Effect of Intramolecular Hydrogen Bond Formation on the Abraham Model Solute Descriptors for Oxybenzone
Abstract
1. Introduction
2. Chemical Materials and Experimental Methodology
3. Results and Discussion
- Group contribution UFZ-LSER estimation [45]—E = 1.50, S = 1.66, A = 0.41, B = 0.71, V = 1.7391, and L = 9.214;
- Group contribution MIT estimation [46]—E = 1.668, S = 1.898, A = 0.431, B = 0.832, V = 1.7391, and L = 9.354;
- Machine learning MIT estimation [46]—E = 1.644, S = 1.714, A = 0.153, B = 0.697, V = 1.7391, and L = 8.915;
- Machine learning AbraLlama estimation [76]—E = 1.467, S = 1.599, A = 0.175, B = 1.049, and V = 1.767.
4. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katritzky, A.R.; Slavov, S.H.; Dobchev, D.A.; Karelson, M. Rapid QSPR model development technique for prediction of vapor pressure of organic compounds. Comp. Chem. Eng. 2007, 31, 1123–1130. [Google Scholar] [CrossRef]
- Zushi, Y. Direct prediction of physicochemical properties and toxicities of chemicals from analytical descriptors by GC-MS. Anal. Chem. 2022, 94, 9149–9157. [Google Scholar] [CrossRef] [PubMed]
- Carande, W.H.; Kazakov, A.; Muzny, C.; Frenkel, M. Quantitative structure-property relationship predictions of critical properties and acentric factors for pure compounds. J. Chem. Eng. Data 2015, 60, 1377–1387. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Petrukhin, R.; Jain, R.; Karelson, M. QSPR analysis of flash points. J. Chem. Inf. Comp. Sci. 2001, 41, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Stoyanova-Slavova, I.B.; Dobchev, D.A.; Karelson, M. QSPR modeling of flash points: An update. J. Mol. Graph. Model. 2007, 26, 529–536. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Chen, J. Quantitative structure-property relationship of distribution coefficients of organic compounds. SAR QSAR Environ. Res. 2020, 31, 585–596. [Google Scholar] [CrossRef]
- Fu, L.; Liu, L.; Yang, Z.-J.; Li, P.; Ding, J.-J.; Yun, Y.-H.; Lu, A.-P.; Hou, T.-J.; Cao, D.-S. Systematic modeling of log D7.4 based on ensemble machine learning, group contribution, and matched molecular pair analysis. J. Chem. Inf. Model. 2020, 60, 63–76. [Google Scholar] [CrossRef]
- Kang, X.; Hu, B.; Perdana, M.C.; Zhao, Y.; Chen, Z. Extreme learning machine models for predicting the n-octanol/water partition coefficient (Kow) data of organic compounds. J. Environ. Chem. Eng. 2022, 10, 108552. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Oliferenko, A.A.; Oliferenko, P.V.; Petrukhin, R.; Tatham, D.B.; Maran, U.; Lomaka, A.; Acree, W.E., Jr. A general treatment of solubility. 2. QSPR prediction of free energies of solvation of specified solutes in ranges of solvents. J. Chem. Inf. Comp. Sci. 2003, 43, 1806–1814. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Oliferenko, A.A.; Oliferenko, P.V.; Petrukhin, R.; Tatham, D.B.; Maran, U.; Lomaka, A.; Acree, W.E., Jr. A general treatment of solubility. 1. The QSPR correlation of solvation free energies of single solutes in series of solvents. J. Chem. Inf. Comp. Sci. 2003, 43, 1794–1805. [Google Scholar] [CrossRef]
- Brown, T.N. QSPRs for predicting equilibrium partitioning in solvent-air systems from the chemical structures of solutes and solvents. J. Solut. Chem. 2022, 51, 1101–1132. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, J.; Li, X.; Wang, Y.; Yu, H. Comparison of prediction methods for octanol-air partition coefficients of diverse organic compounds. Chemosphere 2016, 148, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, W.; Xiao, Z.; Yang, W.; Peng, Y.; Chen, J.; Li, J. Novel quantitative structure activity relationship models for predicting hexadecane/air partition coefficients of organic compounds. J. Environ. Sci. 2023, 124, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Vermeire, F.H.; Wu, H.; Walker, P.J.; Abraham, M.H.; Green, W.H. Group contribution and machine learning approaches to predict Abraham solute parameters, solvation free energy, and solvation enthalpy. J. Chem. Inf. Model. 2022, 62, 433–446. [Google Scholar] [CrossRef]
- Ebert, R.-U.; Kühne, R.; Schüürmann, G. Henry’s law constant—A general-purpose fragment model to predict Log Kaw from molecular structure. Environ. Sci. Technol. 2023, 57, 160–167. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Chen, K.; Wang, Y.; Karelson, M.; Lucic, B.; Trinajstic, N.; Suzuki, T.; Schuurmann, G. Prediction of liquid viscosity for organic compounds by a quantitative structure-property relationship. J. Phys. Org. Chem. 2000, 13, 80–86. [Google Scholar] [CrossRef]
- Mirkhani, S.A.; Gharagheizi, F. Predictive quantitative structure-property relationship model for the estimation of ionic liquid viscosity. Ind. Eng. Chem. Res. 2012, 51, 2470–2477. [Google Scholar] [CrossRef]
- Paduszyński, K.; Domańska, U. Viscosity of ionic liquids: An extensive database and a new group contribution model based on a feed-forward artificial neural network. J. Chem. Inf. Model. 2014, 54, 1311–1324. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ojha, P.K.; Roy, K. Development of a temperature dependent 2D-QSPR model for viscosity of diverse functional ionic liquids. J. Mol. Liq. 2017, 240, 454–467. [Google Scholar] [CrossRef]
- Lazzús, J.A.; Pulgar-Villarroel, G.; Cuturrufo, F.; Vega, P. Development of a group contribution method for estimating surface tension of ionic liquids over a wide range of temperatures. J. Mol. Liq. 2017, 240, 522–531. [Google Scholar] [CrossRef]
- Naef, R. A generally applicable computer algorithm based on the group additivity method for the calculation of seven molecular descriptors: Heat of combustion, log Po/w, log S, refractivity, polarizability, toxicity and log BB of organic compounds; scope and limits of applicability. Molecules 2015, 20, 18279–18351. [Google Scholar] [CrossRef] [PubMed]
