Compensation Relationships in the Solvation Thermodynamics of Proton Acceptors in Aliphatic Alcohols
Abstract
1. Introduction
- No A-S and S-S hydrogen bonding is present.
- A undergoes hydrogen bonding with non-associated solvent S.
- Solute A incapable of hydrogen bonding in associated solvent S.
- Both A-S and S-S hydrogen bonds are present in solution.
2. Methodology
2.1. Definitions and the Framework
2.2. Compensation Relationships Between ΔsolvG and ΔsolvH of Organic Non-Electrolytes
3. Results
4. Discussion
5. Conclusions
0.660·ΔH − TΔS(noncomp.)
6. Experimental
6.1. Materials
6.2. Solution Calorimetry
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Guo, Q.-X. Isokinetic relationship, isoequilibrium relationship, and enthalpy−entropy compensation. Chem. Rev. 2001, 101, 673–696. [Google Scholar] [CrossRef] [PubMed]
- Barclay, I.; Butler, J. The entropy of solution. Trans. Faraday Soc. 1938, 34, 1445–1454. [Google Scholar] [CrossRef]
- Henn, A.R.; Kauzmann, W. New considerations of the Barclay–Butler rule and the behavior of water dissolved in organic solvents. Biophys. Chem. 2002, 100, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Frank, H.S.; Evans, M.W. Free Volume and Entropy in Condensed Systems III. Entropy in Binary Liquid Mixtures; Partial Molal Entropy in Dilute Solutions; Structure and Thermodynamics in Aqueous Electrolytes. J. Chem. Phys. 1945, 13, 507–532. [Google Scholar] [CrossRef]
- Leffler, J.E. The enthalpy-entropy relationship and its implications for organic chemistry. J. Org. Chem. 1955, 20, 1202–1231. [Google Scholar] [CrossRef]
- Lee, B. Solvent reorganization contribution to the transfer thermodynamics of small nonpolar molecules. Biopolymers 1991, 31, 993–1008. [Google Scholar] [CrossRef]
- Fishman, A.I.; Stolov, A.A.; Remizov, A.B. Compensation effect in the thermodynamics of conformational equilibria. Spectrochim. Acta Part A Mol. Spectrosc. 1990, 46, 1037–1043. [Google Scholar] [CrossRef]
- Dunitz, J.D. Win some, lose some: Enthalpy-entropy compensation in weak intermolecular interactions. Chem. Biol. 1995, 2, 709–712. [Google Scholar] [CrossRef]
