An X-ray and Neutron Scattering Study of Aqueous MgCl2 Solution in the Gigapascal Pressure Range
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Solutions
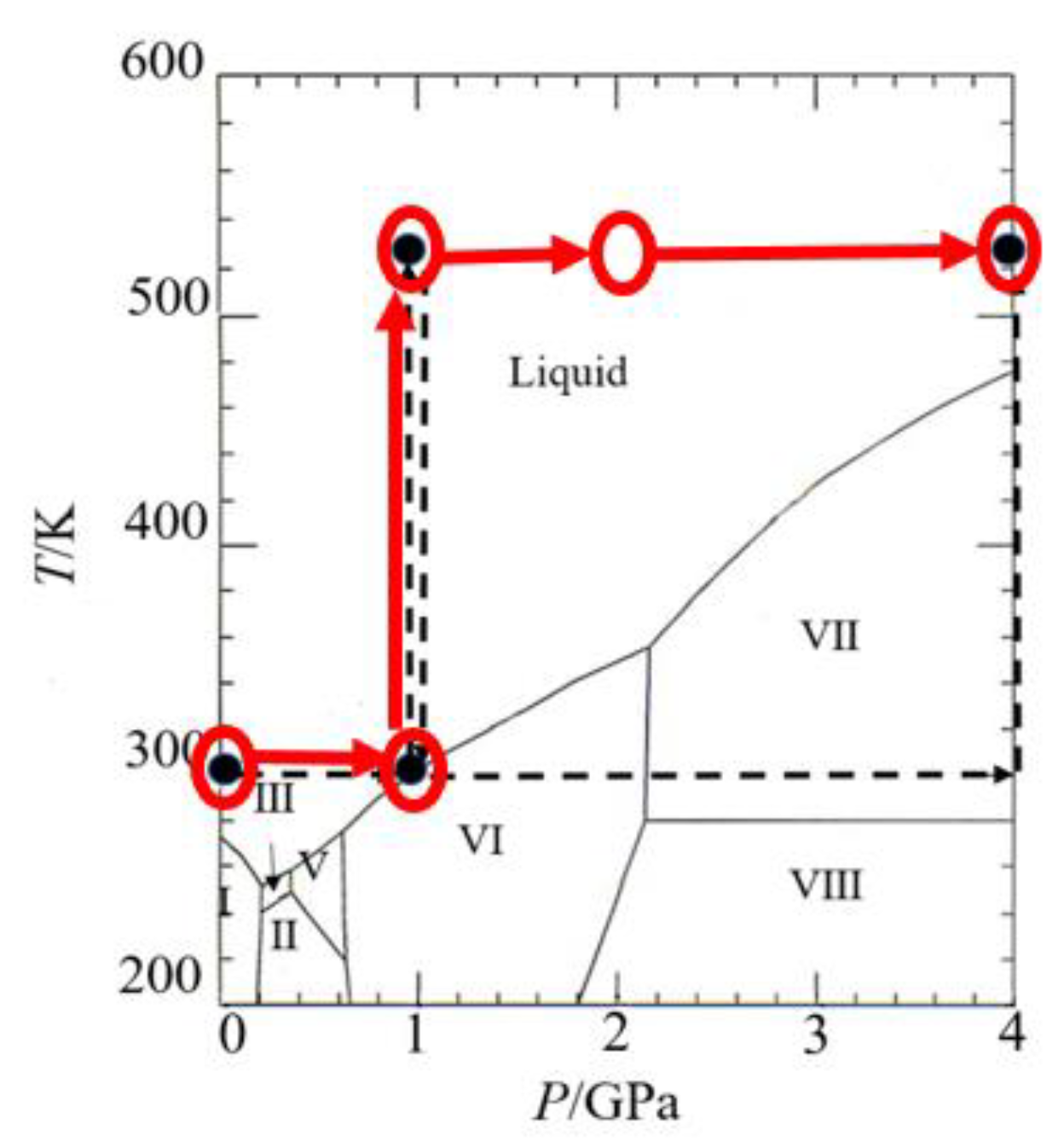
2.2. Experimental PT Condition and Density Estimation
2.3. X-ray Scattering Measurements