- Gharagheizi, F.; Mirkhani, S.A.; Tofangchi Mahyari, A.R. Prediction of standard enthalpy of combustion of pure compounds using a very accurate group-contribution-based method. Energy Fuels 2011, 25, 2651–2654. [Google Scholar] [CrossRef]
- Cardozo, R.L. Prediction of the enthalpy of combustion of organic compounds. AIChE J. 1986, 32, 844–848. [Google Scholar] [CrossRef]
- Hukkerikar, A.S.; Meier, R.J.; Sina, G.; Gani, R. Method to estimate the enthalpy of formation of organic compounds with chemical accuracy. Fluid Phase Equilibria 2013, 348, 23–32. [Google Scholar] [CrossRef]
- Golmohammadi, H.; Dashtbozorgi, Z.; Gholam Samani, M.; Acree, W.E., Jr. QSPR prediction of gas-to-methanol solvation enthalpy of organic compounds using replacement method and support vector machine. Phys. Chem. Liq. 2015, 53, 46–66. [Google Scholar] [CrossRef]
- Toubaei, A.; Golmohammadi, H.; Dashtbozorgi, Z.; Acree, W.E., Jr. QSPR studies for predicting gas to acetone and gas to acetonitrile solvation enthalpies using support vector machine. J. Mol. Liq. 2012, 175, 24–32. [Google Scholar] [CrossRef]
- Khajeh, A.; Modarress, H. Quantitative structure–property relationship prediction of liquid heat capacity at 298.15 K for organic compounds. Ind. Eng. Chem. Res. 2012, 51, 6251–6255. [Google Scholar] [CrossRef]
- Khajeh, A.; Modarress, H. Quantitative structure-property relationship prediction of gas heat capacity for organic compounds. Ind. Eng. Chem. Res. 2012, 51, 13490–13495. [Google Scholar] [CrossRef]
- Naef, R. Calculation of the isobaric heat capacities of the liquid and solid phase of organic compounds at 298.15 K by means of the group-additivity method. Molecules 2020, 25, 1147. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hesse, D.G.; Liebman, J.F. A group additivity approach for the estimation of heat capacities of organic liquids and solids at 298 K. Struct. Chem. 1993, 4, 261–269. [Google Scholar] [CrossRef]
- Acree, W.; Chickos, J.S. Phase transition enthalpy measurements of organic and organometallic compounds and ionic liquids. sublimation, vaporization, and fusion enthalpies from 1880 to 2015. Part 2. C11-C192. J. Phys. Chem. Ref. Data 2017, 46, 013104. [Google Scholar] [CrossRef]
- Kolska, Z.; Kukal, J.; Zabransky, M.; Ruazicka, V. Estimation of the heat capacity of organic liquids as a function of temperature by a three-level group contribution method. Ind. Eng. Chem. Res. 2008, 47, 2075–2085. [Google Scholar] [CrossRef]
- Naef, R.; Acree, W.E., Jr. Calculation of five thermodynamic molecular descriptors by means of a general computer algorithm based on the group-additivity method: Standard enthalpies of vaporization, sublimation and solvation, and entropy of fusion of ordinary organic molecules and total phase-change entropy of liquid crystals. Molecules 2017, 22, 1059. [Google Scholar] [CrossRef]
- Gharagheizi, F.; Ilani-Kashkouli, P.; Acree, W.E., Jr.; Mohammadi, A.H.; Ramjugernath, D. A group contribution model for determining the vaporization enthalpy of organic compounds at the standard reference temperature of 298 K. Fluid Phase Equilibria 2013, 360, 279–292. [Google Scholar] [CrossRef]
- Gharagheizi, F.; Ilani-Kashkouli, P.; Acree, W.E., Jr.; Mohammadi, A.H.; Ramjugernath, D. A group contribution model for determining the sublimation enthalpy of organic compounds at the standard reference temperature. Fluid Phase Equilibria 2013, 354, 265–285. [Google Scholar] [CrossRef]
- Dohnal, V. New QSPR molecular descriptors based on low-cost quantum chemistry computations using DFT/COSMO approach. J. Mol. Liq. 2024, 407, 125256. [Google Scholar] [CrossRef]
- Yan, X.; Lan, T.; Jia, Q.; Yan, F.; Wang, Q. A norm indexes-based QSPR model for predicting the standard vaporization enthalpy and formation enthalpy of organic compounds. Fluid Phase Equilibria 2020, 507, 112437. [Google Scholar] [CrossRef]
- Chickos, J.S.; Acree, W.E., Jr.; Liebman, J.F. Estimating solid-liquid phase change enthalpies and entropies. J. Phys. Chem. Ref. Data 1999, 28, 1535–1673. [Google Scholar] [CrossRef]
- Chickos, J.S.; Acree, W.E., Jr. Total phase change entropies and enthalpies. An update on fusion enthalpies and their estimation. Thermochim. Acta 2009, 495, 5–13. [Google Scholar] [CrossRef]
- Fredenslund, A.; Gmehling, J.; Michelsen, M.L.; Rasmussen, P.; Prausnitz, J.M. Computerized design of multicomponent distillation columns using the UNIFAC group contribution method for calculation of activity coefficients. Ind. Eng. Chem. Proc. Des. Dev. 1977, 16, 450–462. [Google Scholar] [CrossRef]
- Gmehling, J.; Tiegs, D.; Knipp, U. A comparison of the predictive capability of different group contribution methods. Fluid Phase Equilibria 1990, 54, 147–165. [Google Scholar] [CrossRef]
- Gmehling, J.; Lohmann, J.; Jakob, A.; Li, J.; Joh, R. A modified UNIFAC (Dortmund) model. 3. Revision and extension. Ind. Eng. Chem. Res. 1998, 37, 4876–4882. [Google Scholar] [CrossRef]
- Currier, R.P.; O’Connell, J.P. An analysis of the solution of groups method for component activity coefficients. Fluid Phase Equilibria 1987, 33, 245–265. [Google Scholar] [CrossRef]
- Tochigi, K.; Gmehling, J. Determination of ASOG parameters-extension and revision. J. Chem. Eng. Jpn. 2011, 44, 304–306. [Google Scholar] [CrossRef]
- Ulrich, N.; Endo, S.; Brown, T.N.; Watanabe, N.; Bronner, G.; Abraham, M.H.; Goss, K.-U. UFZ-LSER Database; v 3.2.1; Helmholtz Centre for Environmental Research-UFZ: Leipzig, Germany, 2017; Available online: http://www.ufz.de/lserd (accessed on 27 June 2024).
