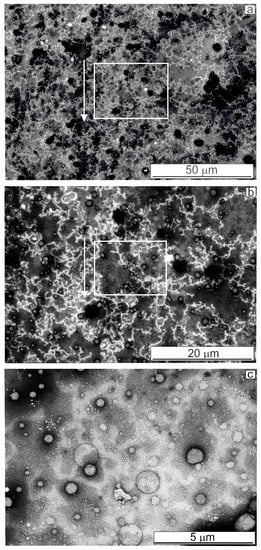
Figure 4.
(a–c) SEM images of structures formed on the GC with different magnifications, obtained in the SE mode. Rectangles and arrows indicate areas with a higher magnification. Droplet as presented in Figure 1c.
On metallic surfaces (Al and Au), bright cubic-shaped nanoobjects ca. 100 nm in size were formed at the ends of the branches of amazing fractal structures [7]. As EDX analysis showed, the chemical composition of these nanoobjects was consistent with sodium chloride (NaCl) since the commercial lyophilized BSA powder used to prepare the solutions contained (in wt %): sodium—0.5; chlorine—0.01. Did such bright nanoobjects form on the surface of carbons? Yes, several times larger individual cubic crystals were observed among the replicas, not associated with lacy structures (Figure 5a).
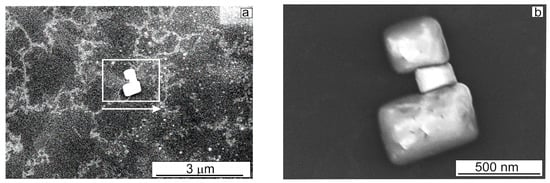
Figure 5.
(a,b) SEM images of large aggregates formed on the HOPG with different magnifications, obtained in the SE mode. Rectangles and arrows indicate areas with a higher magnification. Droplet as presented in Figure 1b.
The features of aggregation processes on the carbon surfaces were revealed: (i) the lace-like replicas were distributed fairly evenly over the entire carbon surface, not only along its defects; (ii) at high magnification, the replicas were made up of “bubbles”, and bubble-like structures were clearly visible; (iii) the visibility of replicas deteriorated significantly at high magnification on the nm scale. Analyzing all SEM images, including previously described in [7] for metallic supports, we concluded that the main reason for differences in the aggregation processes and the formation of the complex branched structures was that BSA molecules were adsorbed and fixed on carbon surfaces mainly due to relatively strong hydrophobic–hydrophobic interactions. The authors proposed the following mechanism of the supramolecular aggregation on carbon supports during drying of aqueous BSA solutions: (globule → dimer, tetramer → oligomers) moving toward the edge of the microdroplet by capillary forces, → (amyloid-like fibrils) fixing on the surface.
4. Conclusions
The features of the aggregation processes on carbon surfaces of highly oriented pyrolytic graphite (HOPG) and glassy carbon (GC) were revealed: (i) the main amount of albumin (more than 80%) was on the edge of the microdroplets, since capillary forces pulled the BSA molecules to the droplet periphery; (ii) the lace-like replicas were formed inside the dried microdroplets of BSA solutions and covered the entire carbon surface not concentrating along the surface defects; (iii) the lacy replicas become barely visible at high magnification due to imaginary “bubbles” formed under the action of a high-energy electron beam. The main reason for the phenomena of the formation of blurred lacy replicas was assumed to be that BSA molecules interacted with each other and the carbon surface and were adsorbed and fixed on HOPG and GC (unlike aluminum and gold in [7]). In addition, the results of the SEM study made it possible to draw a practically important conclusion that obtaining uniform coatings on various solid surfaces is a difficult and non-trivial task. It is necessary to select special conditions and carry out SEM control of fixed homogeneous bioactive films formed on the surface, for example, of indicator electrodes for the development of amperometric biosensors.
