Abstract
Density and viscosity are fundamental properties necessary for processing of red palm oil (RPO). The main fatty acid constituents of RPO were determined to be palmitic acid (C16:0), oleic acid (C18:1), and linoleic acid (C18:2). Rheology measurements confirmed that RPO behaved as a Newtonian fluid. Viscosities and atmospheric densities of RPO were measured at 0.1 MPa and (293 K to 413) K and correlated with the Rodenbush model (0.05% deviation). Dynamic viscosities of RPO were correlated with the Vogel–Fulcher–Tammann model (0.06% deviation) and Doolittle free volume model (0.04% deviation). High-pressure densities of RPO were measured at (10 to 150) MPa and (312 to 352) K. The Tait equation could correlate the high-pressure densities of RPO to within 0.021% deviation and was used to estimate the thermal expansion as 5.1 × 10−4 K−1 (at 312 K, 150 MPa) to 4.8 × 10−4 K−1 (at 352 K, 150 MPa) and isothermal compressibility as 7.3 × 10−4 MPa−1 (at 352 K, 0.1 MPa) to 3.5 × 10−4 MPa−1 (at 352 K, 150 MPa). Parameters for the perturbed-chain statistical associating fluid theory equation of state were determined and gave an average of 0.143% deviation in density. The data and equations developed should be useful in high-pressure food processing as well as in applications considering vegetable oils as heat transfer fluids or as lubricants.
1. Introduction
Red palm oil (RPO) is produced via physical refining of crude palm fruits and contains roughly 46% saturated fatty acids, 42% monounsaturated fatty acids, and 12% polyunsaturated fatty acids [1]. In plant oil processing, the viscosity and density are important physical properties of an oil, because they affect applications in high-pressure microfluidics in crystallization [2], high-pressure homogenization in processing of palm-oil-containing foods [3], and separation of phytonutrients with supercritical fluids [4].
Densities and viscosities of plant oils are directly related to lipid structure characteristics, such as type and composition of fatty acid constituents, number of unsaturated constituents, and chain length of fatty acids that constitute their triacylglycerides. Folayan et al. [5] reported that high-linoleic vegetable oil biomass showed cold flow behavior due to high linoleic acid constituents, a high degree of unsaturation, and long chain length. Anitescu et al. [6] reported that optimum fluid volatility and velocity for the 2-D grade diesel fuel oil process could be estimated by the measurement and modeling of oil (palm oil, soybean oil, and rapeseed oil) density and viscosity. In that study, experimental density and dynamic viscosity of oils at atmospheric pressure were used to predict the biodiesel–benzene fluid behavior with the Grunberg–Nissan model [7].
High-pressure densities of oils are prerequisite information for supercritical fluid extraction processes [8], heat transfer, and tribology of lubricants [9]. For instance, Kok et al. [10] found that increased mass transfer of virgin coconut oil and supercritical CO2 into mangosteen peel was related to the high-pressure density. Regueira et al. [11] measured high-pressure densities of sunflower oil to estimate thermal expansion and isothermal compressibility for applications in power transfer and engine hydraulic fluids. Jasiok et al. [9] measured high-pressure densities of oils for lubricating oil selection and developed a fluctuation theory equation of state to correlate the data.
Even though RPO is of interest to supercritical fluid extraction processing for making functional foods and it is used as a lubricant in food processing machinery in industry due to food safety, experimental densities of RPO at high pressure are unavailable in the literature. In this study, the objective was to measure the density of red palm oil at pressures up to 150 MPa, to measure the viscosity over a wide range of temperatures, and to develop several kinds of correlating equations that are used in the above applications. The Tait equation was used to correlate high-pressure density data and to estimate thermal expansion and isothermal compressibility of RPO. Parameters for the perturbed-chain statistical associating fluid theory (PC-SAFT) equation [12] were determined to allow fundamental description of the pressure–density–temperature behavior of red palm oil and to provide a basis for studying some of the above applications.
2. Materials and Methods
2.1. Materials
Red palm oil (RPO) was supplied by OriferaTM (Selangor, Malaysia). Fatty acid methyl ester (FAME) mix standards (C8 to C22) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
2.2. Gas Chromatography–Flame Ionization Detector (GC-FID)
Fatty acid composition was determined by a gas chromatography–flame ionization detector (GC-FID) (Agilent Technologies 6890N Series Network Gas Chromatograph, Santa Clara, CA, USA) equipped with a BPX70 SGE capillary column (30 m × 320 µm × 0.25 µm). RPO was esterified into methyl esters by following AOCS procedure Ce 2-66 [13] and loaded into an oven at 373 K for 2 min, then heated to 503 K in 6 K min−1. The injection temperature and detector temperature were 523 K. RPOs were injected using split mode (20:1), an injection volume of 1.0 µL, and a total analysis time of 38 min.
