Thermodynamic Constraints on the “Hidden” Folding Intermediates
Abstract
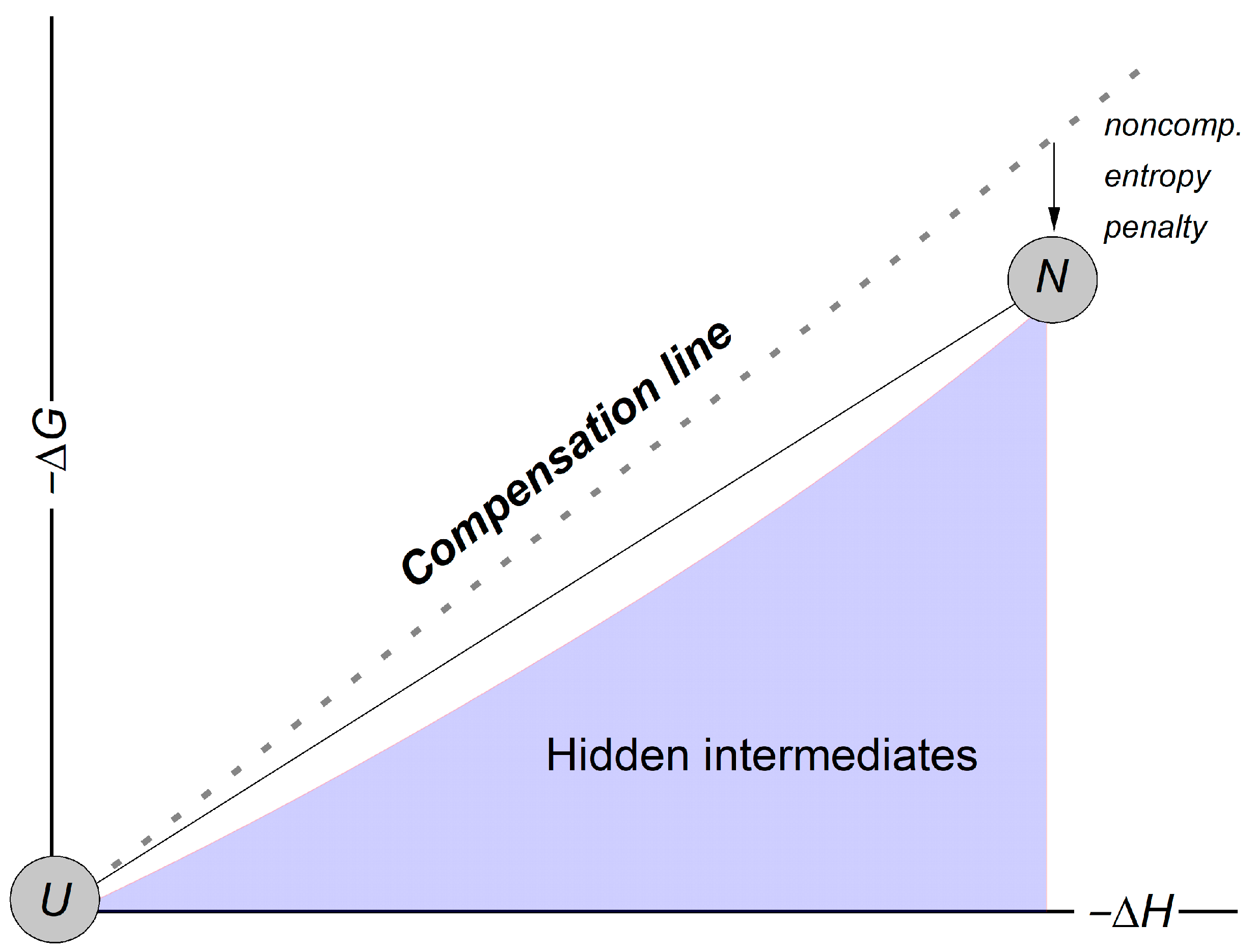
1. Introduction
2. Theoretical Unfolding Curves
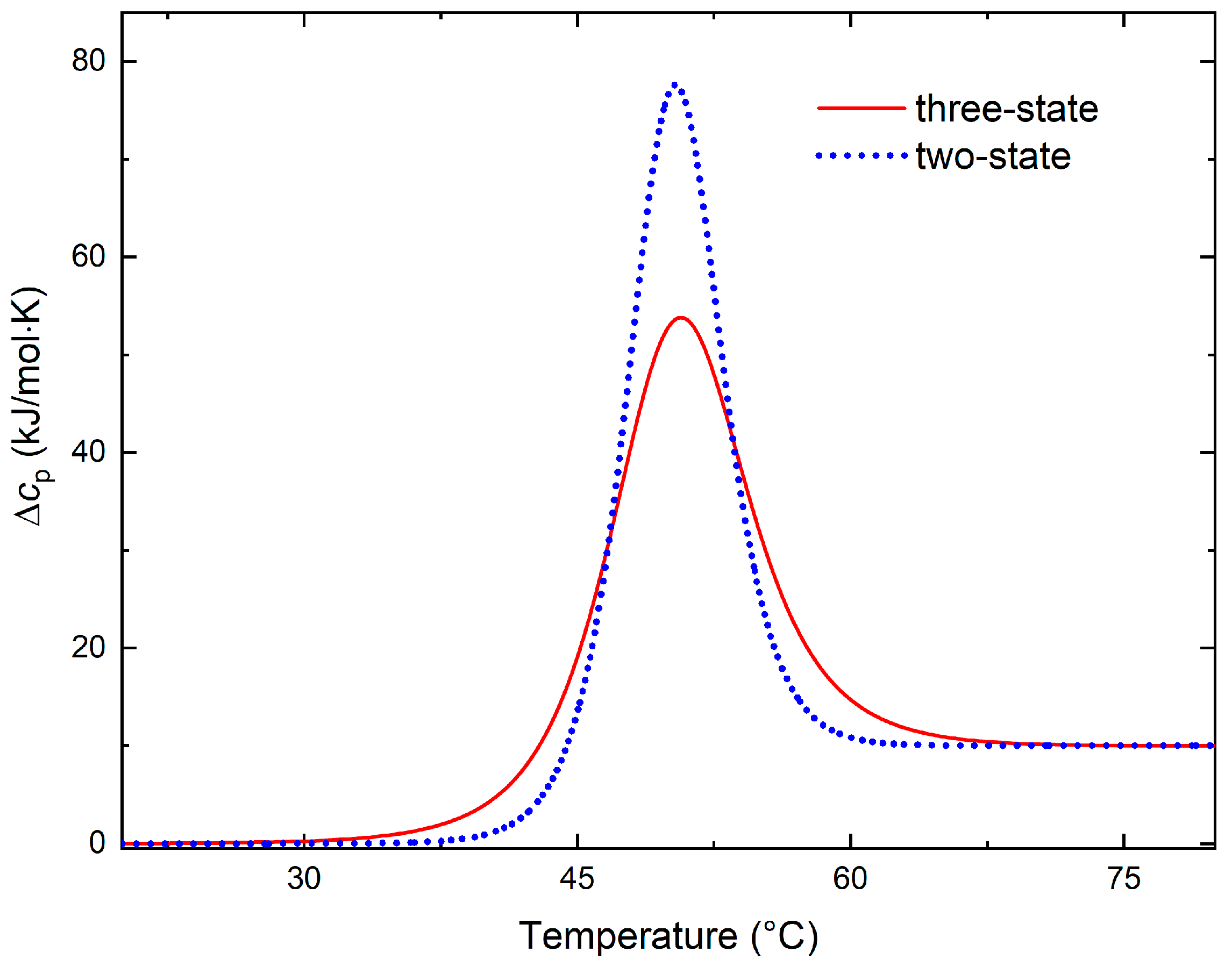
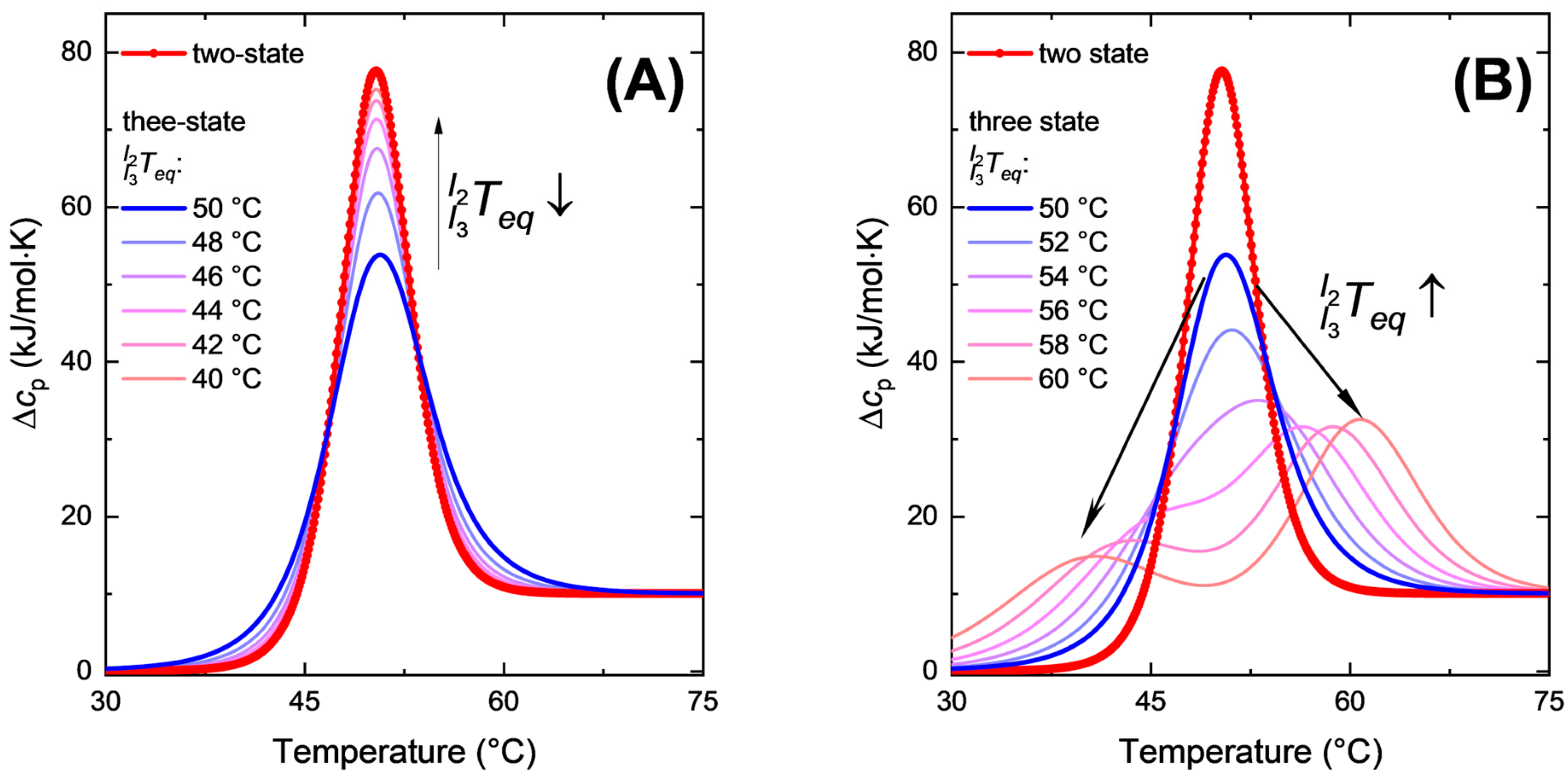
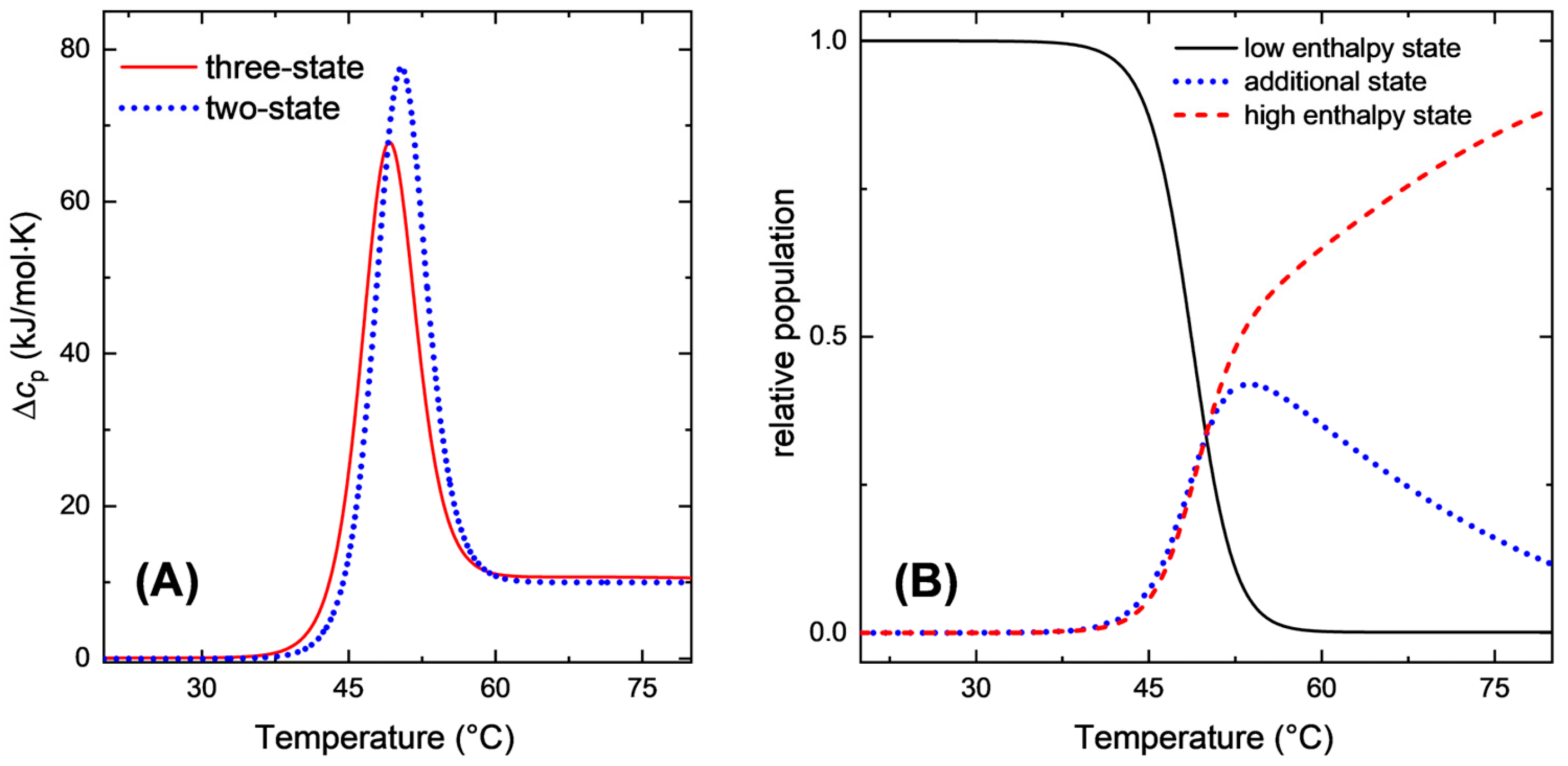
2.1. Equilibrium Calorimetric Curves with More than Two States
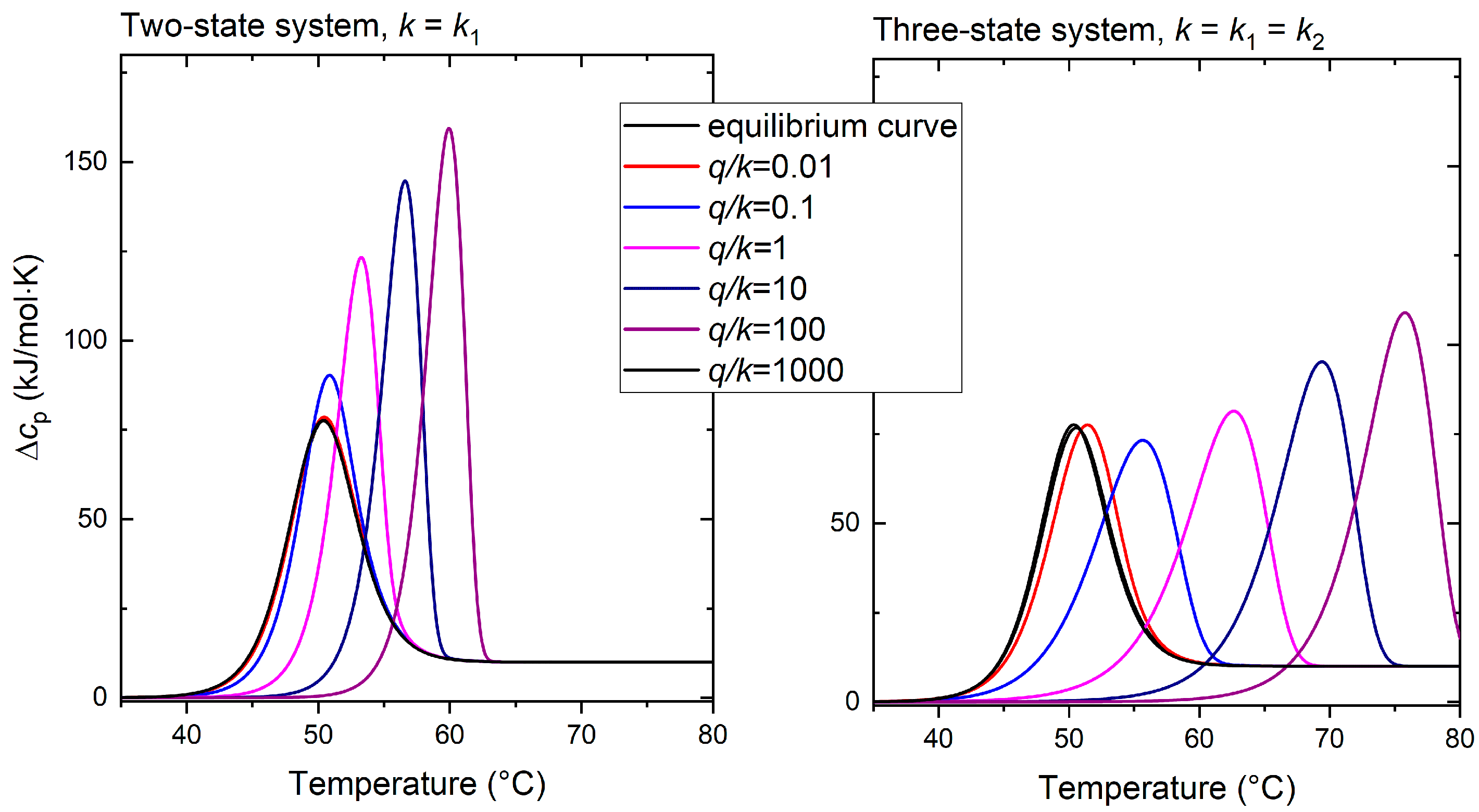
2.2. Kinetic Behavior
3. Practical Considerations
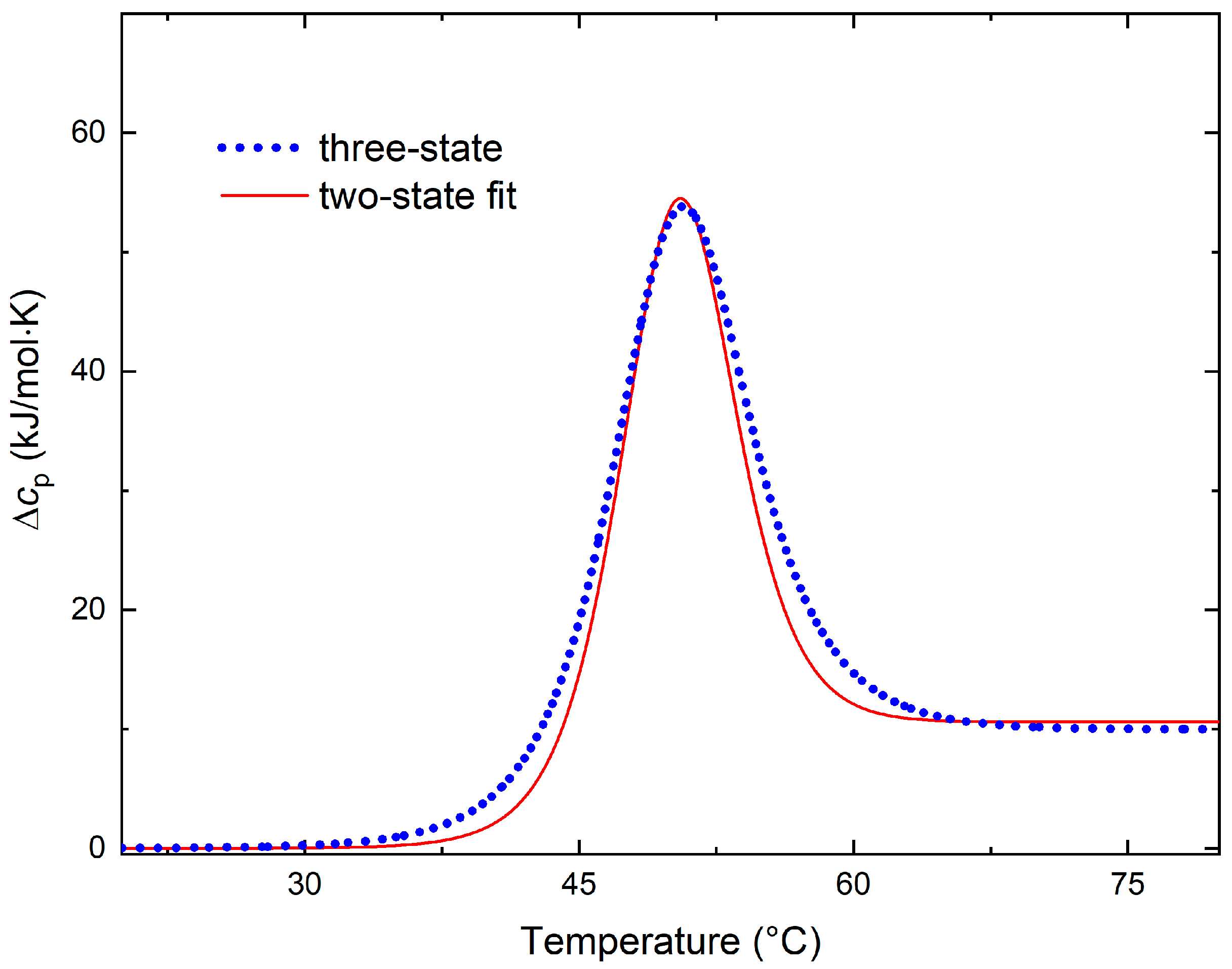
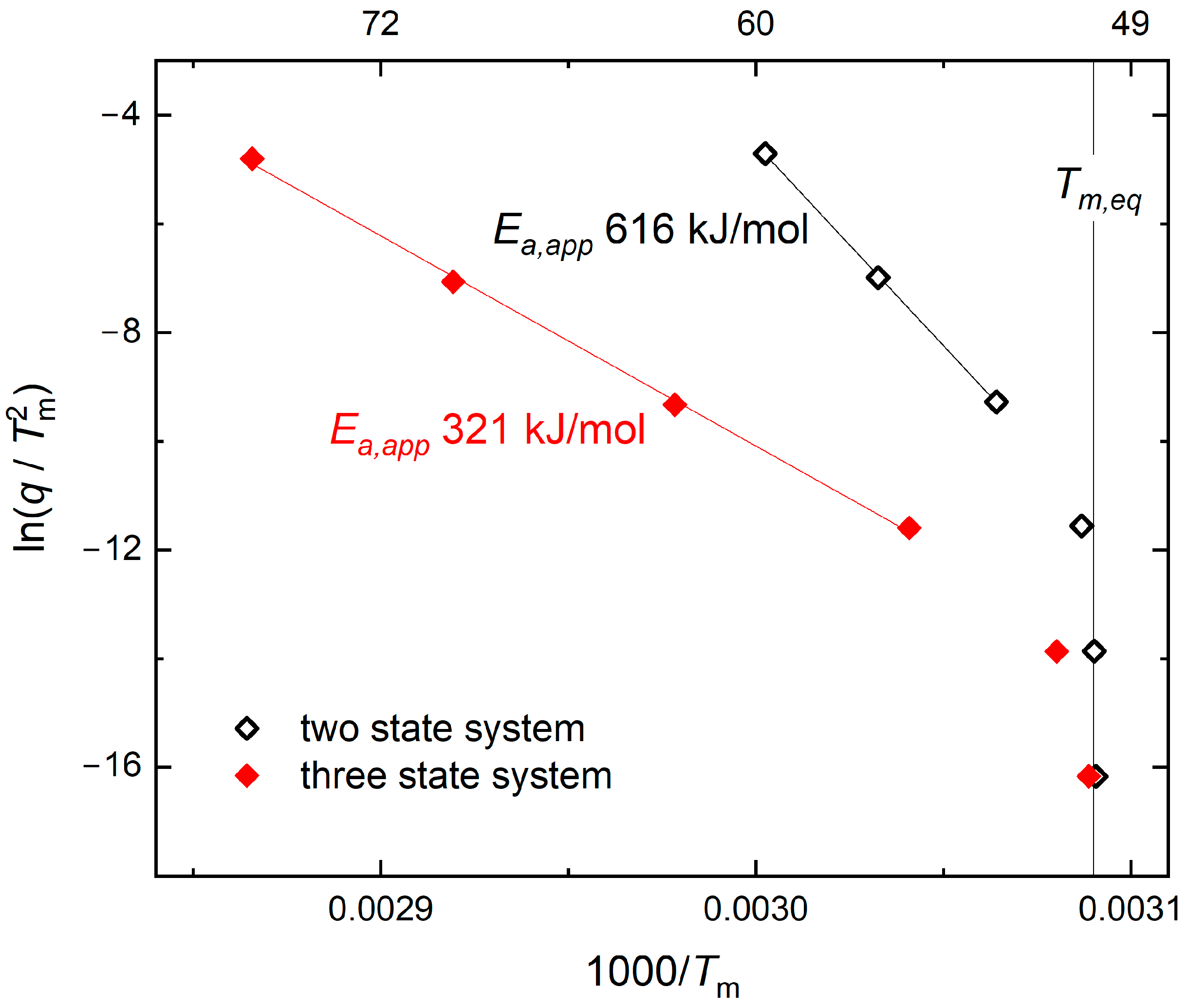
3.1. Kinetic Aspects
3.2. Cold-Denaturation
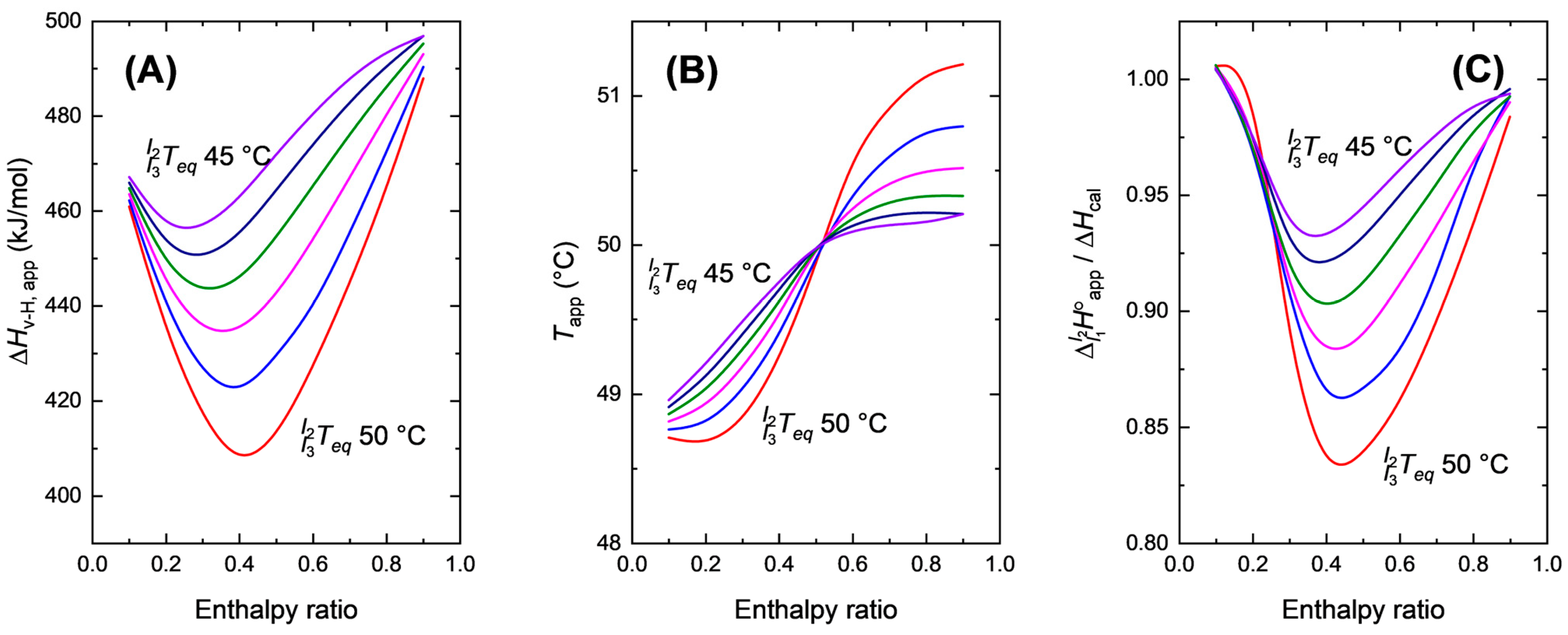
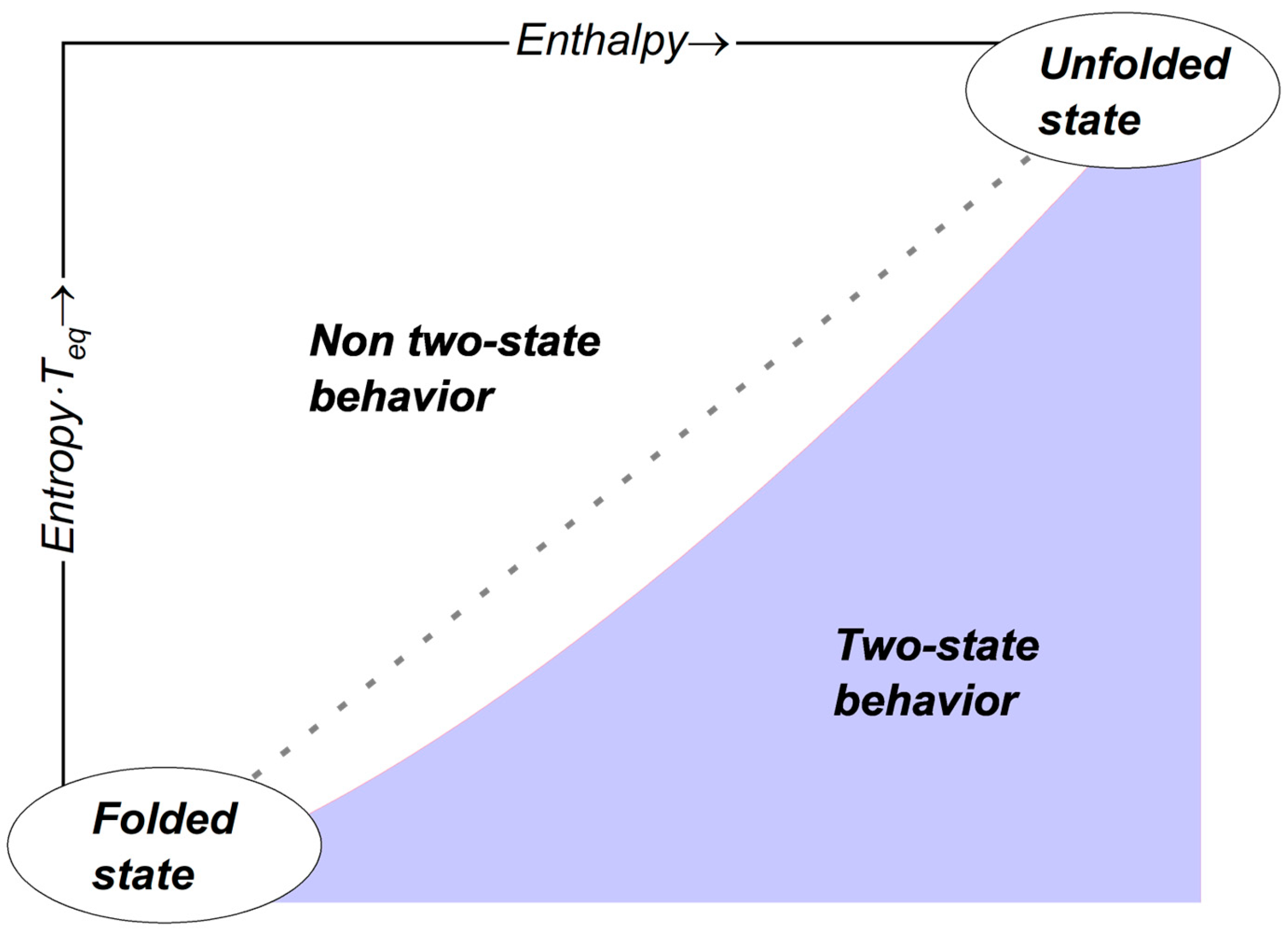
3.3. Thermodynamic Aspects
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Theory
Appendix A.2. The Calorimetric Criterion for a Two-State Folding
Appendix A.3. Calorimetric Curve Generation
References
- Anfinsen, C.B. Principles that govern the folding of protein chains. Science 1973, 181, 223–230. [Google Scholar] [CrossRef]
- Chan, H.S.; Dill, K.A. Protein folding in the landscape perspective: Chevron plots and non-arrhenius kinetics. Proteins Struct. Funct. Genet. 1998, 30, 2–33. [Google Scholar] [CrossRef]
- Dill, K.A. Polymer principles and protein folding. Protein Sci. 1999, 8, 1166–1180. [Google Scholar] [CrossRef] [PubMed]
- Onuchic, J.N.; Luthey-Schulten, Z.; Wolynes, P.G. Theory of Protein Folding: The Energy Landscape Perspective. Annu. Rev. Phys. Chem. 1997, 48, 545–600. [Google Scholar] [CrossRef] [PubMed]