Author Contributions
SEM study and data processing, A.S. (Aleksei Salanov); worked on the Regulus electron microscope, A.S. (Alexandra Serkova) and A.Z.; skillful preparation of the samples (microdroplets) for SEM study, L.P.; writing and supervision, G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation within the governmental order for Boreskov Institute of Catalysis (project AAAA-A21-121011390007-7).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The studies were carried out using facilities of the shared research center “National Center of investigation of catalysts” at the Boreskov Institute of Catalysis. The authors are grateful to Alexandr Kalinkin who donated HOPG and GC samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goldstein, J.; Newbury, D.; Joy, D.; Lyman, C.; Echlin, P.; Lifshin, E.; Sawyer, L.; Michael, J. Scanning Electron Microscopy and X-ray Microanalysis; Springer: New York, NY, USA, 2003; pp. 591–619. [Google Scholar]
- Werny, M.J.; Müller, D.; Hendriksen, C.; Chan, R.; Friederichs, N.A.; Fella, C.; Meirer, F.; Weckhuysen, B.M. Elucidating the Sectioning Fragmentation Mechanism in Silica-Supported Olefin Polymerization Catalysts with Laboratory-Based X-ray and Electron Microscopy. ChemCatChem 2022, 14, e202200067. [Google Scholar] [CrossRef]
- Shams, A.; Mehdizadeh, M.; Teimoury, H.; Emami, M.; Mirmohammadi, S.A.; Sadjadi, S.; Bardají, E.; Poater, A. Effect of the pore architecture of Ziegler-Natta catalyst on its behavior in propylene/1-hexene copolymerization. J. Ind. Eng. Chem. 2022, 116, 359–370. [Google Scholar] [CrossRef]
- Hasanzadeh, R.; Najafi Moghadam, P.; Bahri-Laleh, N.; Sillanpää, M. Effective removal of toxic metal ions from aqueous solutions: 2-Bifunctional magnetic nanocomposite base on novel reactive PGMAMAncopolymer@Fe3O4 nanoparticles. J. Colloid Interface Sci. 2017, 490, 727–746. [Google Scholar] [CrossRef]
- Lv, A.; Chen, Q.; Zhao, C.; Li, S.; Sun, S.; Dong, J.; Li, Z.; Lin, H. Long-wavelength (red to near-infrared) emissive carbon dots: Key factors for synthesis, fluorescence mechanism, and applications in biosensing and cancer theranostics. Chin. Chem. Lett. 2021, 32, 3653–3664. [Google Scholar] [CrossRef]
- Kovalenko, G.A.; Kuznetsova, E.V.; Mogilnykh, Y.I.; Andreeva, I.S.; Kuvshinov, D.G.; Rudina, N.A. Catalytic filamenous carbon for immobilization of biologically active substances and non-growing bacterial cells. Carbon 2001, 39, 1033–1043. [Google Scholar] [CrossRef]
- Salanov, A.; Serkova, A.; Zhirnova, A.; Perminova, L.; Kovalenko, G. Supramolecular Aggregation of Nanoparticles on Aluminum and Gold Surfaces Occurring in Bovine Serum Albumin Solutions. Micro 2022, 2, 334–341. [Google Scholar] [CrossRef]
- Kibena, E.; Marandi, M.; Sammelselg, V.; Tammeveski, K.; Jensen, B.B.E.; Mortensen, A.B.; Lillethorup, M.; Kongsfelt, M.; Pedersen, S.U.; Daasbjerg, K. Electrochemical Behaviour of HOPG and CVD-Grown Graphene Electrodes Modified with Thick Anthraquinone Films by Diazonium Reduction. Electroanalysis 2014, 26, 2619–2630. [Google Scholar] [CrossRef]
- Hu, B.; Bharate, B.; Jimenez, J.D.; Lauterbach, J.; Todoroki, N.; Wadayama, T.; Higashi, K.; Takakusagi, S.; Asakura, K. Abnormal Metal Bond Distances in PtAu Alloy Nanoparticles: In Situ Back-Illumination XAFS Investigations of the Structure of PtAu Nanoparticles on a Flat HOPG Substrate Prepared by Arc Plasma Deposition. J. Phys. Chem. 2022, 126, 1006–1016. [Google Scholar] [CrossRef]
- Baxter, E.T.; Zhang, J.; Tan, S.; Nguyen, M.-T.; Zhang, D.; Yuan, Q.; Cao, W.; Glezakou, V.A.; Johnson, G.E. Functionalization of Electrodes with Tunable [EMIM]x[Cl]x+1-Ionic Liquid Clusters for Electrochemical Separations. Chem. Mater. 2022, 34, 2612–2623. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Xiao, P.; Wu, Y.; Liu, Y.; Jiang, Y.; Wang, X.; Han, J.; Xiao, W. Morphology-Controlled Electrocatalytic Performance of Two-Dimensional VSe2Nanoflakes for Hydrogen Evolution Reactions. ACS Appl. Nano Mater. 2022, 5, 2087–2093. [Google Scholar] [CrossRef]