- Ford, D.M. Enthalpy−Entropy Compensation is Not a General Feature of Weak Association. J. Am. Chem. Soc. 2005, 127, 16167–16170. [Google Scholar] [CrossRef]
- Wickstrom, L.; He, P.; Gallicchio, E.; Levy, R.M. Large Scale Affinity Calculations of Cyclodextrin Host–Guest Complexes: Understanding the Role of Reorganization in the Molecular Recognition Process. J. Chem. Theory Comput. 2013, 9, 3136–3150. [Google Scholar] [CrossRef]
- Qian, H.; Hopfield, J.J. Entropy-enthalpy compensation: Perturbation and relaxation in thermodynamic systems. J. Chem. Phys. 1996, 105, 9292–9298. [Google Scholar] [CrossRef]
- Reynolds, C.H.; Holloway, M.K. Thermodynamics of Ligand Binding and Efficiency. ACS Med. Chem. Lett. 2011, 2, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, E.; Kubo, M.M.; Levy, R.M. Entropy−Enthalpy Compensation in Solvation and Ligand Binding Revisited. J. Am. Chem. Soc. 1998, 120, 4526–4527. [Google Scholar] [CrossRef]
- Exner, O. Concerning the isokinetic relationship. Nature 1964, 201, 488–490. [Google Scholar] [CrossRef]
- Sharp, K. Entropy—Enthalpy compensation: Fact or artifact? Protein Sci. 2001, 10, 661–667. [Google Scholar] [CrossRef]
- Ryde, U. A fundamental view of enthalpy–entropy compensation. MedChemComm 2014, 5, 1324–1336. [Google Scholar] [CrossRef]
- Grunwald, E.; Steel, C. Solvent Reorganization and Thermodynamic Enthalpy-Entropy Compensation. J. Am. Chem. Soc. 1995, 117, 5687–5692. [Google Scholar] [CrossRef]
- van der Vegt, N.F.A.; Trzesniak, D.; Kasumaj, B.; van Gunsteren, W.F. Energy–Entropy Compensation in the Transfer of Nonpolar Solutes from Water to Cosolvent/Water Mixtures. ChemPhysChem 2004, 5, 144–147. [Google Scholar] [CrossRef]
- Bell, R. Relations between the energy and entropy of solution and their significance. Trans. Faraday Soc. 1937, 33, 496–501. [Google Scholar] [CrossRef]
- Korth, M. A quantum chemical view of enthalpy–entropy compensation. MedChemComm 2013, 4, 1025–1033. [Google Scholar] [CrossRef]
- Yu, H.A.; Karplus, M. A thermodynamic analysis of solvation. J. Chem. Phys. 1988, 89, 2366–2379. [Google Scholar] [CrossRef]