- Englander, S.W. Protein Folding Intermediates and Pathways Studied by Hydrogen Exchange. Annu. Rev. Biophys. 2000, 29, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Walters, B.T.; Mayne, L.; Hinshaw, J.R.; Sosnick, T.R.; Englander, S.W. Folding of a large protein at high structural resolution. Proc. Natl. Acad. Sci. USA 2013, 110, 18898–18903. [Google Scholar] [CrossRef]
- Baldwin, R.L. The Search for Folding Intermediates and the Mechanism of Protein Folding. Annu. Rev. Biophys. 2008, 37, 1–21. [Google Scholar] [CrossRef]
- Jain, A.; Stock, G. Identifying Metastable States of Folding Proteins. J. Chem. Theory Comput. 2012, 8, 3810–3819. [Google Scholar] [CrossRef]
- Kumar, S.; Tsai, C.-J.; Nussinov, R. Maximal Stabilities of Reversible Two-State Proteins. Biochemistry 2002, 41, 5359–5374. [Google Scholar] [CrossRef]
- Privalov, P.L.; Khechinashvili, N.N. A thermodynamic approach to the problem of stabilization of globular protein structure: A calorimetric study. J. Mol. Biol. 1974, 86, 665–684. [Google Scholar] [CrossRef]
- Privalov, P.L. Cold Denaturation of Protein. Crit. Rev. Biochem. Mol. Biol. 1990, 25, 281–306. [Google Scholar] [CrossRef]
- Zhou, Y.; Hall, C.K.; Karplus, M. The calorimetric criterion for a two-state process revisited. Protein Sci. 1999, 8, 1064–1074. [Google Scholar] [CrossRef]