2.4. Neutron Scattering Measurements
2.5. EPSR Modeling
3. Results
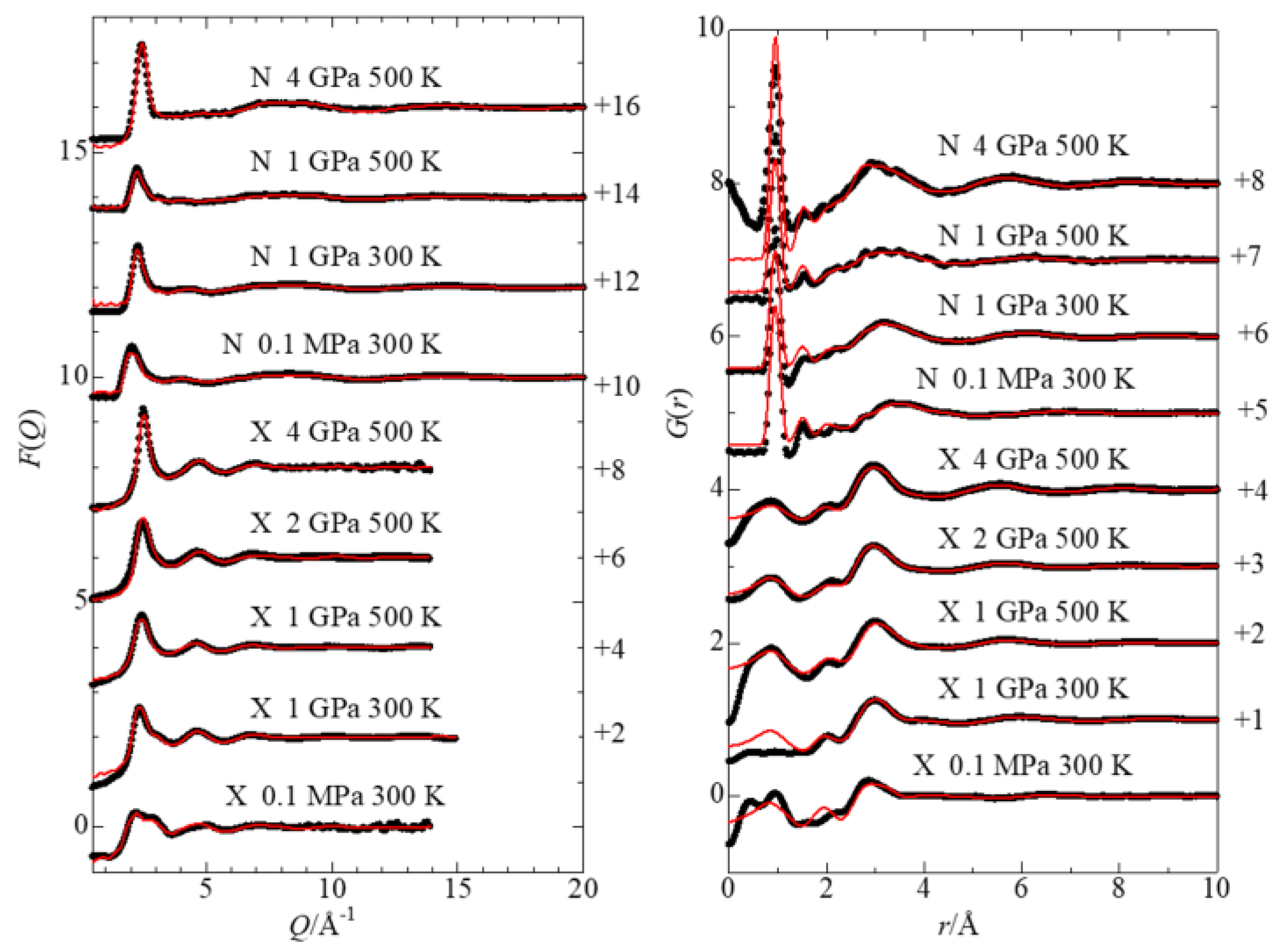
3.1. Interference Functions and Radial Distribution Functions