- Solvation Tools. Available online: https://rmg.mit.edu/database/solvation/search/ (accessed on 27 June 2024).
- Acree, W.E., Jr.; Smart, K.; Abraham, M.H. Abraham model solute descriptors reveal strong intramolecular hydrogen bonding in 1,4-dihydroxyanthraquinone and 1,8-dihydroxyanthraquinone. Phys. Chem. Liq. 2018, 56, 416–420. [Google Scholar] [CrossRef]
- Merrill, J.R. Measurements of intramolecular hydrogen bonding by nuclear magnetic resonance and infrared spectroscopy. J. Phys. Chem. 1961, 65, 2023–2026. [Google Scholar] [CrossRef]
- Strat, M.; Umreiko, D.S.; Khovratovich, N.N. Spectroscopic manifestation of an intramolecular hydrogen bond in 2-hydroxybenzophenone derivatives. Zhurnal Prikl. Spektrosk. 1973, 19, 103–108. [Google Scholar]
- Liebich, B.W.; Parthe, E. 2-Hydroxy-4-methoxybenzophenone. Acta Crystallogr. B 1974, 30B, 2522–2524. [Google Scholar] [CrossRef]
- Liebich, B.W. The crystal and molecular structure of 2,4-dihydroxybenzophenone (HHB). Acta Crystallogr. B 1979, B35, 1186–1190. [Google Scholar] [CrossRef]
- Baumstark, A.L.; Boykin, D.W. Oxygen-17 NMR spectroscopy: Multiple intramolecular hydrogen-bonded phenolic carbonyl systems. Nouv. J. Chim. 1992, 16, 357–360. [Google Scholar]
- Zahedi-Tabrizi, M.; Tayyari, S.F.; Badalkhani-Khamseh, F.; Ghomi, R.; Afshar-Qahremani, F. Molecular structure and intramolecular hydrogen bonding in 2-hydroxybenzophenones: A theoretical study. J. Chem. Sci. 2014, 126, 919–929. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, X.; Wu, F.; Huang, B.; Au, C.; Yi, B. Effect of substituent groups on the strength of intramolecular hydrogen bonds in 2,4-dihydroxybenzophenone UV absorbers. Molecules 2023, 28, 5017. [Google Scholar] [CrossRef] [PubMed]
- Baughman, B.M.; Stennett, E.; Lipner, R.E.; Rudawsky, A.C.; Schmidtke, S.J. Structural and spectroscopic studies of the photophysical properties of benzophenone derivatives. J. Phys. Chem. A 2009, 113, 8011–8019. [Google Scholar] [CrossRef]
- Litwinienko, G.; Di Labio, G.A.; Mulder, P.; Korth, H.-G.; Ingold, K.U. Intramolecular and intermolecular hydrogen bond formation by some ortho-substituted phenols: Some surprising results from an experimental and theoretical investigation. J. Phys. Chem. A 2009, 113, 6275–6288. [Google Scholar] [CrossRef]
- Bureiko, S.F.; Golubev, N.S.; Pihlaja, K. Spectroscopic studies of bifurcated hydrogen bonds in solution. J. Mol. Struct. 1999, 480–481, 297–301. [Google Scholar] [CrossRef]
- Bureiko, S.F.; Golubev, N.S.; Mattinen, J.; Pihlaja, K. On the existence of bifurcated hydrogen bond in liquids: Interaction of 2,6-disubstituted phenols with proton acceptors. J. Mol. Liq. 1990, 45, 139–145. [Google Scholar] [CrossRef]
- Liao, C.; Kannan, K. Widespread occurrence of benzophenone-type UV light filters in personal care products from China and the United States: An assessment of human exposure. Environ. Sci. Technol. 2014, 48, 4103–4109. [Google Scholar] [CrossRef] [PubMed]
- Scheele, A.; Sutter, K.; Karatum, O.; Danley-Thomson, A.A.; Redfern, L.K. Environmental impacts of the ultraviolet filter oxybenzone. Sci. Total Environ. 2023, 863, 160966. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Law, J.C.F.; Lam, T.K.; Leung, K.S.Y. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total Environ. 2021, 755, 142486. [Google Scholar] [CrossRef]
- Downs, C.A.; Bishop, E.; Diaz-Cruz, M.S.; Haghshenas, S.A.; Stien, D.; Rodrigues, A.M.S.; Woodley, C.M.; Sunyer-Caldu, A.; Doust, S.N.; Espero, W.; et al. Oxybenzone contamination from sunscreen pollution and its ecological threat to Hanauma Bay, Oahu, Hawaii, U.S.A. Chemosphere 2022, 291, 132880. [Google Scholar] [CrossRef]