- Chialvo, A.A. On the Elusive Links Between Solution Microstructure, Dynamics, and Solvation Thermodynamics: Demystifying the Path Through a Bridge over Troubled Conjectures and Misinterpretations. J. Phys. Chem. B 2023, 127, 10792–10813. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.H.; Bergman, R.G.; Raymond, K.N. Enthalpy−Entropy Compensation Reveals Solvent Reorganization as a Driving Force for Supramolecular Encapsulation in Water. J. Am. Chem. Soc. 2008, 130, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Solomonov, B.N.; Yagofarov, M.I. Compensation relationship in Thermodynamics of solvation and vaporization: Features and applications. I. Non-hydrogen-bonded systems. J. Mol. Liq. 2022, 368, 120762. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Yagofarov, M.I. Compensation relationship in thermodynamics of solvation and vaporization: Features and applications. II. Hydrogen-bonded systems. J. Mol. Liq. 2023, 372, 121205. [Google Scholar] [CrossRef]
- Yagofarov, M.I.; Solomonov, B.N. Compensation relationship in thermodynamics of vaporization of aromatic and aliphatic compounds and their heat capacities. J. Mol. Liq. 2023, 390, 123075. [Google Scholar] [CrossRef]
- Sedov, I.A.; Stolov, M.A.; Solomonov, B.N. Solvophobic effects and relationships between the Gibbs energy and enthalpy for the solvation process. J. Phys. Org. Chem. 2011, 24, 1088–1094. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Sedov, I.A. The hydrophobic effect Gibbs energy. J. Mol. Liq. 2008, 139, 89–97. [Google Scholar] [CrossRef]
- Abraham, M.H.; McGowan, J. The use of characteristic volumes to measure cavity terms in reversed phase liquid chromatography. Chromatographia 1987, 23, 243–246. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Khisamiev, M.B.; Yagofarov, M.I. Calculation of the formation enthalpies of charge-transfer complexes with iodine from the binding constants at 298.15 K. J. Mol. Liq. 2024, 411, 125690. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Yagofarov, M.I. Can the hydrogen bonding enthalpy be calculated from the binding constant at 298.15 K? J. Mol. Liq. 2024, 409, 125353. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Khisamiev, M.B.; Yagofarov, M.I. Calculation of Hydrogen Bonding Enthalpy Using the Two-Parameter Abraham Equation. Liquids 2024, 4, 624–631. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Yagofarov, M.I. The relationship between the Gibbs energies and enthalpies of hydrogen bonding and charge-transfer complex formation in non-electrolytes solutions. Is it a rule? J. Mol. Liq. 2025, 424, 127053. [Google Scholar] [CrossRef]
