Fluoroquinolone Metalloantibiotics: Fighting Staphylococcus aureus Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spectroscopic Measurements
2.2. Fluoroquinolone and Metalloantibiotic Preparation
2.3. Liposome Preparation
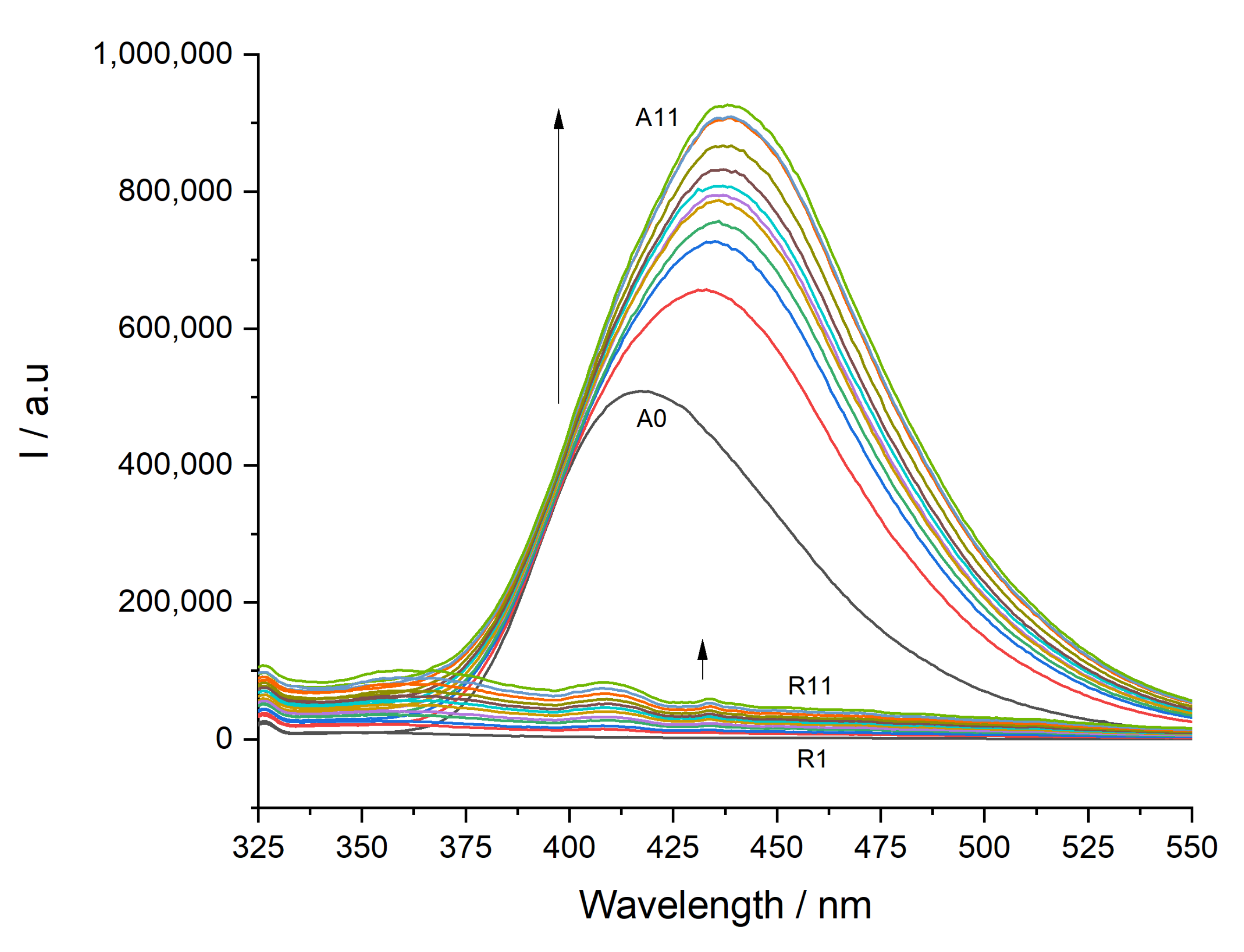
2.4. Partition Constants Determined by Steady-State Fluorescence Spectroscopy