2.3. High-Performance Liquid Chromatography (HPLC)
Carotenoid and vitamin E content were analyzed according to the method of Lee et al. [14]. Carotenoid content was determined by high-performance liquid chromatography (HPLC) (Agilent Technologies 1200 chromatographic system, Santa Clara, CA, USA) with a Develosil RP-Aqueous C30 column (250 mm × 4.6 mm, 5 µm) supplied from Hinode-cho Seto, Japan. The mobile phase (acetonitrile/chloroform 80/20% v/v) was delivered isocratically at 1 mL/min and analytes were detected at a 450 nm wavelength with a UV detector. Vitamin E was identified with a Phenomenex Kinetex PFP column (250 mm × 4.6 mm, 5 µm) supplied from Madrid Avenue, Torrance, CA, USA. Methanol and water as the mobile phase were used in gradient mode at 1 mL/min and Ex/Em: 295/330 nm wavelength for detection of analytes with a fluorescence detector.
2.4. Rheology
The shear rate of RPO was measured by a rotational rheometer (Haake, RheoStress 6000, Waltham, MA, USA) to confirm Newtonian behavior and to provide reference values for the Stabinger viscometer. RPO was loaded between a lower plate (222-1891 TMP 60, diameter of 60 mm) and cone that was fixed at a 0.105 mm gap from the attachment (222-1867 cone, C60/2° tilt). One cycle began from (0.1 to 1000) s−1 and then was reversed from (1000 to 0.1) s−1 for two temperatures, 313.2 K and 353.2 K. The shear stress is proportional to shear rate and was determined from the following equations:
In Equations (1)–(3), is the dynamic density in units of Pa∙s, is the shear stress in units of Pa, and is shear rate in units of s−1.
2.5. Measurements of Density and Viscosity at Atmospheric Pressure
Measurements of density and viscosity of RPO were conducted with a Stabinger viscometer (Anton Paar, SVM 3000, Graz, Styria, Austria), from (293.15 to 353.15) K at intervals of 10 K increments. The average of eight measurements comprised four cycles. The first cycle started from a low to high temperature and the second cycle from a high temperature to low temperature.
2.6. Viscosity Model
The Rodenbush et al. [15] model was used to determine the relationship between density and viscosity as follows:
In Equation (4), is density in units of , is viscosity in units of mPa∙s, and are fitting parameters. The Vogel–Fulcher–Tammann model [16,17] was used to correlate the viscosity as follows:
In Equation (5), is the absolute temperature, (mPa∙s), (K) and (K) are the fitting parameters. The Doolittle free volume (FV) model [18] correlated viscosity based on temperature and free volume fraction as follows:
In Equations (6) and (7), A and B are fitting parameters based on the atmospheric viscosities, is the free volume fraction in units of , Vs,free is the free volume, Vs is the liquid volume in units of , and Vs,occ is the occupied volume in units of . In Equation (7), the free volume can be estimated from the excluded volume of the liquid [19], however, in this study, Vs,occ was treated as a fitting parameter.
2.7. Measurement of Density at High Pressure
Densities at high pressure were measured with a bellows dilatometer under vacuum (ca. 3 Pa) and at ambient temperature as described in previous publications [20,21,22,23,24]. Briefly, RPO was poured into a dilatometer and loaded into the pressure vessel and then the chamber was pressurized. Measurements were made from (313 to 353) K in intervals of 10 K and from (10 to 150) MPa in intervals of 10 MPa. The volume of the bellows was obtained by its linear length expansion or contraction that was measured with a calibrated linear variable differential transformer (LVDT). An additional sequence of measurements was made after the full sequence of measurements from low temperature to high temperature to verify the equipment and the integrity and stability of RPO.