- Mintz, C.; Clark, M.; Acree, W.E.; Abraham, M.H. Enthalpy of Solvation Correlations for Gaseous Solutes Dissolved in Water and in 1-Octanol Based on the Abraham Model. J. Chem. Inf. Model. 2007, 47, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Sprunger, L.M.; Achi, S.S.; Acree, W.E.; Abraham, M.H. Development of correlations for describing solute transfer into acyclic alcohol solvents based on the Abraham model and fragment-specific equation coefficients. Fluid Phase Equilibr. 2010, 288, 139–144. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Novikov, V.B.; Varfolomeev, M.A.; Mileshko, N.M. A new method for the extraction of specific interaction enthalpy from the enthalpy of solvation. J. Phys. Org. Chem. 2005, 18, 49–61. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Lang, A. Acree Enthalpy of Solvation Dataset; Figshare: London, UK, 2015. [Google Scholar] [CrossRef]
- Li, J.; Zhu, T.; Hawkins, G.D.; Winget, P.; Liotard, D.A.; Cramer, C.J.; Truhlar, D.G. Extension of the platform of applicability of the SM5.42R universal solvation model. Theor. Chem. Acc. 1999, 103, 9–63. [Google Scholar] [CrossRef]
- Zaitseva, K.V.; Varfolomeev, M.A.; Solomonov, B.N. Thermodynamics of specific interactions of pyridines in aliphatic alcohols: Gibbs energy, entropy, and degree of binding. Russ. J. Gen. Chem. 2013, 83, 438–444. [Google Scholar] [CrossRef]
- Zaitseva, K.V.; Varfolomeev, M.A.; Solomonov, B.N. Hydrogen bonding of aliphatic and aromatic amines in aqueous solution: Thermochemistry of solvation. Russ. J. Gen. Chem. 2012, 82, 1669–1674. [Google Scholar] [CrossRef]
- Zaitseva, K.V.; Varfolomeev, M.A.; Novikov, V.B.; Solomonov, B. Enthalpy of cooperative hydrogen bonding in complexes of tertiary amines with aliphatic alcohols: Calorimetric study. J. Chem. Thermodyn. 2011, 43, 1083–1090. [Google Scholar] [CrossRef]