- Narla, S.; Lim, H.W. Sunscreen: FDA regulation, and environmental and health impact. Photochem. Photobiol. Sci. 2020, 19, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Pergolizzi, J.V., Jr.; Taylor, R., Jr.; Kitzen, J.M. Sunscreen bans: Coral reefs and skin cancer. J. Clin. Pharm. Ther. 2019, 44, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.L.; Lim, H.W. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J. Am. Acad. Dermatol. 2019, 80, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Rodil, R.; Moeder, M. Development of a method for the determination of UV filters in water samples using stir bar sorptive extraction and thermal desorption–gas chromatography–mass spectrometry. J. Chromatogr. A 2008, 1179, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; Smith, R.E.; Luchtefeld, R.; Boorem, A.J.; Luo, R.; Acree, W.E., Jr. Prediction of solubility of drugs and other compounds in organic solvents. J. Pharm. Sci. 2010, 99, 1500–1515. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Horton, M.Y.; Higgins, E.; Abraham, M.H. Abraham model linear free energy relationships as a means of extending solubility studies to include the estimation of solute solubilities in additional organic solvents. J. Chem. Thermodyn. 2016, 102, 392–397. [Google Scholar] [CrossRef]
- Jalan, A.; Ashcraft, R.W.; West, R.H.; Green, W.H. Predicting solvation energies for kinetic modeling. Annu. Rep. Prog. Chem Sec. C Phys. Chem. 2010, 106, 211–258. [Google Scholar] [CrossRef]
- Clarke, E.D. Beyond physical properties–application of Abraham descriptors and LFER analysis in agrochemical research. Bioorg. Med. Chem. 2009, 17, 4153–4159. [Google Scholar] [CrossRef]
- Poole, C.F.; Ariyasena, T.C.; Lenca, N. Estimation of the environmental properties of compounds from chromatographic properties and the solvation parameter method. J. Chromatogr. A 2013, 1317, 85–104. [Google Scholar] [CrossRef]
- Poole, C.F.; Atapattu, S.N. Recent advances for estimating environmental properties for small molecules from chromatographic measurements and the solvation parameter model. J. Chromatogr. A 2022, 1687, 463682. [Google Scholar] [CrossRef]
- Endo, S.; Goss, K.-U. Applications of polyparameter linear free energy relationships in environmental chemistry. Environ. Sci. Technol. 2014, 48, 12477–12491. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; McGowan, J.C. The use of characteristic volumes to measure cavity terms in reversed phase liquid chromatography. Chromatographia 1987, 23, 243–246. [Google Scholar] [CrossRef]
- Sinha, S.; Yang, C.; Wu, E.; Acree, W.E., Jr. Abraham solvation parameter model: Examination of possible intramolecular hydrogen-bonding using calculated solute descriptors. Liquids 2022, 2, 131–146. [Google Scholar] [CrossRef]
- Lang, A.S.I.D.; Lee, Y. AbraLlama Hugging Face App: Predicting Abraham Model Solute Descriptors and Modified Solvent Parameters Using Llama. Available online: https://huggingface.co/spaces/ttmn/AbraLlama (accessed on 21 July 2024).
- Hansen, P.K. A Spectroscopic overview of intramolecular hydrogen bonds of NH…O,S,N type. Molecules 2021, 26, 2409. [Google Scholar] [CrossRef]
- Bertolasi, V.; Ferretti, V.; Gilli, P.; Gilli, G.; lssaI, Y.M.; Sherif, O.E. Intramolecular N-H O hydrogen bonding assisted by resonance. Part 2. Intercorrelation between structural and spectroscopic parameters for five 1.3-diketone arylhydrazones derived from dibenzoylmethane. J. Chem. Soc. Perkin Trans. 2 1993, 2223–2228. [Google Scholar] [CrossRef]
- Marrero, J.; Gani, R. Group-contribution based estimation of pure component properties. Fluid Phase Equilibria 2001, 183–184, 183–208. [Google Scholar] [CrossRef]
- SciFinder. Chemical Abstracts Service: Columbus, OH. Carbon-13 NMR Spectrum; Spectrum ID CC-03-C_SPC-3734; RN 50-52-2. Available online: https://scifinder.cas.org (accessed on 21 July 2024).