2.8. Tait Equation
In the Tait equation, correlation of density data was made with the following equations:
In Equations (8)–(10), is the density, is the atmospheric pressure, is the absolute temperature, is the pressure, and ,, , , , , and are fitting parameters. In the analysis with the Tait equation, the coefficient of thermal expansion (K−1) and isothermal compressibility (MPa−1) of RPO were calculated from:
2.9. Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT) Equation
In perturbation theory, a fluid corresponding to a hard chain (hc) fluid is used as a reference and perturbations that account for dispersion (disp) or van der Waals forces are summed to form the dimensionless residual Helmholtz function () as follows [12,25]:
The equation of state can be determined from the derivative of the Helmholtz function to obtain the compressibility factor as follows:
In Equation (14), is the residual hard chain contribution and is the residual dispersion contribution. The terms in Equation (14) are defined as follows:
In Equations (15)–(21), is the single pure component of oil, NC is the number of components (NC = 1), is equal to the packing fraction, is the mole fraction of chains of a single pure component (), is the mean segment number of molecule , is the number of segments in a chain of molecule , is the radial pair distribution function of molecule in a hard sphere system, superscript hs indicates quantities of the hard sphere system, is the total number density of molecule , and temperature-dependent segment diameter is calculated in units of Å by the following equation [12]:
In Equation (22), is temperature-independent segments in units of , is dispersion energy divided by the Boltzmann constant in units of K, is pair potential in units of , kB is the average kinetic energy of one particle related to absolute temperature (1.380649 × 10−23 ), and is absolute temperature in units of . The dispersion contribution is given by the following equations:
In Equations (23)–(29), is the reduced density (), , are functions of molecule , is shorthand notation for a function, is a function for the derivative of , and are integrals of the perturbation theory given by Equations (28) and (29) and thus the compressibility factor can be obtained from the Helmholtz function using standard thermodynamic relationships [12], while and are computational coefficients:
In Equations (30) and (31), to andto are a set of 42 universal constants of the model that is based on pure components [12]. Parameters and are determined by Berthelot–Lorentz combining rules for a pair of unlike segments:
In Equations (32) and (33), is a binary interaction parameter that does not apply for this work (), because the oils studied are considered to be a single type of triglyceride molecule.
2.10. Objective Function
The following objective functions were used for determining model parameters: Average relative deviation () of calculated () and experimental () viscosities () and densities () were applied as objective functions in calculating fitting parameters of the Rodenbush et al. [15] model:
In Equations (34) and (35), is number of data and is an index. The objective function of Equation (34) was used to minimize the deviation of experimental and calculated viscosity by correlation of Vogel–Fulcher–Tammann and Doolittle free volume models. For the Tait equation, atmospheric pressure density data with multiple linear regression was used to determine parameters (, , ) and high-pressure density data with non-linear minimization of Equation (35) were used to determine parameters (, ).
2.11. Optimal Parameter Analysis
For density and viscosity models, Equations (2)–(8), the ARD% values were plotted against a single fitting parameter and the remaining parameters were adjusted to confirm a global minimum. Optimization of the fitting parameter set was performed step-wise according to the sequences’ change in the equations. In Equation (2), initially the parameter value was set equal to zero and the value was optimized and afterwards the two parameters (, ) were optimized together. This was applied to Equations (2)–(8).
3. Results and Discussion
3.1. Characterization of Red Palm Oil
Characterization of red palm oil (RPO) fatty acid composition by GC-FID analyses is shown in Table 1. The main fatty acid constituents of RPO were determined to be palmitic acid (C16:0), oleic acid (C18:1), and linoleic acid (C18:2). The RPO has similar main fatty acid constituents to palm oil (Table S1), while RPO constituents are different from those of other common plant oils. According to its constituents, RPO should have similar physical properties to palm oil as discussed below.

Table 1.
Analysis of fatty acid constituents in red palm oil (RPO).
Figure 1 shows the shear stress versus shear rate curve of RPO (Figure 1a) and residuals of straight-line fits (Figure 1b) measured with a rotational. As shown in Figure 1a, the shear stress was directly proportional to the shear rate at both 303.2 K and 353.2 K, meaning that RPO behaves as a Newtonian fluid.
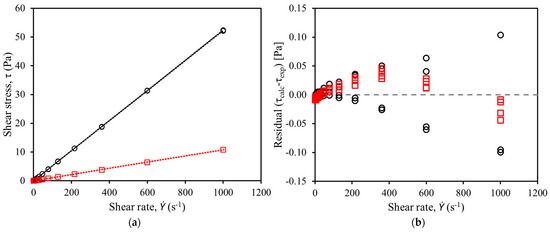
Figure 1.
Shear stress versus shear rate curve of red palm oil measured with rotational rheometer at: (○) 303.2 K and (□) 353.2 K. (a) Linear regression of data with R2 = 0.99; (b) residuals of fits.
Measurements of dynamic viscosities and atmospheric densities for red palm oil (RPO) at (293 to 353) K are shown in Table 2. Combined extended uncertainties in density and viscosity were estimated to be 1 kg m−3 and 0.73%, respectively, at a 95% level of confidence.

Table 2.
Dynamic viscosities and atmospheric densities of red palm oil at (293 to 353) K.
Figure 2a shows dynamic viscosities and atmospheric densities of red palm oil (RPO) measured with a Stabinger viscometer and correlated with the Rodenbush et al. [15] model. As shown in Figure 2a, the Rodenbush et al. [15] model could correlate the experimental RPO densities well (0.05%) and when the set of parameters was used to predict the viscosities, an average deviation of 0.08% was obtained. The Rodenbush model was able to correlate both viscosity and density as shown by the ARD values in Table 3. It should be noted that the VFT and FV models provide a set of parameters that allow them to predict RPO densities via the Rodenbush et al. [15] model.