- Mintz, C.; Ladlie, T.; Burton, K.; Clark, M.; Acree, W.E., Jr.; Abraham, M.H. Enthalpy of Solvation Correlations for Gaseous Solutes Dissolved in Alcohol Solvents based on the Abraham Model. QSAR Comb. Sci. 2008, 27, 627–635. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Tulp, I.; Fara, D.C.; Lauria, A.; Maran, U.; Acree, W.E. A General Treatment of Solubility. 3. Principal Component Analysis (PCA) of the Solubilities of Diverse Solutes in Diverse Solvents. J. Chem. Inf. Model. 2005, 45, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Majer, V.; Svoboda, V. Enthalpies of Vaporization of Organic Compounds: A Critical Review and Data Compilation; Blackwell Scientific Publications: Oxford, UK, 1986. [Google Scholar]
- Freitas, V.L.S.; Gomes, J.R.B.; Gales, L.; Damas, A.M.; Ribeiro da Silva, M.D.M.C. Experimental and Computational Studies on the Structural and Thermodynamic Properties of Two Sulfur Heterocyclic Keto Compounds. J. Chem. Eng. Data 2010, 55, 5009–5017. [Google Scholar] [CrossRef]
- Arnett, E.M.; Chawla, B.; Bell, L.; Taagepera, M.; Hehre, W.J.; Taft, R.W. Solvation and hydrogen bonding of pyridinium ions. J. Am. Chem. Soc. 1977, 99, 5729–5738. [Google Scholar] [CrossRef]
- Sedov, I.A.; Solomonov, B.N. Distinctive thermodynamic properties of solute–solvent hydrogen bonds in self-associated solvents. J. Phys. Org. Chem. 2012, 25, 1144–1152. [Google Scholar] [CrossRef]
- Hart, E.; Damini, G.; Heidi, Z.; Victoria, K.; Acree, W.E.; Abraham, M.H. Development of Abraham model expressions for predicting the enthalpies of solvation of solutes dissolved in acetic acid. Phys. Chem. Liq. 2016, 54, 141–154. [Google Scholar] [CrossRef]
- Benson, S.W. Thermochemical Kinetics: Methods for the Estimation of Thermochemical Data and Rate Parameters; Wiley: Hoboken, NJ, USA, 1976; 320p. [Google Scholar]
- Armarego, W.L. Purification of Laboratory Chemicals; Butterworth-Heinemann: Oxford, UK, 2017; 1198p. [Google Scholar]
- Notfullin, A.A.; Bolmatenkov, D.N.; Sokolov, A.A.; Balakhontsev, I.S.; Kachmarzhik, A.D.; Solomonov, B.N.; Yagofarov, M.I. Phase transition thermodynamics of organic semiconductors N,N,N′,N′-tetraphenyl-p-phenylenediamine, N,N′-diphenyl-N,N′-di-p-tolylbenzene-1,4-diamine, and 4,4′-bis(m-tolylphenylamino)biphenyl. J. Chem. Thermodyn. 2025, 206, 107470. [Google Scholar] [CrossRef]