- Domanska, U. Solubility of acetyl-substituted naphthols in binary solvent mixtures. Fluid Phase Equilibria 1990, 55, 125–145. [Google Scholar] [CrossRef]
- Buchowski, H.; Domanska, U. Solubility and hydrogen bonding. Part VI. Evidence for intramolecular hydrogen bonds. Pol. J. Chem. 1980, 54, 97–102. [Google Scholar]
- Skulski, L. Studies on intramolecular hydrogen bonding by ultraviolet spectroscopy. VI. Ultraviolet absorption spectra of acetyl- and benzoyl-substituted naphthols. Bull. Acad. Polon. Sci. Ser. Sci. Chim. 1969, 17, 253–258. [Google Scholar]
- Skulski, L.; Waclawek, W. Studies on intramolecular hydrogen bonding by ultraviolet-visible absorption spectroscopy. VIII. Correlations between the spectral solvent effects and dioxane effect in the dipolometric measurements. Bull. Acad. Polon. Sci. Ser. Sci. Chim. 1971, 19, 277–286. [Google Scholar]
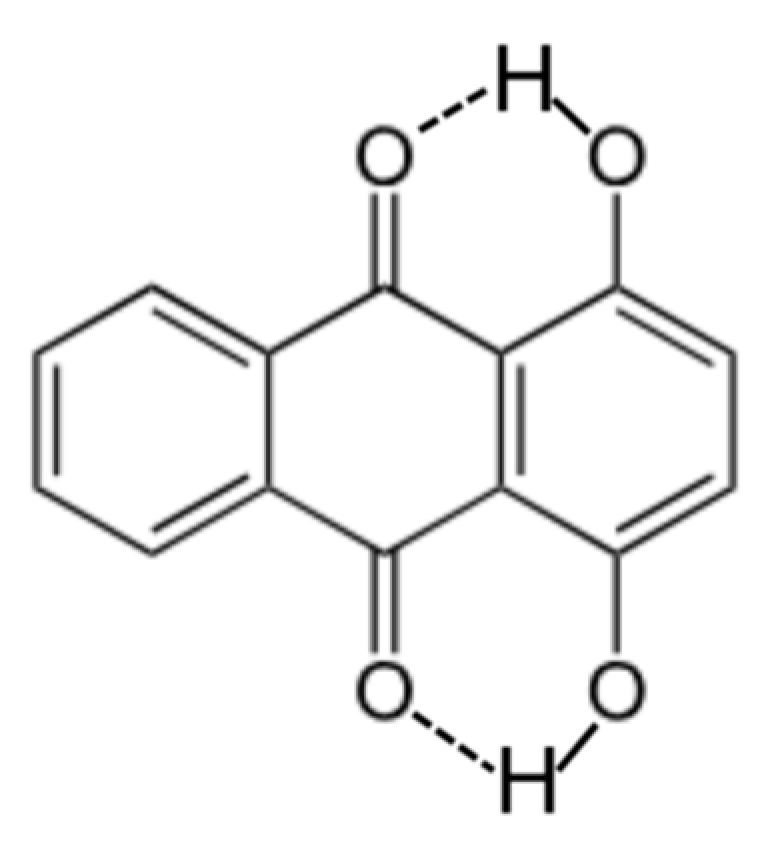
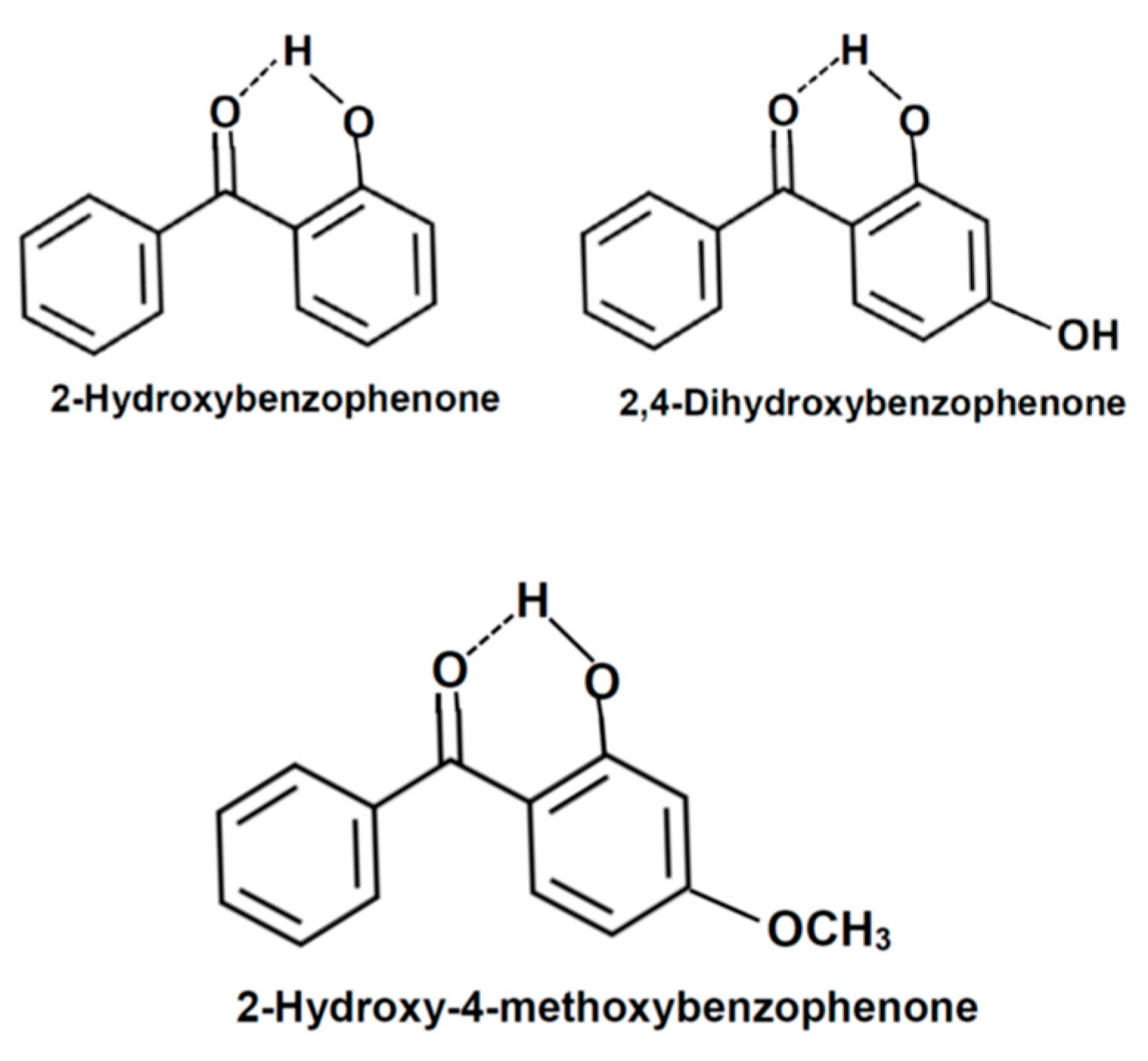
| Organic Solvent | XS,organic | Chemical Name | XS,organic |
|---|---|---|---|
| Hexane | 0.01298 | Heptan-1-ol | 0.03054 |
| Heptane | 0.01467 | Octan-1-ol | 0.04069 |
| Octane | 0.01554 | Butan-2-ol | 0.01698 |
| Dodecane | 0.02101 | 2-Methylpropan-1-ol | 0.01161 |
| Cyclohexane | 0.01994 | 2-Methylbutan-1-ol | 0.02262 |
| Methylcyclohexane | 0.02604 | 3-Methylbutan-1-ol | 0.02313 |
| 2,2,4-Trimethylpentane | 0.01264 | 4-Methylpentan-2-ol | 0.02695 |
| Diisopropyl ether | 0.06958 | Ethyl-1-hexan-1-ol | 0.02726 |
| Propan-1-ol | 0.02016 | Cyclopentanol | 0.05380 |
| Butan-1-ol | 0.01819 | Ethylene glycol | 0.000994 |
| Pentan-1-ol | 0.01633 |
| Solvent | Log CS,organicexp | Log CS,organiccalc; Equation (1) a | Log CS,organiccalc; Equation (2) a |
|---|---|---|---|
| Hexane | −1.008 | −1.102 | −1.077 |
| Heptane | −1.004 | −1.039 | −1.070 |
| Octane | −1.023 | −1.031 | −1.048 |
| Dodecane | −1.035 | −1.101 | −0.999 |
| Cyclohexane | −0.744 | −0.678 | −0.810 |
| Methylcyclohexane | −0.698 | −0.716 | −0.822 |
| Isooctane | −1.120 | −1.184 | −1.219 |
| Propan-1-ol | −0.585 | −0.659 | −0.700 |
| Butan-1-ol | −0.713 | −0.631 | −0.677 |
| Pentan-1-ol | −0.822 | −0.636 | −0.682 |
| Heptan-1-ol | −0.675 | −0.601 | −0.603 |
| Octan-1-ol | −0.594 | −0.613 | −0.627 |
| 2-Methylpropan-1-ol | −0.908 | −0.703 | −0.732 |
| Butan-2-ol | −0.744 | −0.673 | −0.670 |
| 2-Methylbutan-1-ol | −0.687 | −0.667 | −0.702 |
| 3-Methylbutan-1-ol | −0.684 | −0.669 | −0.687 |
| 4-Methylpentan-2-ol | −0.680 | −0.707 | −0.733 |
| 2-Ethylhexan-1-ol | −0.764 | −0.677 | −0.653 |
| Cyclopentanol | −0.236 | −0.455 | −0.380 |
| Ethylene glycol | −1.751 | −1.554 | −1.581 |