- Steady-state fluorescence data analysis
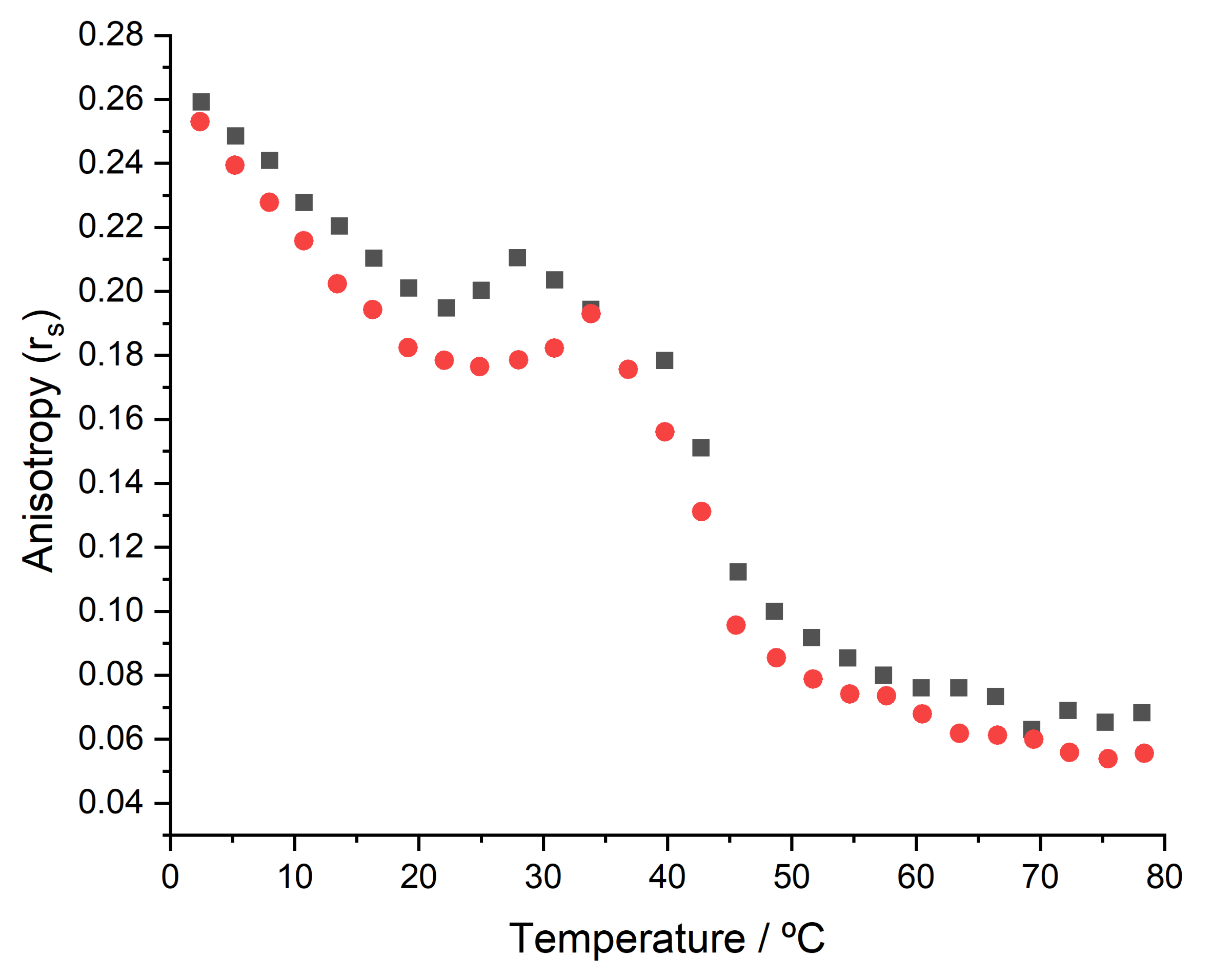
2.5. Thermotropic Properties Determined by Steady-State Fluorescence Anisotropy
- Steady-state fluorescence anisotropy data analysis
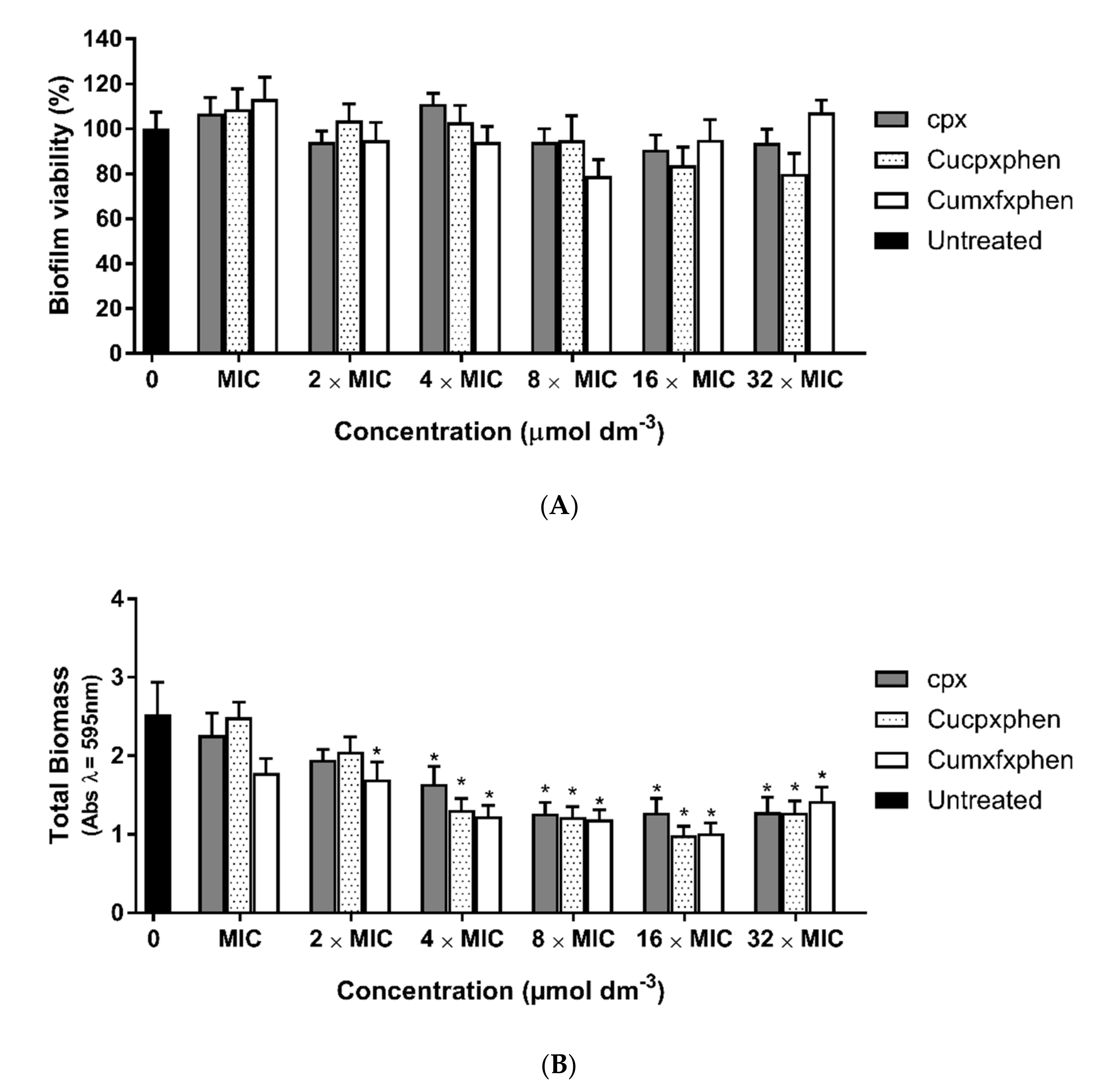
2.6. Antibiofilm Activity
2.6.1. Bacterial Strains and Growth Conditions
2.6.2. Biofilm Treatment Assay
- MTT Assay
- Crystal Violet Assay
2.7. Membrane Fluidity Studies
- Laurdan generalized polarization measurements
3. Results and Discussion
3.1. Determination of Partition Constants
3.2. Thermotropic Properties of Membranes
3.3. Antibiofilm Activity
3.4. Membrane Fluidity Assays
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 4 May 2022).