- Nunes, N.; Reis, M.; Moreira, L.; Elvas-Leitão, R.; Martins, F. Solution enthalpies of 1,4-dioxane: Study of solvent effects through quantitative structure–property relationships. Thermochim. Acta 2013, 574, 85–87. [Google Scholar] [CrossRef][Green Version]
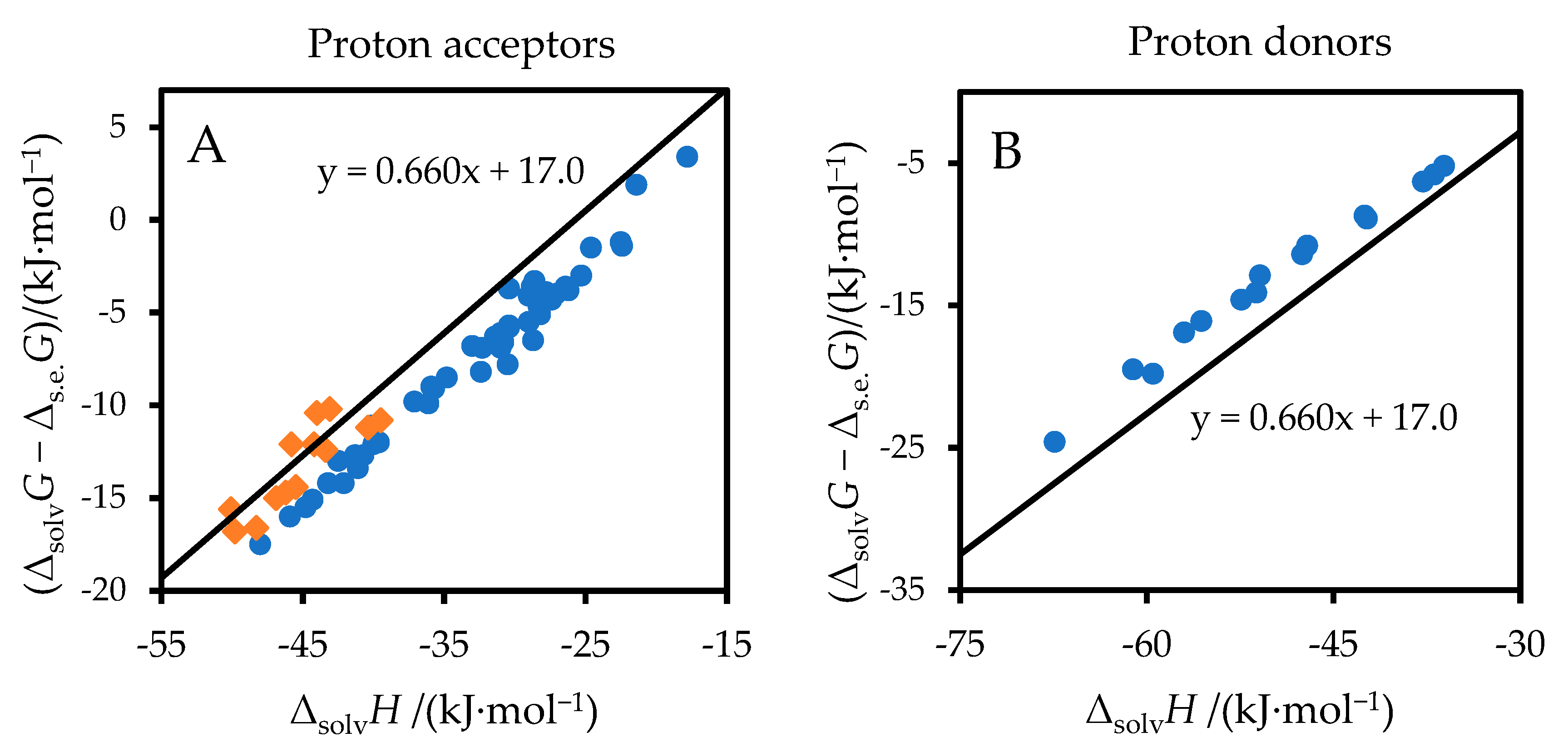
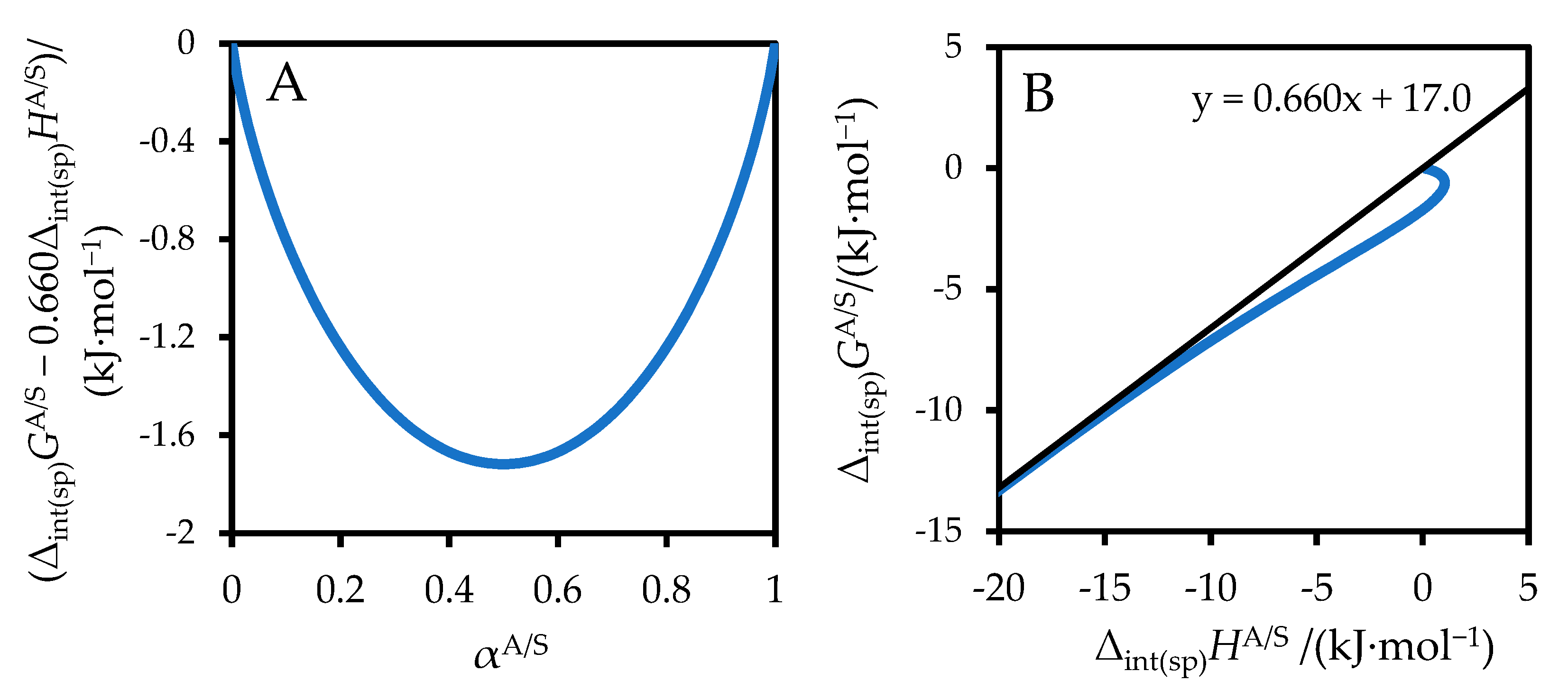
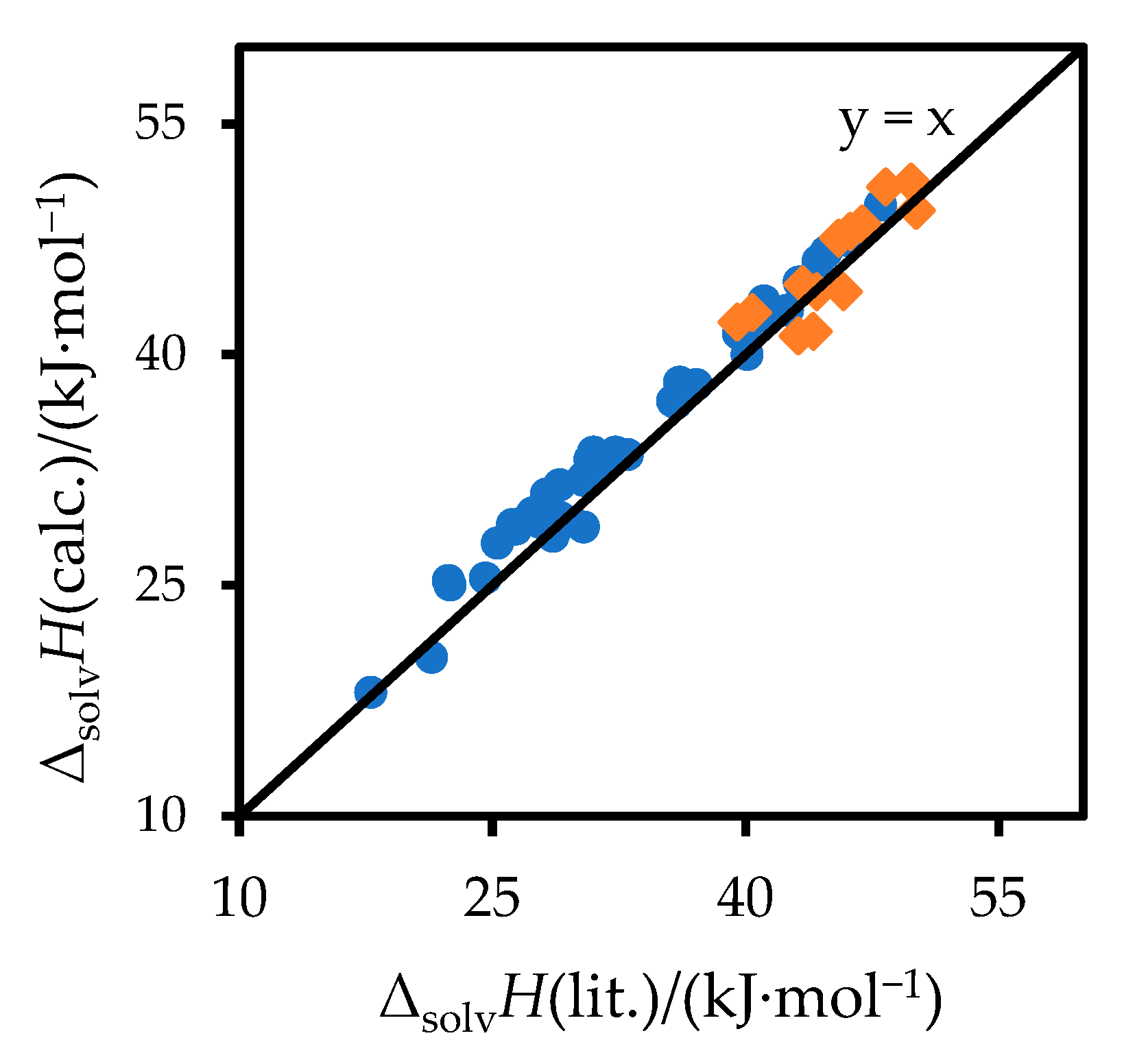
| Solute | Solvent | −ΔsolvG (Lit) | Δs.e.G (Equation (8)) | Δs.e.G − ΔsolvGA/S(Lit) | −ΔsolvH (Lit) | Δs.e.G − ΔsolvGA/S (Equation (14)) | Δ a | Ref. ΔsolvH | Ref. ΔsolvG |
|---|---|---|---|---|---|---|---|---|---|
| kJ·mol−1 | |||||||||
| Pyridine | Methanol | 8.4 | 3.7 | 12.1 | 44.2 | 12.2 | −0.1 | [42,46], this work | [39] |
| 2-Methylpyridine | Methanol | 11.2 | 4.4 | 15.6 | 50.1 | 16.1 | −0.4 | [40,41] | [39] |
| 3-Methylpyridine | Methanol | 12.4 | 4.4 | 16.8 | 49.8 | 15.9 | 1.0 | [37] | [39] |
| 2-Hexanone | Methanol | 5.9 | 5.2 | 11.1 | 40.1 | 9.5 | 1.7 | [42] | [35] |
| 2-Heptanone | Methanol | 9.1 | 6.0 | 15.1 | 44.3 | 12.2 | 2.8 | [42] | [35] |
| 2-Pentanone | Methanol | 4.5 | 4.5 | 9.0 | 35.9 | 6.7 | 2.4 | [42] | [43] |
| 4-Heptanone | Methanol | 8.2 | 6.0 | 14.2 | 43.2 | 11.5 | 2.7 | [42] | [43] |
| Acetone | Methanol | 0.6 | 3.1 | 3.6 | 28.8 | 2.0 | 1.6 | [42] | [35] |
| Butanone | Methanol | 3.2 | 3.8 | 6.9 | 32.3 | 4.3 | 2.7 | [42] | [35] |
| Butyl acetate | Methanol | 6.5 | 5.5 | 12.1 | 40.0 | 9.4 | 2.7 | [37] | [35] |
| N,N-Dimethylformamide | Methanol | 13.9 | 3.6 | 17.5 | 48.0 | 14.7 | 2.9 | [36] | [43] |
| Methyl acetate | Methanol | 0.8 | 3.4 | 4.1 | 29.0 | 2.1 | 2.0 | [42] | [35] |
| Methyl pentanoate | Methanol | 6.4 | 5.5 | 12.0 | 39.6 | 9.1 | 2.8 | [37] | [35] |
| Propyl acetate | Methanol | 4.2 | 4.8 | 9.1 | 35.7 | 6.6 | 2.5 | [37] | [35] |
| Tetrahydrofuran | Methanol | 2.6 | 3.4 | 6.1 | 31.0 | 3.5 | 2.7 | [42] | [35] |
| Acetonitrile | Methanol | 1.0 | 2.3 | 3.3 | 28.6 | 1.9 | 1.4 | [42] | [35] |
| 1,4-Dioxane | Methanol | 4.8 | 3.8 | 8.5 | 34.8 | 6.0 | 2.5 | [42] | [35] |
| Pyridine | Ethanol | 8.9 | 3.5 | 12.4 | 43.4 | 11.6 | 0.7 | [42] | [35] |