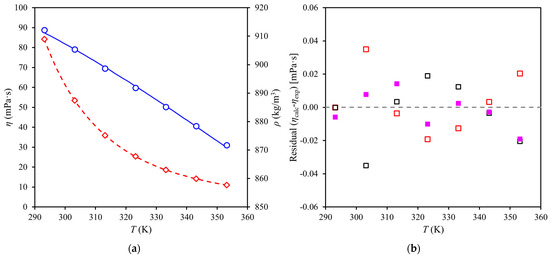
Figure 2.
Experimental viscosity and density data for red palm oil at (293 to 353) K and 0.1 MPa with correlation by Rodenbush et al. [15] model: (a) (◊) dynamic viscosities and correlation (▬ ▬) and (○) liquid densities and (▬▬) correlation; (b) residuals, (□) Rodenbush et al. [15] model; (□) Vogel–Fulcher–Tammann model; (■) Doolittle free volume model.

Table 3.
Parameters and statistical details of Rodenbush et al. [15] model, Vogel–Fulcher–Tammann (VFT) model, Doolittle free volume (FV) model for red palm oil.
Dynamic viscosities of RPO at (293 to 353) K and 0.1 MPa were correlated with the Vogel–Fulcher–Tammann (VFT) and Doolittle free volume (FV) models (Table 3) and residuals of all models are plotted in Figure 2b. The VFT and FV models could correlate the experimental RPO viscosities at low deviations (0.04 to 0.06)%, however, the residuals of VFT and FV models showed systematic deviations (quadratic) that indicate that additional terms could be added to the models, because about half of the residuals fall outside of the 95% confidence interval (Figure S1).
The 95% confidence interval (Figure S1) was similar for all models which means that the correlation of the data is not so different. Residuals of the FV model all fall within the 95% confidence interval (Figure S1), which means the FV model is the most reliable among the correlating equations (Figure S2).
The dynamic viscosities and atmospheric densities of RPO were compared with palm oil, sunflower oil, soybean oil, and corn oil (Figure S3). From Figure S3, viscosities of RPO and palm oil were high, with low densities, while those of sunflower oil and corn oil were low, with high densities. This means that fluid behavior of RPO was similar to that of palm oil at atmospheric pressure [26].
3.2. High-Pressure Densities of Red Palm Oil (RPO)
Table 4 shows the measurement of high-pressure density for red palm oil (RPO) at (312 to 351) K and (10 to 150) MPa. For Table 4, high-pressure liquid densities of RPO increased with increasing pressure. The last column in Table 5 is a replicate run after completing the series of density measurements and shows that the oil maintained its integrity, because the maximum density differences observed were 0.1 kg m−3 to 0.2 kg m−3.

Table 4.
Measurement of liquid densities a for red palm oil at (312 to 351) K and (10 to 150) MPa.

Table 5.
Summary of Tait equation parameters and average relative deviation (ARD) for experimental liquid densities of oils at high pressure.
High-pressure densities of red palm oil (RPO) at (312 to 351) K and (10 to 150) MPa were correlated with the Tait equation (Figure 3a) and residuals were plotted (Figure 3b). As shown in Figure 3a, the Tait equation could correlate the trends of the data at low deviations (0.021%), however, the residuals (Figure 3b) showed quadratic dependence in pressure that implies that an additional term in pressure could be added to the model, especially since about half of the residuals fall outside of the 95% confidence interval.
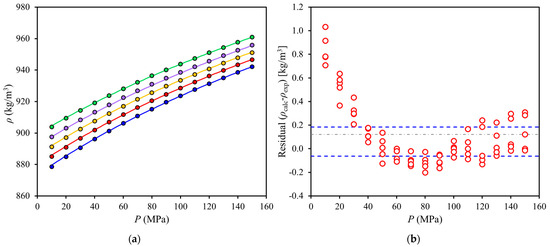
Figure 3.
High-pressure densities of red palm oil at (312 to 352) K and (10 to 150) MPa: (a) experimental data and correlation with Tait equation and (b) ( ) calculated minus experimental density values (residuals). Symbols: (blue,
) calculated minus experimental density values (residuals). Symbols: (blue,  ) 352 K; (red,
) 352 K; (red,  ) 342 K; (yellow,
) 342 K; (yellow,  ) 332 K; (purple,
) 332 K; (purple,  ) 322 K; (green,
) 322 K; (green,  ) 312 K. Lines: (⸺) Tait equation correlation, (- - -) 95% confidence interval, (-∙-) mean.