- Spellberg, B.; Gilbert, D.N. The future of antibiotics and resistance: A tribute to a career of leadership by John Bartlett. Clin. Infect. Dis. 2014, 59 (Suppl. S2), S71–S75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shlaes, D.M.; Sahm, D.; Opiela, C.; Spellberg, B. The FDA reboot of antibiotic development. Antimicrob. Agents Chemother. 2013, 57, 4605–4607. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Fàbrega, A.; Madurga, S.; Giralt, E.; Vila, J. Mechanism of action of and resistance to quinolones. Microb. Biotechnol. 2009, 2, 40–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gellert, M.; Mizuuchi, K.; O’Dea, M.H.; Itoh, T.; Tomizawa, J.I. Nalidixic acid resistance: A second genetic character involved in DNA gyrase activity. Proc. Natl. Acad. Sci. USA 1977, 74, 4772–4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, K.L.; Davies, R.H.; Threlfall, E.J. Mechanisms of quinolone resistance in Escherichia coli and Salmonella: Recent developments. Int. J. Antimicrob. Agents 2005, 25, 358–373. [Google Scholar] [CrossRef]
- Ruiz, J. Transferable Mechanisms of Quinolone Resistance from 1998 Onward. Clin. Microbiol. Rev. 2019, 32, e00007–e00019. [Google Scholar] [CrossRef]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef] [Green Version]
- Lopes, S.C.; Ribeiro, C.; Gameiro, P. A new approach to counteract bacteria resistance: A comparative study between moxifloxacin and a new moxifloxacin derivative in different model systems of bacterial membrane. Chem. Biol. Drug Des. 2013, 81, 265–274. [Google Scholar] [CrossRef]
- López-Gresa, M.P.; Ortiz, R.; Perelló, L.; Latorre, J.; Liu-González, M.; García-Granda, S.; Pérez-Priede, M.; Cantón, E. Interactions of metal ions with two quinolone antimicrobial agents (cinoxacin and ciprofloxacin). Spectroscopic and X-ray structural characterization. Antibacterial studies. J. Inorg. Biochem. 2002, 92, 65–74. [Google Scholar] [CrossRef]
- Wu, G.; Wang, G.; Fu, X.; Zhu, L. Synthesis, Crystal Structure, Stacking Effect and Antibacterial Studies of a Novel Quaternary Copper (II) Complex with Quinolone. Molecules 2003, 8, 287–296. [Google Scholar] [CrossRef]
- Macías, B.; Villa, M.V.; Rubio, I.; Castiñeiras, A.; Borrás, J. Complexes of Ni(II) and Cu(II) with ofloxacin. Crystal structure of a new Cu(II) ofloxacin complex. J. Inorg. Biochem. 2001, 84, 163–170. [Google Scholar] [CrossRef]
- Ferreira, M.; Gameiro, P. Fluoroquinolone-Transition Metal Complexes: A Strategy to Overcome Bacterial Resistance. Microorganisms 2021, 9, 1506. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Gameiro, P. Ciprofloxacin metalloantibiotic: An effective antibiotic with an influx route strongly dependent on lipid interaction? J. Membr. Biol. 2015, 248, 125–136. [Google Scholar] [CrossRef]
- Ribeiro, C.; Lopes, S.C.; Gameiro, P. New Insights into the Translocation Route of Enrofloxacin and Its Metalloantibiotics. J. Membr. Biol. 2011, 241, 117–125. [Google Scholar] [CrossRef]
- Feio, M.J.; Sousa, I.; Ferreira, M.; Cunha-Silva, L.; Saraiva, R.G.; Queirós, C.; Alexandre, J.G.; Claro, V.; Mendes, A.; Ortiz, R.; et al. Fluoroquinolone–metal complexes: A route to counteract bacterial resistance? J. Inorg. Biochem. 2014, 138, 129–143. [Google Scholar] [CrossRef]
- Ferreira, M.; Bessa, L.J.; Sousa, C.F.; Eaton, P.; Bongiorno, D.; Stefani, S.; Campanile, F.; Gameiro, P. Fluoroquinolone Metalloantibiotics: A Promising Approach against Methicillin-Resistant Staphylococcus aureus. Int. J. Environ. Res. Public Health 2020, 17, 3127. [Google Scholar] [CrossRef]