| Diisopropyl ether | −0.324 | −0.533 | −0.491 |
| Log (P or CS,org/CS,water) | cp | ep | sp | ap | bp | vp |
|---|---|---|---|---|---|---|
| Octan-1-ol (wet) | 0.088 | 0.562 | −1.054 | 0.034 | −3.460 | 3.814 |
| Hexane | 0.333 | 0.560 | −1.710 | −3.578 | −4.939 | 4.463 |
| Heptane | 0.297 | 0.634 | −1.755 | −3.571 | −4.946 | 4.488 |
| Octane | 0.241 | 0.690 | −1.769 | −3.545 | −5.011 | 4.511 |
| Dodecane | 0.114 | 0.668 | −1.644 | −3.545 | −5.006 | 4.459 |
| Cyclohexane | 0.159 | 0.784 | −1.678 | −3.740 | −4.929 | 4.577 |
| Methylcyclohexane | 0.246 | 0.782 | −1.982 | −3.517 | −4.293 | 4.528 |
| Isooctane | 0.318 | 0.555 | −1.737 | −3.677 | −4.864 | 4.417 |
| Air-to-water | −0.994 | 0.577 | 2.549 | 3.813 | 4.841 | −0.869 |
| Propan-1-ol | 0.139 | 0.405 | −1.029 | 0.247 | −3.767 | 3.986 |
| Butan-1-ol | 0.165 | 0.401 | −1.011 | 0.056 | −3.958 | 4.044 |
| Pentan-1-ol | 0.150 | 0.536 | −1.229 | 0.141 | −3.864 | 4.077 |
| Heptan-1-ol | 0.035 | 0.398 | −1.063 | 0.002 | −4.342 | 4.317 |
| Octan-1-ol | −0.034 | 0.489 | −1.044 | −0.024 | −4.235 | 4.218 |
| 2-Methylpropan-1-ol | 0.188 | 0.354 | −1.127 | 0.016 | −3.568 | 3.986 |
| Butan-2-ol | 0.127 | 0.253 | −0.976 | 0.158 | −3.882 | 4.114 |
| 2-Methylbutan-1-ol | 0.143 | 0.388 | −1.173 | −0.024 | −3.817 | 4.129 |
| 3-Methylbutan-1-ol | 0.111 | 0.337 | −1.180 | 0.063 | −3.880 | 4.218 |
| 4-Methylpentan-2-ol | 0.096 | 0.301 | −1.100 | 0.039 | −4.081 | 4.242 |
| 2-Ethylhexan-1-ol | −0.033 | 0.566 | −1.233 | −0.068 | −3.912 | 4.153 |
| Cyclopentanol | 0.332 | 0.522 | −1.034 | −0.106 | −3.756 | 3.892 |
| Ethylene glycol | −0.270 | 0.578 | −0.511 | 0.715 | −2.619 | 2.729 |
| Diisopropyl ether | 0.181 | 0.285 | −0.954 | −0.956 | −5.077 | 4.542 |
| Log (K or CS,org/CS,gas) | ck | ek | sk | ak | bk | lk |
| Octan-1-ol (wet) | −0.198 | 0.002 | 0.709 | 3.519 | 1.429 | 0.858 |
| Hexane | 0.320 | 0.000 | 0.000 | 0.000 | 0.000 | 0.945 |
| Heptane | 0.284 | 0.000 | 0.000 | 0.000 | 0.000 | 0.950 |
| Octane | 0.219 | 0.000 | 0.000 | 0.000 | 0.000 | 0.960 |
| Dodecane | 0.017 | 0.000 | 0.000 | 0.000 | 0.000 | 0.989 |
| Cyclohexane | 0.163 | −0.110 | 0.000 | 0.000 | 0.000 | 1.013 |
| Methylcyclohexane | 0.318 | −0.215 | 0.000 | 0.000 | 0.000 | 1.012 |
| Isooctane | 0.264 | −0.230 | 0.000 | 0.000 | 0.000 | 0.975 |
| Air-to-water | −1.271 | 0.822 | 2.743 | 3.904 | 4.814 | −0.213 |
| Propan-1-ol | −0.042 | −0.246 | 0.749 | 3.888 | 1.076 | 0.874 |
| Butan-1-ol | −0.004 | −0.285 | 0.768 | 3.705 | 0.879 | 0.890 |
| Pentan-1-ol | −0.002 | −0.161 | 0.535 | 3.778 | 0.960 | 0.900 |