) 312 K. Lines: (⸺) Tait equation correlation, (- - -) 95% confidence interval, (-∙-) mean.
 ) calculated minus experimental density values (residuals). Symbols: (blue,
) calculated minus experimental density values (residuals). Symbols: (blue,  ) 352 K; (red,
) 352 K; (red,  ) 342 K; (yellow,
) 342 K; (yellow,  ) 332 K; (purple,
) 332 K; (purple,  ) 322 K; (green,
) 322 K; (green,  ) 312 K. Lines: (⸺) Tait equation correlation, (- - -) 95% confidence interval, (-∙-) mean.
) 312 K. Lines: (⸺) Tait equation correlation, (- - -) 95% confidence interval, (-∙-) mean.
High-pressure densities of red palm oil (RPO) were correlated with the PC-SAFT equation (Figure 4a) and residuals were plotted (Figure 4b). As shown in Figure 4a, the PC-SAFT model could correlate the trends of the data with moderate deviations (0.143%), however, the residuals (Figure 4b) showed quadratic dependence in pressure as well as a systematic deviation in temperature, indicating that additional terms in temperature and density could be added to the model, because about one third of the residuals fall outside of the 95% confidence interval.
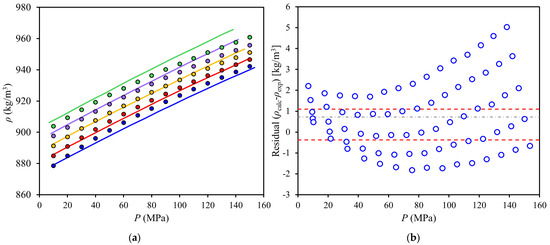
Figure 4.
High-pressure densities of red palm oil at (312 to 352) K and (10 to 150) MPa: (a) experimental data and correlation with PC-SAFT equation and (b) ( ) calculated minus experimental density values (residuals). Symbols: (blue,
) calculated minus experimental density values (residuals). Symbols: (blue,  ) 352 K; (red,
) 352 K; (red,  ) 342 K; (yellow,
) 342 K; (yellow,  ) 332 K; (purple,
) 332 K; (purple,  ) 322 K; (green,
) 322 K; (green,  ) 312 K. Lines: (⸺) PC-SAFT correlation, (- - -) 95% confidence interval, (-∙-) mean.
) 312 K. Lines: (⸺) PC-SAFT correlation, (- - -) 95% confidence interval, (-∙-) mean.
 ) calculated minus experimental density values (residuals). Symbols: (blue,
) calculated minus experimental density values (residuals). Symbols: (blue,  ) 352 K; (red,
) 352 K; (red,  ) 342 K; (yellow,
) 342 K; (yellow,  ) 332 K; (purple,
) 332 K; (purple,  ) 322 K; (green,
) 322 K; (green,  ) 312 K. Lines: (⸺) PC-SAFT correlation, (- - -) 95% confidence interval, (-∙-) mean.
) 312 K. Lines: (⸺) PC-SAFT correlation, (- - -) 95% confidence interval, (-∙-) mean.
Comparing the Tait and the PC-SAFT equations, it is clear that the Tait equation gives deviations that are roughly seven times lower than those of the PC-SAFT equation for liquid density correlation. Nevertheless, deviations in density with either equation are relatively low compared with cubic equations of state (e.g., 2% to 6%) and the molecular parameters of the PC-SAFT equation allow it to be applied to calculate other physical properties as well as phase equilibria of mixtures.
Table 5 shows a summary of Tait equation parameters and ARD values for high-pressure density of RPO. In Table 5, the optimal parameter values (Figure S4) of RPO were close to those of palm oil (Table S2), but different from those of olive oil, despite RPO having similar fatty acid constituents to palm oil and olive oil (Table S1).
Table 6 shows the PC-SAFT model parameters and ARD values for high-pressure RPO densities. In Table 6, the PC-SAFT parameter of RPO is slightly larger than those of palm oil or olive oil (Table S3), but the parameter of RPO was in between their values (Table S3), which means that the interaction strength parameter is the main reason for the differences in the densities of these oils.

Table 6.
Summary parameter and statistic details of Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT) model with molecular weight of red palm oil (RPO) for correlation of liquid densities at high pressure.
Figure 5 shows derivative properties of red palm oil (RPO) derived from the Tait equation. The thermal expansion decreased with increasing temperature (Figure 5a) and the isothermal compressibility decreased with increasing pressure (Figure 5b). The thermal expansion of RPO was lower than that of palm oil at a given pressure (Figure S5a) and the isothermal compressibility was lower than that of palm oil at a given temperature (Figure S5b). These differences in derivative properties between RPO and palm oil (Figure S5) are as high as 10% depending on conditions of temperature and pressure and may be important for some applications where low thermal expansion and low compressibility are required.