- Knott, M.; Chan, H.S. Criteria for downhill protein folding: Calorimetry, chevron plot, kinetic relaxation, and single-molecule radius of gyration in chain models with subdued degrees of cooperativity. Proteins Struct. Funct. Bioinform. 2006, 65, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Chan, H.S. Towards a consistent modeling of protein thermodynamic and kinetic cooperativity: How applicable is the transition state picture to folding and unfolding? J. Mol. Biol. 2002, 315, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.; Chan, H.S. Polymer principles of protein calorimetric two-state cooperativity. Proteins Struct. Funct. Bioinform. 2000, 40, 637–661. [Google Scholar] [CrossRef]
- Akmal, A.; Muñoz, V. The nature of the free energy barriers to two-state folding. Proteins Struct. Funct. Bioinform. 2004, 57, 142–152. [Google Scholar] [CrossRef]
- Seelig, J.; Seelig, A. Protein Unfolding-Thermodynamic Perspectives and Unfolding Models. Int. J. Mol. Sci. 2023, 24, 5457. [Google Scholar] [CrossRef]
- Sánchez, I.E.; Kiefhaber, T. Evidence for Sequential Barriers and Obligatory Intermediates in Apparent Two-state Protein Folding. J. Mol. Biol. 2003, 325, 367–376. [Google Scholar] [CrossRef]
- Wildegger, G.; Kiefhaber, T. Three-state model for lysozyme folding: Triangular folding mechanism with an energetically trapped intermediate11Edited by A. R. Fresht. J. Mol. Biol. 1997, 270, 294–304. [Google Scholar] [CrossRef]