| Heptan-1-ol | −0.056 | −0.216 | 0.554 | 3.596 | 0.803 | 0.933 |
| Octan-1-ol | −0.147 | −0.214 | 0.561 | 3.507 | 0.749 | 0.943 |
| 2-Methylpropan-1-ol | −0.003 | −0.357 | 0.699 | 3.595 | 1.247 | 0.881 |
| Butan-2-ol | −0.034 | −0.387 | 0.719 | 3.736 | 1.088 | 0.905 |
| 2-Methylbutan-1-ol | −0.055 | −0.348 | 0.601 | 3.565 | 0.996 | 0.925 |
| 3-Methylbutan-1-ol | −0.040 | −0.408 | 0.648 | 3.599 | 0.905 | 0.932 |
| 4-Methylpentan-2-ol | −0.013 | −0.606 | 0.687 | 3.622 | 0.436 | 0.985 |
| 2-Ethylhexan-1-ol | −0.127 | −0.339 | 0.551 | 3.397 | 0.722 | 0.963 |
| Cyclopentanol | −0.151 | −0.314 | 0.693 | 3.549 | 0.914 | 0.956 |
| Ethylene glycol | −0.887 | 0.132 | 1.657 | 4.457 | 2.355 | 0.565 |
| Diisopropyl ether | 0.139 | −0.473 | 0.610 | 2.568 | 0.000 | 1.016 |
| Solute Descriptor Estimation Method | SD (Log Units) | Log CS,water | Log CS,gas |
|---|---|---|---|
| Experiment-Based Values | 0.115 | −4.558 | −9.581 |
| UFZ-LSER Group Contribution Model | 0.896 | −3.100 | −11.485 |
| MIT Group Contribution Model | 1.025 | −2.642 | −11.551 |
| MIT Machine Learning Model | 0.439 | −3.729 | −10.347 |
| AbraLlama Machine Learning Model | 0.707 a | −2.397 a |
| Solute | Informed User-Modified Canonical SMILES Code a |
|---|---|
| Oxybenzone | O=C(C=1C=CC=CC1)C2=CC=C(OC)C=C2Ohb |
| 2-Hydroxybenzophenone | O=C(C=1C=CC=CC1)C=2C=CC=CC2Ohb |
| 2,4-Dihydroxybenzophenone | O=C(C=1C=CC=CC1)C2=CC=C(O)C=C2Ohb |
| 5-Hydroxyflavone | O=C1C=C(OC=2C=CC=C(Ohb)C12)C=3C=CC=CC3 |
| 3,5-Dihydroxyflavone | O=C1C(O)=C(OC=2C=CC=C(Ohb)C12)C=3C=CC=CC3 |
| 5,6-Dihydroxyflavone | O=C1C=C(OC2=CC=C(O)C(Ohb)=C12)C=3C=CC=CC3 |
| 5,7-Dihydroxyflavone | O=C1C=C(OC=2C=C(O)C=C(Ohb)C12)C=3C=CC=CC3 |
| 5,4′-Dihydroxyflavone | O=C1C=C(OC=2C=CC=C(Ohb)C12)C=3C=CC(O)=CC3 |
| 5,2′-Dihydroxyflavone | O=C1C=C(OC=2C=CC=C(Ohb)C12)C=3C=CC=CC3O |
| 5,3′-Dihydroxyflavone | O=C1C=C(OC=2C=CC=C(Ohb)C12)C=3C=CC=C(O)C3 |
| 5,4′-Dihydroxy-7-methoxyisoflavone | O=C1C(=COC=2C=C(OhbC)C=C(O)C12)C=3C=CC(O)=CC3 |
| 1-Hydroxyanthraquinone | O=C1C=2C=CC=CC2C(=O)C=3C(Ohb)=CC=CC13 |
| 1,4-Dihydroxyanthraquinone | O=C1C=2C=CC=CC2C(=O)C=3C(Ohb)=CC=C(Ohb)C13 |
| 1,8-Dihydroxyanthraquinone | O=C1C=2C=CC=C(Ohb)C2C(=O)C=3C(Ohb)=CC=CC13 |
| 4,5-Dihydroxyanthraquinone-2-carboxylic acid | O=C(O)C1=CC(Ohb)=C2C(=O)C=3C(Ohb)=CC=CC3C(=O)C2=C1 |
| 2′-Hydroxyacetophenone | O=C(C=1C=CC=CC1Ohb)C |
| 2-Nitrophenol | O=N(=O)C=1C=CC=CC1Ohb |
| 2,4-Dinitrophenol | O=N(=O)C1=CC=C(Ohb)C(=C1)N(=O)=O |
| 2,5-Dinitrophenol | O=N(=O)C1=CC=C(C(Ohb)=C1)N(=O)=O |
| 2,6-Dinitrophenol | O=N(=O)C1=CC=CC(=C1Ohb)N(=O)=O |
| 2-Methoxyphenol | OhbC=1C=CC=CC1OC |