- Mita, M.; Matsushima, H.; Ueda, M.; Ito, H. In-situ high-speed atomic force microscopy observation of dynamic nanobubbles during water electrolysis. J. Colloid Interface Sci. 2022, 614, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.T.; Mellado, J.M.R.; Medina, A. Rapid Electrochemical Determination of Antioxidant Capacity Using Glassy Carbon Electrodes Modified with Copper and Polyaniline. Application to Ascorbic and Gallic acids. Biointerface Res. Appl. Chem. 2022, 13, 23. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Fakayode, O.J.; Mamba, B.B.; Nkambule, T.T.I. Determination of humic acid (HA) and sodium alginate in water using Fe2O3 and CuO nanoparticle-modified glassy carbon electrode. Int. J. Environ. Anal. Chem. 2022, 102, 736–756. [Google Scholar] [CrossRef]
- Lavanya, A.L.; Bala Kumari, K.G.; Gowri, K.P.; Brahman, P.K. Fabrication of electrochemical sensor based on electrochemically co-deposited Ru-Co bimetallic nanoparticles on glassy carbon electrode: An analytical measurement tool for monitoring of hydrazine in water samples. Int. J. Environ. Anal. Chem. 2022, 102, 720–735. [Google Scholar] [CrossRef]
- Besharati, M.; Tabrizi, M.A.; Molaabasi, F.; Saber, R.; Shamsipur, M.; Hamedi, J.; Hosseinkhani, S. Novel enzyme-based electrochemical and colorimetric biosensors for tetracycline monitoring in mil. Biotechnol. Appl. Biochem. 2022, 69, 41–50. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, H.; Wang, P.; Zhang, C.; Wu, M.; Yang, L. A stable biosensor for organophosphorus pesticide detection based on chitosan modified graphene. Biotechnol. Appl. Biochem. 2022, 69, 567–575. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, T.; Fu, D.; Liu, M.; Cheng, Z.; Hua, Y.; Liu, J. The construction of molecularly imprinted electrochemical biosensor for selective glucose sensing based on the synergistic enzyme-enzyme mimic catalytic system. Talanta 2022, 242, 123279. [Google Scholar] [CrossRef]
- Kalaw, J.M.; Kitagawa, M.; Shigemitsu, H.; Kida, T. Highly Regulated Supramolecular Assembly of 2-O-Methylated α-Cyclodextrin to Construct Vertically Oriented Microrods on Graphite. Langmuir 2022, 38, 5149–5155. [Google Scholar] [CrossRef]
- Kong, H.; Liu, B.; Yang, G.; Chen, Y.; Wei, G. Tailoring Peptide Self-Assembly and Formation of 2D Nanoribbons on Mica and HOPG Surface. Materials 2022, 15, 310. [Google Scholar] [CrossRef]
- Ukraintsev, E.; Houska, V.; Rezek, B. Small angle symmetry splitting of helicene-based molecular wires on pyrolytic graphite. Carbon 2022, 193, 171–181. [Google Scholar] [CrossRef]
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal structure of human serum albumin at 2. 5 A resolution. Protein Eng. 1999, 12, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Jain, N.; Mukhopadhyay, S. Insights into the mechanism of aggregation and fibril formation from bovine serum albumin. J. Phys. Chem. B 2011, 115, 4195–4205. [Google Scholar] [CrossRef] [PubMed]
- Qingmin, Y.; Jing, C.; Facui, Y.; Yongchun, L.; Mengmeng, C.; Rongrong, Q.; Lixin, C.; Peng, Y. Amyloid-like aggregates of bovine serum albumin for extraction of gold from ores and electronic waste. Chem. Eng. J. 2021, 416, 129066. [Google Scholar]
- Nagy, D.; Toth, A.; Savina, I.; Mikhalovsky, S.; Mikhalovska, L.; Geissler, T.; Laszlo, K. Double probe approach to protein adsorption on porous carbon surfaces. Carbon 2017, 112, 103–110. [Google Scholar] [CrossRef]
- Duggal, R.; Hussain, F.; Pasquali, M. Self-Assembly of Single-Walled Carbon Nanotubes into a Sheet by Drop Drying. Adv. Mater. 2006, 18, 29–34. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).