- Radford, S.E.; Dobson, C.M.; Evans, P.A. The folding of hen lysozyme involves partially structured intermediates and multiple pathways. Nature 1992, 358, 302–307. [Google Scholar] [CrossRef]
- Fatkhutdinova, A.; Mukhametzyanov, T.; Schick, C. Refolding of Lysozyme in Glycerol as Studied by Fast Scanning Calorimetry. Int. J. Mol. Sci. 2022, 23, 2773. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, E.; Makshakova, O.; Zuev, Y.; Sedov, I. Beta-rich intermediates in denaturation of lysozyme: Accelerated molecular dynamics simulations. J. Biomol. Struct. Dyn. 2022, 40, 13953–13964. [Google Scholar] [CrossRef] [PubMed]
- Magsumov, T.; Ziying, L.; Sedov, I. Comparative study of the protein denaturing ability of different organic cosolvents. Int. J. Biol. Macromol. 2020, 160, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Magsumov, T.; Fatkhutdinova, A.; Mukhametzyanov, T.; Sedov, I. The Effect of Dimethyl Sulfoxide on the Lysozyme Unfolding Kinetics, Thermodynamics, and Mechanism. Biomolecules 2019, 9, 547. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783319141756. [Google Scholar]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Mukhametzyanov, T.A.; Sedov, I.A.; Solomonov, B.N.; Schick, C. Fast scanning calorimetry of lysozyme unfolding at scanning rates from 5 K/min to 500,000 K/min. Biochim. Biophys. Acta-Gen. Subj. 2018, 1862, 2024–2030. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Dill, K.A.; MacCallum, J.L. The Protein-Folding Problem, 50 Years on. Science 2012, 338, 1042–1046. [Google Scholar] [CrossRef]
- Bartlett, A.I.; Radford, S.E. An expanding arsenal of experimental methods yields an explosion of insights into protein folding mechanisms. Nat. Struct. Mol. Biol. 2009, 16, 582–588. [Google Scholar] [CrossRef]
- Lyubarev, A.; Kurganov, B. Analysis of DSC data relating to proteins undergoing irreversible thermal denaturation. J. Therm. Anal. Calorim. 2000, 62, 51–62. [Google Scholar] [CrossRef]
- Splinter, R.; van Herwaarden, A.W.; van Wetten, I.A.; Pfreundt, A.; Svendsen, W.E. Fast differential scanning calorimetry of liquid samples with chips. Thermochim. Acta 2015, 603, 162–171. [Google Scholar] [CrossRef]
- Ibarra-Molero, B.; Naganathan, A.N.; Sanchez-Ruiz, J.M.; Muñoz, V. Modern Analysis of Protein Folding by Differential Scanning Calorimetry. In Methods in Enzymology; Academic Press Inc.: San Diego, CA, USA, 2016; Volume 567, pp. 281–318. [Google Scholar]
- Pace, C.N.; Laurents, D.V. A new method for determining the heat capacity change for protein folding. Biochemistry 1989, 28, 2520–2525. [Google Scholar] [CrossRef]