- Sousa, C.F.; Coimbra, J.T.S.; Ferreira, M.; Pereira-Leite, C.; Reis, S.; Ramos, M.J.; Fernandes, P.A.; Gameiro, P. Passive Diffusion of Ciprofloxacin and its Metalloantibiotic: A Computational and Experimental study. J. Mol. Biol. 2021, 433, 166911. [Google Scholar] [CrossRef]
- Sousa, C.F.; Coimbra, J.T.S.; Gomes, I.; Franco, R.; Fernandes, P.A.; Gameiro, P. The binding of free and copper-complexed fluoroquinolones to OmpF porins: An experimental and molecular docking study. RSC Adv. 2017, 7, 10009–10019. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.; Sousa, C.F.; Gameiro, P. Fluoroquinolone Metalloantibiotics to Bypass Antimicrobial Resistance Mechanisms: Decreased Permeation through Porins. Membranes 2021, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Mohamed, Z.J.; Artim, C.M.; Thornlow, D.N.; Hassler, J.F.; Rigoglioso, V.P.; Daniel, S.; Alabi, C.A. Antibacterial isoamphipathic oligomers highlight the importance of multimeric lipid aggregation for antibacterial potency. Commun. Biol. 2018, 1, 220. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Epand, R.F. Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim. Biophys. Acta. 2009, 1788, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, T. Fluorescence Inner-Filtering Correction for Determining the Humification Index of Dissolved Organic Matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Cox, L.; Celis, R.; Hermosín, M.C.; Cornejo, J.; Zsolnay, A.; Zeller, K. Effect of Organic Amendments on Herbicide Sorption as Related to the Nature of the Dissolved Organic Matter. Environ. Sci. Technol. 2000, 34, 4600–4605. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H. Thin-Film Hydration Followed by Extrusion Method for Liposome Preparation. Methods Mol. Biol. 2017, 1522, 17–22. [Google Scholar] [CrossRef] [PubMed]
- McClare, C.W. An accurate and convenient organic phosphorus assay. Anal Biochem. 1971, 39, 527–530. [Google Scholar] [CrossRef]
- Bartlett, G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959, 234, 466–468. [Google Scholar] [CrossRef]
- Coutinho, A.; Prieto, M. Ribonuclease T1 and alcohol dehydrogenase fluorescence quenching by acrylamide: A laboratory experiment for undergraduate students. J. Chem. Educ. 1993, 70, 425. [Google Scholar] [CrossRef]
- Rodrigues, C.; Gameiro, P.; Reis, S.; Lima, J.L.F.C.; de Castro, B. Interaction of Grepafloxacin with Large Unilamellar Liposomes: Partition and Fluorescence Studies Reveal the Importance of Charge Interactions. Langmuir 2002, 18, 10231–10236. [Google Scholar] [CrossRef]
- Coutinho, A.; Prieto, M. Self-association of the polyene antibiotic nystatin in dipalmitoylphosphatidylcholine vesicles: A time-resolved fluorescence study. Biophys. J. 1995, 69, 2541–2557. [Google Scholar] [CrossRef] [Green Version]
- Lopes, S.; Neves, C.S.; Eaton, P.; Gameiro, P. Cardiolipin, a key component to mimic the E. coli bacterial membrane in model systems revealed by dynamic light scattering and steady-state fluorescence anisotropy. Anal Bioanal. Chem. 2010, 398, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Phase Transition Temperatures for Glycerophospholipids. Available online: https://avantilipids.com/tech-support/physical-properties/phase-transition-temps (accessed on 21 April 2022).