| 2-Methylpyridine | Ethanol | 10.9 | 4.1 | 15.0 | 46.9 | 14.0 | 1.0 | [37] | [39] |
| 3-Methylpyridine | Ethanol | 12.5 | 4.1 | 16.6 | 48.3 | 14.9 | 1.7 | [37] | [39] |
| Triethylamine | Ethanol | 5.4 | 5.0 | 10.4 | 44.0 | 12.0 | −1.6 | [42] | [35] |
| Acetone | Ethanol | 0.8 | 3.0 | 3.8 | 26.2 | 0.3 | 3.5 | [42] | [35] |
| Butanone | Ethanol | 3.0 | 3.6 | 6.6 | 30.8 | 3.3 | 3.3 | [42] | [35] |
| Acetonitrile | Ethanol | 1.6 | 2.4 | 4.0 | 27.1 | 0.9 | 3.1 | [42] | [35] |
| N,N-Dimethylformamide | Ethanol | 12.1 | 3.4 | 15.5 | 44.8 | 12.6 | 3.0 | [42] | [43] |
| Tetrahydrofuran | Ethanol | 2.4 | 3.3 | 5.7 | 30.4 | 3.1 | 2.6 | [42] | [43] |
| Ethyl acetate | Ethanol | 2.0 | 3.8 | 5.8 | 30.4 | 3.1 | 2.7 | [42] | [35] |
| 1,4-Dioxane | Ethanol | 4.6 | 3.5 | 8.2 | 32.4 | 4.4 | 3.8 | [44] | [35] |
| Pyridine | 1-Butanol | 8.7 | 2.6 | 11.2 | 40.4 | 9.7 | 1.6 | [42] | [35] |
| 2-Methylpyridine | 1-Butanol | 11.7 | 3.0 | 14.7 | 46.2 | 13.5 | 1.2 | [40,41] | [39] |
| Triethylamine | 1-Butanol | 6.4 | 3.7 | 10.2 | 43.1 | 11.4 | −1.3 | [42] | [35] |
| Butylamine | 1-Butanol | 9.2 | 2.9 | 12.1 | 45.8 | 13.2 | −1.1 | [42] | [38] |
| Acetone | 1-Butanol | 0.8 | 2.2 | 3.0 | 25.3 | −0.3 | 3.2 | [42] | [35] |
| Butyl ether | 1-Butanol | 8.5 | 4.5 | 13.0 | 42.5 | 11.1 | 1.9 | [42] | [35] |
| Ethyl acetate | 1-Butanol | 2.7 | 2.8 | 5.5 | 29.0 | 2.1 | 3.4 | [42] | [35] |
| Methyl ether | 1-Butanol | −5.3 | 1.9 | −3.4 | 17.8 | −5.3 | 1.8 | [42] | [35] |
| Propionitrile | 1-Butanol | 2.9 | 2.2 | 5.1 | 28.2 | 1.6 | 3.5 | [44], this work | [43] |
| 1,4-Dioxane | 1-Butanol | 5.2 | 2.6 | 7.8 | 30.5 | 3.1 | 4.7 | [44], this work | [35] |
| Pyridine | 1-Octanol | 9.8 | 1.0 | 10.8 | 39.5 | 9.1 | 1.7 | [36] | [39] |
| 2-Methylpyridine | 1-Octanol | 13.2 | 1.2 | 14.4 | 45.5 | 13.0 | 1.3 | [40,41] | [39] |
| Acetone | 1-Octanol | 0.7 | 0.8 | 1.4 | 22.4 | −2.2 | 3.7 | [34] | [35] |
| Butanone | 1-Octanol | 3.3 | 1.0 | 4.3 | 27.4 | 1.1 | 3.2 | [34] | [35] |
| 2-Pentanone | 1-Octanol | 5.7 | 1.2 | 6.9 | 31.0 | 3.5 | 3.4 | [34] | [35] |
| 2-Hexanone | 1-Octanol | 8.5 | 1.4 | 9.9 | 36.1 | 6.8 | 3.0 | [34] | [35] |
| 2-Heptanone | 1-Octanol | 11.2 | 1.6 | 12.7 | 40.7 | 9.9 | 2.9 | [36] | [35] |