- Bieri, O.; Kiefhaber, T. Origin of apparent fast and non-exponential kinetics of lysozyme folding measured in pulsed hydrogen exchange experiment. J. Mol. Biol. 2001, 310, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, Q.-X. Isokinetic Relationship, Isoequilibrium Relationship, and Enthalpy−Entropy Compensation. Chem. Rev. 2001, 101, 673–696. [Google Scholar] [CrossRef] [PubMed]
- Dumas, P. Enthalpy-Entropy Compensation. bioRxiv 2025. [Google Scholar] [CrossRef]
- Li, J.; Hou, C.; Ma, X.; Guo, S.; Zhang, H.; Shi, L.; Liao, C.; Zheng, B.; Ye, L.; Yang, L.; et al. Entropy-Enthalpy Compensations Fold Proteins in Precise Ways. Int. J. Mol. Sci. 2021, 22, 9653. [Google Scholar] [CrossRef] [PubMed]
- Solomonov, B.N.; Yagofarov, M.I. The relationship between the Gibbs energies and enthalpies of hydrogen bonding and charge-transfer complex formation in non-electrolytes solutions. Is it a rule? J. Mol. Liq. 2025, 424, 127053. [Google Scholar] [CrossRef]
- Azizi, K.; Laio, A.; Hassanali, A. Solvation thermodynamics from cavity shapes of amino acids. Proc. Natl. Acad. Sci. USA 2023, 2, pgad239. [Google Scholar] [CrossRef]
- Sedov, I.A.; Stolov, M.A.; Solomonov, B.N. Solvophobic effects and relationships between the Gibbs energy and enthalpy for the solvation process. J. Phys. Org. Chem. 2011, 24, 1088–1094. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Yagofarov, M.I. Compensation relationship in thermodynamics of solvation and vaporization: Features and applications. II. Hydrogen-bonded systems. J. Mol. Liq. 2023, 372, 121205. [Google Scholar] [CrossRef]