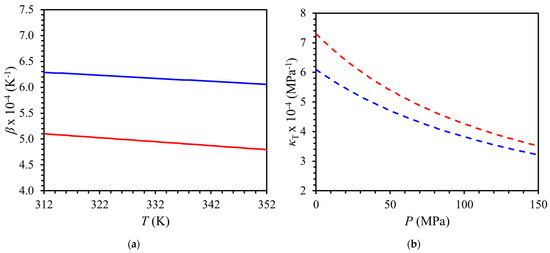
Figure 5.
Calculation of volumetric behavior of red palm oil with Tait model for (a) thermal expansion () at 50 MPa and 150 MPa at (312 to 352) K and (b) isothermal compressibility () at (0.1 to 150) MPa for 312 K and 352 K. Red palm oil (This work): (▬▬) 50 MPa; (▬▬) 150 MPa; (- - -) 312 K; (- - -) 352 K.
4. Conclusions
In this study, high-pressure densities and densities and viscosities at atmospheric pressure were measured for red palm oil (RPO). Shear rate versus shear stress measurements showed that RPO had Newtonian behavior with maximum shear stress (52 Pa) and shear rate (1000 s−1) at 303 K. The viscosity and density of RPO could be correlated with Vogel–Fulcher–Tammann (VFT) and Doolittle free volume (FV) models at deviations lower than about 0.08%. For high-pressure densities, the Tait equation provided data correlation with low deviations (0.02%). Variation of the thermal expansion and isothermal compressibility of RPO with temperature and pressure estimated with the Tait equation showed typical trends for vegetable oils. From analysis of the residuals, it is likely that high-pressure density data correlation with the Tait equation can be improved by adding another parameter in pressure. Although molecular parameters for RPO could be estimated for the PC-SAFT equation of state, systematic deviations (0.14%) in density were apparent in both temperature and pressure. Correlation of high-pressure density data with the PC-SAFT equation can probably be improved by fitting isotherms and examining the temperature dependence of the fitted parameters.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/liquids5020013/s1, Figure S1: residual deviation of viscosities model [15]; Figure S2: optimal parameter analysis of viscosities model [15]; Figure S3: experimental of RPO viscosities and densities and compared with calculated oils [27]; Figure S4: optimal parameter analysis of Tait equation; Figure S5: volumetric behavior of RPO and compared with palm oil; Table S1: Fatty acid constituents of vegetable oils [27,28,29,30,31,32]; Table S2: Tait equation parameters and average relative deviation; Table S3: PC-SAFT equation molecular parameters of RPO, palm oil, and olive oil.
Author Contributions
J.L.L.: Writing—Original Draft, Data curation, Investigation, Formal analysis, Visualization, Software. G.H.C.: Conceptualization, Methodology, Validation, Investigation, Resources, Writing—Reviewing and Editing, Supervision, Project administration, Funding acquisition. Y.H.: Methodology, Validation, Formal analysis, Investigation. Y.S.: Supervision, Methodology, Resources. M.O.: Supervision, Methodology, Resources. R.L.S.J.: Conceptualization, Supervision, Methodology, Formal analysis, Project administration, Resources, Funding acquisition, Writing—Reviewing and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the Ministry of Higher Education (MOHE), Fundamental Research Grant Scheme (FRGS) of MOHE project code FRGS/1/2024/TK05/UPM/02/4 for funding for this study.
Data Availability Statement
All data are available from the authors upon reasonable request.