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Boston, MA, USA, 2006; p. 954. [Google Scholar] [CrossRef]
- Merino-Montero, S.; Montero, M.T.; Hernández-Borrell, J. Effects of lactose permease of Escherichia coli on the anisotropy and electrostatic surface potential of liposomes. Biophys. Chem. 2006, 119, 101–105. [Google Scholar] [CrossRef]
- Bessa, L.J.; Eaton, P.; Dematei, A.; Plácido, A.; Vale, N.; Gomes, P.; Delerue-Matos, C.; Leite, J.R.S.; Gameiro, P. Synergistic and antibiofilm properties of ocellatin peptides against multidrug-resistant Pseudomonas aeruginosa. Future Microbiol. 2018, 13, 151–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessa, L.J.; Ferreira, M.; Gameiro, P. Evaluation of membrane fluidity of multidrug-resistant isolates of Escherichia coli and Staphylococcus aureus in presence and absence of antibiotics. J. Photochem. Photobiol. B. 2018, 181, 150–156. [Google Scholar] [CrossRef]
- Jay, A.G.; Hamilton, J.A. Disorder Amidst Membrane Order: Standardizing Laurdan Generalized Polarization and Membrane Fluidity Terms. J. Fluoresc. 2017, 27, 243–249. [Google Scholar] [CrossRef]
- Esteves, F.; Moutinho, C.; Matos, C. Correlation between octanol/water and liposome/water distribution coefficients and drug absorption of a set of pharmacologically active compounds. J. Liposome Res. 2013, 23, 83–93. [Google Scholar] [CrossRef]
- Pávez, P.; Herrera, B.; Toro-Labbé, A.; Encinas, M.V. Structure and medium effects on the photochemical behavior of nonfluorinated quinolone antibiotics. Photochem. Photobiol. 2007, 83, 511–519. [Google Scholar] [CrossRef]
- Strahl, H.; Errington, J. Bacterial. Membranes: Structure, Domains, and Function. Annu. Rev. Microbiol. 2017, 71, 519–538. [Google Scholar] [CrossRef]
- Jouhet, J. Importance of the hexagonal lipid phase in biological membrane organization. Front. Plant Sci. 2013, 4, 494. [Google Scholar] [CrossRef] [Green Version]
- ISO 22196:2011; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. International Organization for Standardization: Geneva, Switzerland, 2011.