- ten Wolde, P.R.; Chandler, D. Drying-induced hydrophobic polymer collapse. Proc. Natl. Acad. Sci. USA 2002, 99, 6539–6543. [Google Scholar] [CrossRef]
- Azizi, K.; Laio, A.; Hassanali, A. Model Folded Hydrophobic Polymers Reside in Highly Branched Voids. J. Phys. Chem. Lett. 2022, 13, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lapidus, L.J. Exploring the top of the protein folding funnel by experiment. Curr. Opin. Struct. Biol. 2013, 23, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Cicero, N.; Vasi, S.; Dugo, G.; Stanley, H.E. Dynamical changes in hydration water accompanying lysozyme thermal denaturation. Front. Phys. 2015, 10, 106104. [Google Scholar] [CrossRef]
- Mallamace, D.; Fazio, E.; Mallamace, F.; Corsaro, C. The Role of Hydrogen Bonding in the Folding/Unfolding Process of Hydrated Lysozyme: A Review of Recent NMR and FTIR Results. Int. J. Mol. Sci. 2018, 19, 3825. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.T.; LeBlanc, M.-A.; Perkins, T.T. Modulation of a protein-folding landscape revealed by AFM-based force spectroscopy notwithstanding instrumental limitations. Proc. Natl. Acad. Sci. USA 2021, 118, e2015728118. [Google Scholar] [CrossRef]