| Acetonitrile | 1-Octanol | 0.7 | 0.6 | 1.2 | 22.5 | −2.2 | 3.4 | [37] | [35] |
| Propionitrile | 1-Octanol | 2.8 | 0.8 | 3.6 | 26.4 | 0.4 | 3.2 | [37] | [35] |
| 2-Octanone | 1-Octanol | 14.2 | 1.8 | 16.0 | 45.9 | 13.3 | 2.7 | [37] | [38] |
| Methyl acetate | 1-Octanol | 0.7 | 0.9 | 1.5 | 24.6 | −0.8 | 2.3 | [34] | [35] |
| Propyl acetate | 1-Octanol | 5.6 | 1.3 | 6.8 | 33.0 | 4.8 | 2.0 | [34] | [35] |
| Butyl acetate | 1-Octanol | 8.3 | 1.5 | 9.8 | 37.1 | 7.5 | 2.3 | [34] | [35] |
| Butyronitrile | 1-Octanol | 5.3 | 1.0 | 6.3 | 31.4 | 3.7 | 2.5 | [34] | [35] |
| Ethyl acetate | 1-Octanol | 2.9 | 1.1 | 3.9 | 27.8 | 1.3 | 2.6 | [34] | [35] |
| Pentyl acetate | 1-Octanol | 11.0 | 1.7 | 12.7 | 41.3 | 10.3 | 2.4 | [34] | [35] |
| Propyl formate | 1-Octanol | 2.6 | 1.1 | 3.7 | 30.4 | 3.1 | 0.7 | [34] | [35] |
| Methyl formate | 1-Octanol | −2.6 | 0.7 | −1.9 | 21.4 | −2.9 | 1.0 | [34] | [35] |
| Tetrahydrofuran | 1-Octanol | 3.8 | 0.9 | 4.7 | 28.3 | 1.7 | 3.0 | [34] | [35] |
| N,N-Dimethylformamide | 1-Octanol | 12.5 | 0.9 | 13.4 | 41.1 | 10.1 | 3.3 | [37] | [35] |
| Benzonitrile | 1-Octanol | 12.9 | 1.3 | 14.2 | 42.1 | 10.8 | 3.4 | [37] | [35] |
| 1,4-Dioxane | 1-Octanol | 5.6 | 1.0 | 6.5 | 28.7 | 1.9 | 4.6 | [34] | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solomonov, B.N.; Khisamiev, M.B.; Yagofarov, M.I. Compensation Relationships in the Solvation Thermodynamics of Proton Acceptors in Aliphatic Alcohols. Liquids 2025, 5, 17. https://doi.org/10.3390/liquids5020017
Solomonov BN, Khisamiev MB, Yagofarov MI. Compensation Relationships in the Solvation Thermodynamics of Proton Acceptors in Aliphatic Alcohols. Liquids. 2025; 5(2):17. https://doi.org/10.3390/liquids5020017
Chicago/Turabian StyleSolomonov, Boris N., Mansur B. Khisamiev, and Mikhail I. Yagofarov. 2025. "Compensation Relationships in the Solvation Thermodynamics of Proton Acceptors in Aliphatic Alcohols" Liquids 5, no. 2: 17. https://doi.org/10.3390/liquids5020017
APA StyleSolomonov, B. N., Khisamiev, M. B., & Yagofarov, M. I. (2025). Compensation Relationships in the Solvation Thermodynamics of Proton Acceptors in Aliphatic Alcohols. Liquids, 5(2), 17. https://doi.org/10.3390/liquids5020017