- Macia, M.D.; Rojo-Molinero, E.; Oliver, A. Antimicrobial. susceptibility. testing. in. biofilm-growing. bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miquel, S.; Lagrafeuille, R.; Souweine, B.; Forestier, C. Anti-biofilm Activity as a Health Issue. Front. Microbiol. 2016, 7, 592. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Ferreira, M.; Gameiro, P. Data on Laurdan spectroscopic analyses to compare membrane fluidity between susceptible and multidrug-resistant bacteria. Data Brief 2018, 21, 128–132. [Google Scholar] [CrossRef] [PubMed]




| Membrane Mimetic System | cpx | mxfx | Cucpxphen | Cumxfxphen |
|---|---|---|---|---|
| POPG | 2.72 ± 0.09 | 3.46 ± 0.07 | 3.54 ± 0.07 | 5.20 ± 0.30 |
| POPG:CL (58:42) | 2.75 ± 0.08 | 3.70 ± 0.10 | 3.73 ± 0.07 | N.D. |
| POPG/CL/DAG (11:5:4) | 2.70 ± 0.10 | N.D. | N.D. | 3.89 ± 0.04 |
| Membrane Mimetic System | Absence of Compound | cpx | mxfx | Cucpxphen | Cumxfxphen |
|---|---|---|---|---|---|
| POPG:CL (58:42) | 10.4 ± 0.3 | 8.9 ± 0.9 | 8.0 ± 0.8 | 9.5 ± 0.1 | 9.1 ± 0.4 |
| POPG/CL/DAG (11:5:4) | 10.7 ± 0.1 | 9.7 ± 0.2 | 9.8 ± 0.1 | 10.3 ± 0.1 | 10.6 ± 0.1 |
| POPG:CL (58:42) | 44.8 ± 0.4 | 46.4 ± 0.1 | 47.7 ± 0.5 | 47.4 ± 0.5 | 45.1 ± 0.4 |
| POPG/CL/DAG (11:5:4) | 43.6 ± 0.2 | 42.5 ± 0.1 | 42.5 ± 0.2 | 41.1 ± 0.1 | 40.9 ± 0.3 |
| Values Determined at 37.0 ± 0.1 °C | |||||
| POPG | 5.92 × 10−2 ± 8.00 × 10−4 | 6.44 × 10−2 ± 1.00 × 10−4 | 6.20 × 10−2 ± 1.10 × 10−3 | 7.46 × 10−2 ± 5.00 × 10−4 | 8.01 × 10−2 ± 4.00 × 10−4 |
| MRSA Sa3 | [cpx]/ μmoldm−3 | [Cucpxphen]/ μmoldm−3 | ||
|---|---|---|---|---|
| Control | 0.245 ± 0.008 | 0 | 0.243 ± 0.009 | 0 |
| ½× MIC | 0.266 ± 0.005 | 193 | 0.409 ± 0.014 | 45 |
| MIC | 0.280 ± 0.006 | 386 | 0.426 ± 0.004 | 90 |
| 2× MIC | 0.298 ± 0.005 | 773 | 0.418 ± 0.012 | 180 |
| MRSA Sa3 | [mxfx]/ μmoldm−3 | [Cumxfxphen]/ μmoldm−3 | ||
| Control | 0.257 ± 0.006 | 0 | 0.243 ± 0.009 | 0 |
| ½× MIC | 0.285 ± 0.005 | 146 | 0.251 ± 0.004 | 5 |
| MIC | 0.295 ± 0.013 | 292 | 0.264 ± 0.014 | 10 |
| 2× MIC | 0.289 ± 0.008 | 585 | 0.326 ± 0.044 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.; Ribeiro, B.; Seabra, C.L.; Ferreira, A.R.; Gameiro, P. Fluoroquinolone Metalloantibiotics: Fighting Staphylococcus aureus Biofilms. Micro 2022, 2, 410-425. https://doi.org/10.3390/micro2030027
Ferreira M, Ribeiro B, Seabra CL, Ferreira AR, Gameiro P. Fluoroquinolone Metalloantibiotics: Fighting Staphylococcus aureus Biofilms. Micro. 2022; 2(3):410-425. https://doi.org/10.3390/micro2030027
Chicago/Turabian StyleFerreira, Mariana, Bruno Ribeiro, Catarina Leal Seabra, Ana Rita Ferreira, and Paula Gameiro. 2022. "Fluoroquinolone Metalloantibiotics: Fighting Staphylococcus aureus Biofilms" Micro 2, no. 3: 410-425. https://doi.org/10.3390/micro2030027
APA StyleFerreira, M., Ribeiro, B., Seabra, C. L., Ferreira, A. R., & Gameiro, P. (2022). Fluoroquinolone Metalloantibiotics: Fighting Staphylococcus aureus Biofilms. Micro, 2(3), 410-425. https://doi.org/10.3390/micro2030027