Acknowledgments
Partial support of this project in the form of facilities and resources of the Research Center of Supercritical Fluid Technology, Tohoku University is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, Z.; Zhu, Y.; Jin, J.; Jin, Q.; Wang, X. Chemical-Physical Properties of Red Palm Oils and Their Application in the Manufacture of Aerated Emulsions with Improved Whipping Capabilities. Foods 2023, 12, 3933. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, L.; Li, B.; Zhao, L.; Liu, G.; Liu, X.; Wang, X. Effect of High Pressure Microfluidization on the Crystallization Behavior of Palm Stearin—Palm Olein Blends. Molecules 2014, 19, 5348–5359. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.; Mustafa, W.; Donsì, F. Use of agri-food residues for oil structuring and functionalization. Chem. Eng. Trans. 2017, 57, 1831–1836. [Google Scholar] [CrossRef]
- Hoe, B.C.; Chan, E.S.; Nagasundara Ramanan, R.; Ooi, C.W. Recent development and challenges in extraction of phytonutrients from palm oil. Compr. Rev. Food Sci. Food Saf. 2020, 19, 4031–4061. [Google Scholar] [CrossRef]
- Folayan, A.J.; Anawe, P.A.L.; Aladejare, A.E.; Ayeni, A.O. Experimental investigation of the effect of fatty acids configuration, chain length, branching and degree of unsaturation on biodiesel fuel properties obtained from lauric oils, high-oleic and high-linoleic vegetable oil biomass. Energy Rep. 2019, 5, 793–806. [Google Scholar] [CrossRef]
- Anitescu, G.; Bruno, T.J. Liquid Biofuels: Fluid Properties to Optimize Feedstock Selection, Processing, Refining/Blending, Storage/Transportation, and Combustion. Energy Fuels 2011, 26, 324–348. [Google Scholar] [CrossRef]
- Geacai, S.; Iulian, O.; Nita, I. Measurement, correlation and prediction of biodiesel blends viscosity. Fuel 2015, 143, 268–274. [Google Scholar] [CrossRef]
- Anitescu, G.; Bruno, T.J. Fluid properties needed in supercritical transesterification of triglyceride feedstocks to biodiesel fuels for efficient and clean combustion—A review. J. Supercrit. Fluids 2012, 63, 133–149. [Google Scholar] [CrossRef]
- Jasiok, B.; Lowe, A.R.; Postnikov, E.B.; Feder-Kubis, J.; Chorążewski, M. High-Pressure Densities of Industrial Lubricants and Complex Oils Predicted by the Fluctuation Theory-Based Equation of State. Ind. Eng. Chem. Res. 2018, 57, 11797–11803. [Google Scholar] [CrossRef]
- Kok, S.L.; Lee, W.J.; Smith, R.L.; Suleiman, N.; Jom, K.N.; Vangnai, K.; Bin Sharaai, A.H.; Chong, G.H. Role of virgin coconut oil (VCO) as co-extractant for obtaining xanthones from mangosteen (Garcinia mangostana) pericarp with supercritical carbon dioxide extraction. J. Supercrit. Fluids 2021, 176, 105305. [Google Scholar] [CrossRef]
- Regueira, T.; Lugo, L.; Fandiño, O.; López, E.R.; Fernández, J. Compressibilities and viscosities of reference and vegetable oils for their use as hydraulic fluids and lubricants. Green. Chem. 2011, 13, 1293–1302. [Google Scholar] [CrossRef]
- Gross, J.; Sadowski, G. Perturbed-Chain SAFT: An Equation of State Based on a Perturbation Theory for Chain Molecules. Ind. Eng. Chem. Res. 2001, 40, 1244–1260. [Google Scholar] [CrossRef]
- Brühl, L. Official Methods and Recommended Practices of the American Oil Chemist’s Society, Physical and Chemical Characteristics of Oils, Fats and Waxes, Section I. Ed. The AOCS Methods Editor and the AOCS Technical Department. 54 pages. AOCS Press, Champaign, 1996. Lipid/Fett 1997, 99, 197. [Google Scholar] [CrossRef]
- Lee, W.J.; Tan, C.P.; Sulaiman, R.; Smith, R.L.; Chong, G.H. Microencapsulation of red palm oil as an oil-in-water emulsion with supercritical carbon dioxide solution-enhanced dispersion. J. Food Eng. 2018, 222, 100–109. [Google Scholar] [CrossRef]
- Rodenbush, C.M.; Hsieh, F.H.; Viswanath, D.S. Density and Viscosity of Vegetable Oils. J. Am. Oil Chem. Soc. 1999, 76, 1415–1419. [Google Scholar] [CrossRef]
- Ikeda, M.; Aniya, M. Understanding the Vogel–Fulcher–Tammann law in terms of the bond strength–coordination number fluctuation model. J. Non-Cryst. Solids 2013, 371–372, 53–57. [Google Scholar] [CrossRef]
- Harris, K.R. Temperature and Pressure Dependence of the Viscosities of Krytox GPL102 Oil and Di(pentaerythritol) Hexa(isononanoate). J. Chem. Eng. Data 2015, 60, 1510–1519. [Google Scholar] [CrossRef]