- Kamagata, K.; Arai, M.; Kuwajima, K. Unification of the Folding Mechanisms of Non-two-state and Two-state Proteins. J. Mol. Biol. 2004, 339, 951–965. [Google Scholar] [CrossRef]
- Kuwajima, K. The Molten Globule, and Two-State vs. Non-Two-State Folding of Globular Proteins. Biomolecules 2020, 10, 407. [Google Scholar] [CrossRef]
- Freire, E. Differential scanning calorimetry. In Protein Stability and Folding Theory Practice; Humana Press: Totowa, NJ, USA, 1995; pp. 191–218. [Google Scholar]
- Malakhov, D.V.; Abou Khatwa, M.K. Constructinga self-consistent integral baseline by using cubic splines. J. Therm. Anal. Calorim. 2007, 87, 595–599. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhametzyanov, T.A.; Yagofarov, M.I.; Schick, C. Thermodynamic Constraints on the “Hidden” Folding Intermediates. Liquids 2025, 5, 24. https://doi.org/10.3390/liquids5030024
Mukhametzyanov TA, Yagofarov MI, Schick C. Thermodynamic Constraints on the “Hidden” Folding Intermediates. Liquids. 2025; 5(3):24. https://doi.org/10.3390/liquids5030024
Chicago/Turabian StyleMukhametzyanov, Timur A., Mikhail I. Yagofarov, and Christoph Schick. 2025. "Thermodynamic Constraints on the “Hidden” Folding Intermediates" Liquids 5, no. 3: 24. https://doi.org/10.3390/liquids5030024
APA StyleMukhametzyanov, T. A., Yagofarov, M. I., & Schick, C. (2025). Thermodynamic Constraints on the “Hidden” Folding Intermediates. Liquids, 5(3), 24. https://doi.org/10.3390/liquids5030024