| 2-Methyl-4,6-dinitrophenol | O=N(=O)C=1C=C(C(Ohb)=C(C1)C)N(=O)=O |
| Solute | E | S | A | B | V | L |
|---|---|---|---|---|---|---|
| Oxybenzone | 1.500 | 1.413 | 0.000 | 0.617 | 1.7391 | 8.660 |
| 2-Hydroxybenzophenone | 1.54 | 1.46 | 0.00 | 0.46 | 1.5395 | 7.95 |
| 2,4-Dihydroxybenzophenone | 1.73 | 2.03 | 0.49 | 0.70 | 1.5982 | 9.062 |
| 5-Hydroxyflavone | 1.91 | 1.91 | 0.02 | 0.52 | 1.7284 | 9.41 |
| 5,3-Dihydroxyflavone | 2.09 | 2.07 | 0.37 | 0.73 | 1.7871 | 10.13 |
| 5,6-Dihydroxyflavone | 2.06 | 1.96 | 0.63 | 0.73 | 1.7871 | 10.08 |
| 5,7-Dihydroxyflavone | 2.13 | 1.98 | 0.69 | 0.73 | 1.7871 | 10.22 |
| 5,4′-Dihydroxyflavone | 2.14 | 1.94 | 0.71 | 0.98 | 1.7871 | 10.18 |
| 5,2′-Dihydroxyflavone | 2.14 | 1.90 | 0.42 | 0.84 | 1.7871 | 10.08 |
| 5,3′-Dihydroxyflavone | 2.14 | 1.90 | 0.67 | 0.85 | 1.7871 | 10.14 |
| 1-Hydroxyanthraquinone | 1.504 | 1.491 | 0.050 | 0.539 | 1.5874 | 9.075 |
| 1,4-Dihydroxyanthraquinone | 2.455 | 1.743 | 0.000 | 0.669 | 1.6462 | 9.919 |
| 1,8-Dihydroxyanthraquinone | 2.455 | 1.786 | 0.000 | 0.558 | 1.6462 | 9.907 |
| 4,5-Dihydroxyanthraquinone-2-carboxylic acid | 2.340 | 2.195 | 0.755 | 0.596 | 1.8615 | 11.073 |
| 2′-Hydroxyacetophenone | 0.948 | 1.32 | 0.00 | 0.37 | 1.0726 | 5.341 |
| 2-Nitrophenol | 1.015 | 1.05 | 0.06 | 0.35 | 0.9493 | 4.778 |
| 2,4-Dinitrophenol | 1.200 | 1.57 | 0.09 | 0.54 | 1.1235 | 6.078 |
| 2,5-Dinitrophenol | 1.200 | 1.46 | 0.14 | 0.51 | 1.1235 | 5.990 |
| 2,6-Dinitrophenol | 1.220 | 2.11 | 0.16 | 0.45 | 1.1235 | 6.565 |
| 2-Methoxyphenol | 0.837 | 0.92 | 0.16 | 0.54 | 0.9747 | 4.494 |
| 2-Methyl-4,6-dinitrophenol | 1.200 | 1.59 | 0.04 | 0.52 | 1.2644 | 5.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Chen, A.; Yang, Y.; Acree, W.E. Effect of Intramolecular Hydrogen Bond Formation on the Abraham Model Solute Descriptors for Oxybenzone. Liquids 2024, 4, 647-662. https://doi.org/10.3390/liquids4030036
Chen J, Chen A, Yang Y, Acree WE. Effect of Intramolecular Hydrogen Bond Formation on the Abraham Model Solute Descriptors for Oxybenzone. Liquids. 2024; 4(3):647-662. https://doi.org/10.3390/liquids4030036
Chicago/Turabian StyleChen, Jocelyn, Audrey Chen, Yixuan Yang, and William E. Acree. 2024. "Effect of Intramolecular Hydrogen Bond Formation on the Abraham Model Solute Descriptors for Oxybenzone" Liquids 4, no. 3: 647-662. https://doi.org/10.3390/liquids4030036
APA StyleChen, J., Chen, A., Yang, Y., & Acree, W. E. (2024). Effect of Intramolecular Hydrogen Bond Formation on the Abraham Model Solute Descriptors for Oxybenzone. Liquids, 4(3), 647-662. https://doi.org/10.3390/liquids4030036