- Doolittle, A.K. Studies in Newtonian Flow. II. The Dependence of the Viscosity of Liquids on Free-Space. J. Appl. Phys. 1951, 22, 1471–1475. [Google Scholar] [CrossRef]
- Opdam, J.; Gandhi, P.; Kuhnhold, A.; Schilling, T.; Tuinier, R. Excluded volume interactions and phase stability in mixtures of hard spheres and hard rods. Phys. Chem. Chem. Phys. 2022, 24, 11820–11827. [Google Scholar] [CrossRef]
- Hiraga, Y.; Kato, A.; Sato, Y.; Smith, R.L. Densities at Pressures up to 200 MPa and Atmospheric Pressure Viscosities of Ionic Liquids 1-Ethyl-3-methylimidazolium Methylphosphate, 1-Ethyl-3-methylimidazolium Diethylphosphate, 1-Butyl-3-methylimidazolium Acetate, and 1-Butyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide. J. Chem. Eng. Data 2015, 60, 876–885. [Google Scholar] [CrossRef]
- Iguchi, M.; Hiraga, Y.; Sato, Y.; Aida, T.M.; Watanabe, M.; Smith, R.L. Measurement of High-Pressure Densities and Atmospheric Viscosities of Ionic Liquids: 1-Hexyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide and 1-Hexyl-3-methylimidazolium Chloride. J. Chem. Eng. Data 2014, 59, 709–717. [Google Scholar] [CrossRef]
- Machida, H.; Sato, Y.; Smith, R.L. Pressure–volume–temperature (PVT) measurements of ionic liquids ([bmim+][PF6−], [bmim+][BF4−], [bmim+][OcSO4−]) and analysis with the Sanchez–Lacombe equation of state. Fluid. Phase Equilibria 2008, 264, 147–155. [Google Scholar] [CrossRef]
- Machida, H.; Taguchi, R.; Sato, Y.; Smith, R.L. Measurement and Correlation of High Pressure Densities of Ionic Liquids, 1-Ethyl-3-methylimidazolium l-Lactate ([emim][Lactate]), 2-Hydroxyethyl-trimethylammonium l-Lactate ([(C2H4OH)(CH3)3N][Lactate]), and 1-Butyl-3-methylimidazolium Chloride ([bmim][Cl]). J. Chem. Eng. Data 2010, 56, 923–928. [Google Scholar] [CrossRef]
- Taguchi, R.; Machida, H.; Sato, Y.; Smith, R.L. High-Pressure Densities of 1-Alkyl-3-methylimidazolium Hexafluorophosphates and 1-Alkyl-3-methylimidazolium Tetrafluoroborates at Temperatures from (313 to 473) K and at Pressures up to 200 MPa. J. Chem. Eng. Data 2009, 54, 22–27. [Google Scholar] [CrossRef]
- Mimoune, Z.; Anoune, I.; Madani, H. Implementation of PC-SAFT for Predicting thermodynamic properties of pure refrigerants and vapor-liquid equilibria of refrigerants binary mixtures. Fluid. Phase Equilibria 2023, 573, 113868. [Google Scholar] [CrossRef]
- de Almeida, E.S.; da Silva Damaceno, D.; Carvalho, L.; Victor, P.A.; dos Passos, R.M.; de Almeida Pontes, P.V.; Cunha-Filho, M.; Sampaio, K.A.; Monteiro, S. Thermal and Physical Properties of Crude Palm Oil with Higher Oleic Content. Appl. Sci. 2021, 11, 7094. [Google Scholar] [CrossRef]
- Esteban, B.; Riba, J.-R.; Baquero, G.; Rius, A.; Puig, R. Temperature dependence of density and viscosity of vegetable oils. Biomass Bioenergy 2012, 42, 164–171. [Google Scholar] [CrossRef]
- Freitas, S.V.D.; e Silva, F.A.; Pastoriza-Gallego, M.J.; Piñeiro, M.M.; Lima, Á.S.; Coutinho, J.A.P. Measurement and Prediction of Densities of Vegetable Oils at Pressures up to 45 MPa. J. Chem. Eng. Data 2013, 58, 3046–3053. [Google Scholar] [CrossRef]
- Kim, N.H.; Jung, S.Y.; Park, Y.A.; Lee, Y.J.; Jo, J.Y.; Lee, S.M.; Oh, Y.H. Fatty acid composition and characterisation of commercial vegetable oils with chemometric approaches. Int. Food Res. J. 2020, 27, 270–279. [Google Scholar]
- Revelou, P.K.; Xagoraris, M.; Alexandropoulou, A.; Kanakis, C.D.; Papadopoulos, G.K.; Pappas, C.S.; Tarantilis, P.A. Chemometric Study of Fatty Acid Composition of Virgin Olive Oil from Four Widespread Greek Cultivars. Molecules 2021, 26, 4151. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Guo, X.; Qiang, J.; Cao, Y.; Zhang, S.; Yu, X. Influence of triacylglycerol structure on the formation of lipid oxidation products in different vegetable oils during frying process. Food Chem. 2025, 464, 141783. [Google Scholar] [CrossRef] [PubMed]
- Indelicato, S.; Bongiorno, D.; Pitonzo, R.; Di Stefano, V.; Calabrese, V.; Indelicato, S.; Avellone, G. Triacylglycerols in edible oils: Determination, characterization, quantitation, chemometric approach and evaluation of adulterations. J. Chromatogr. A 2017, 1515, 1–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).