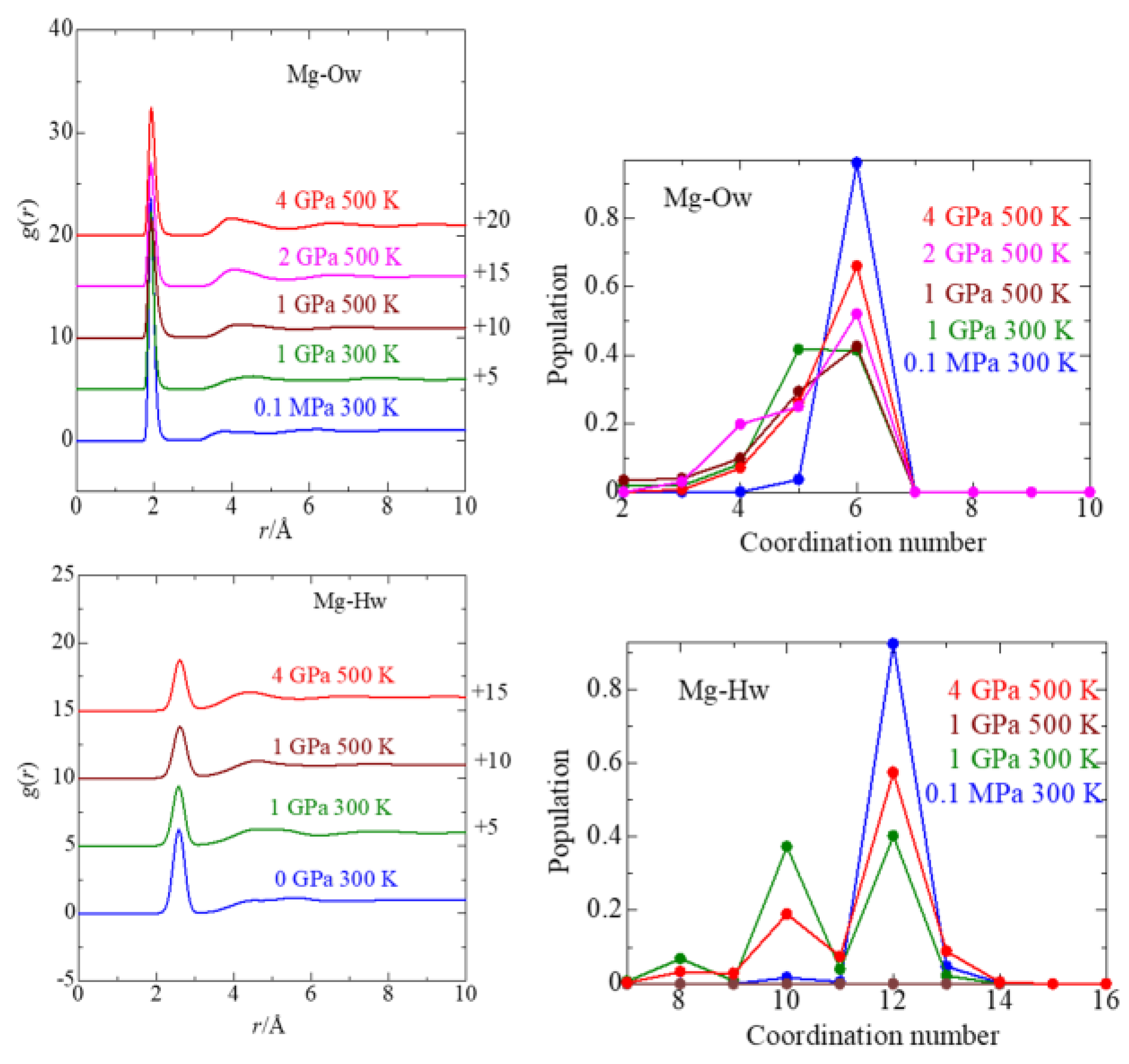
3.2. Mg2+ Solvation
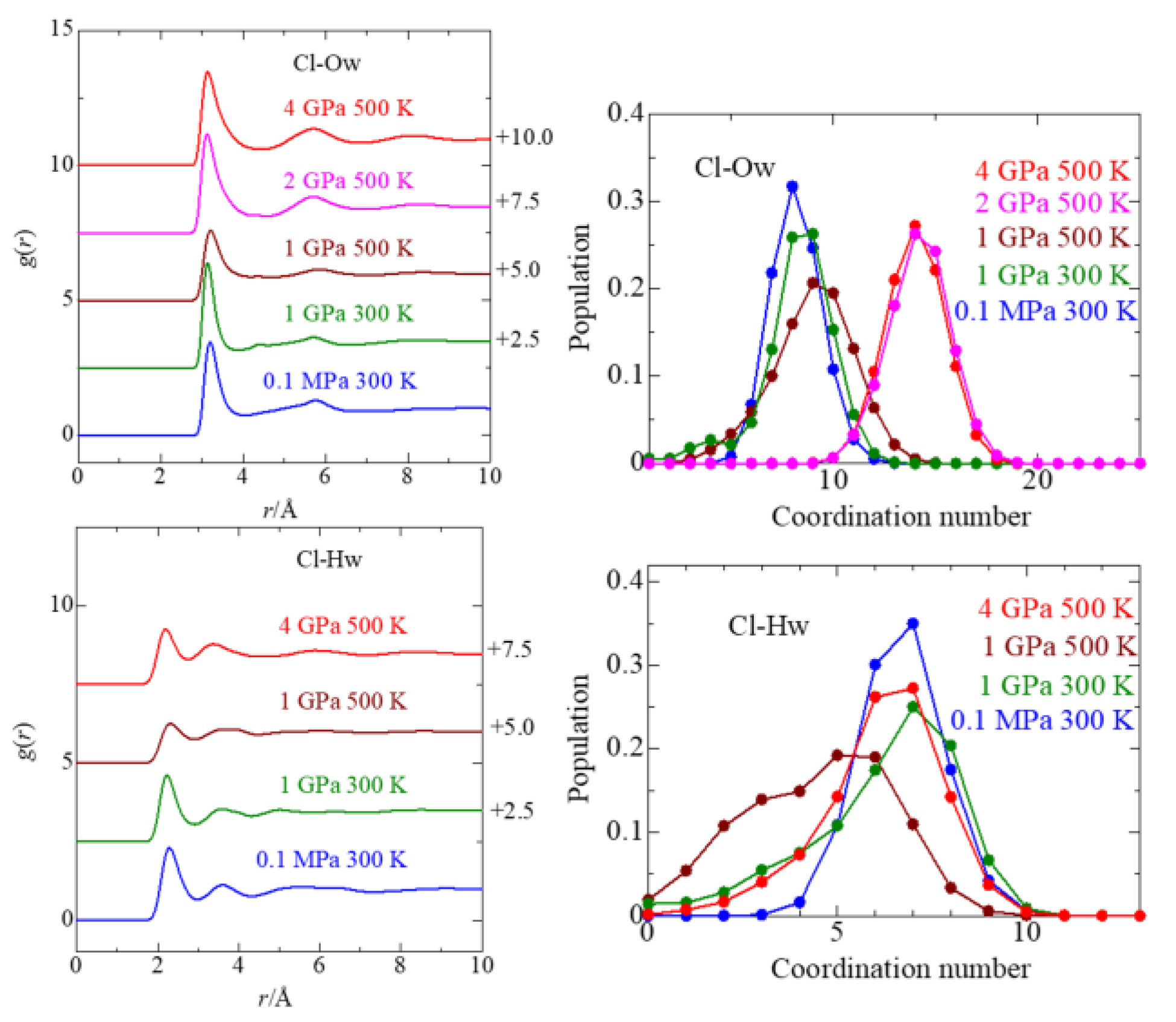
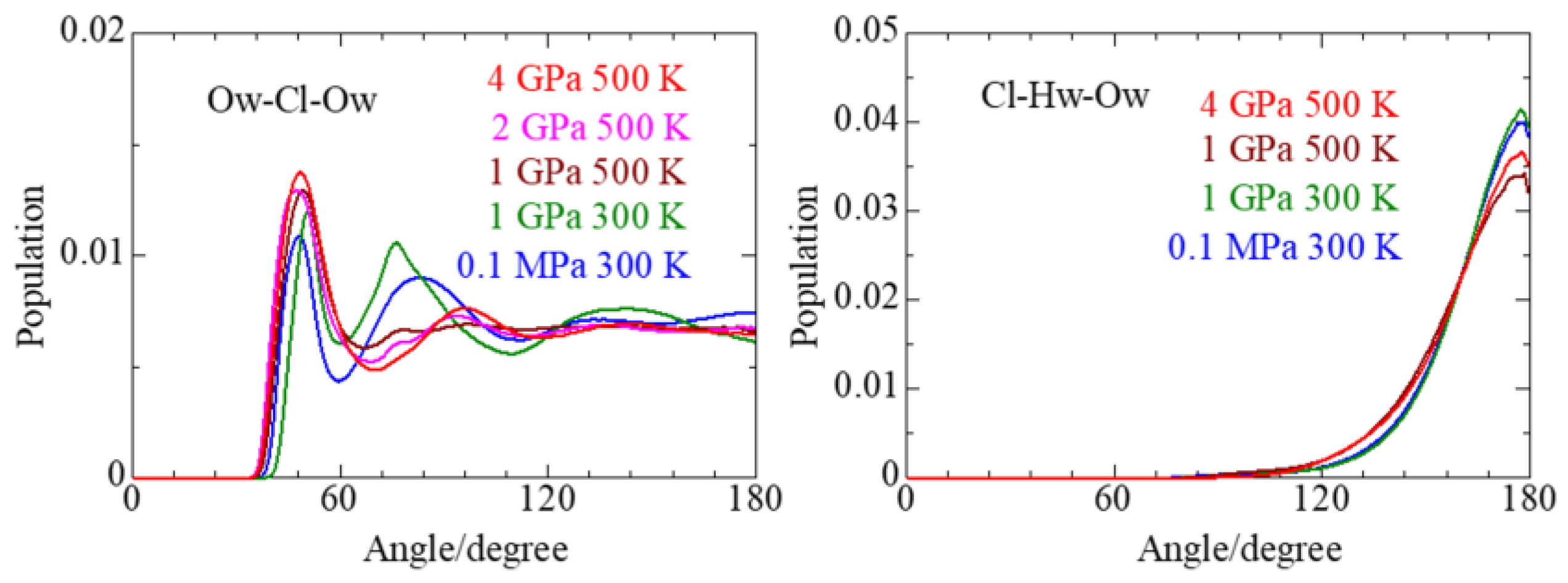
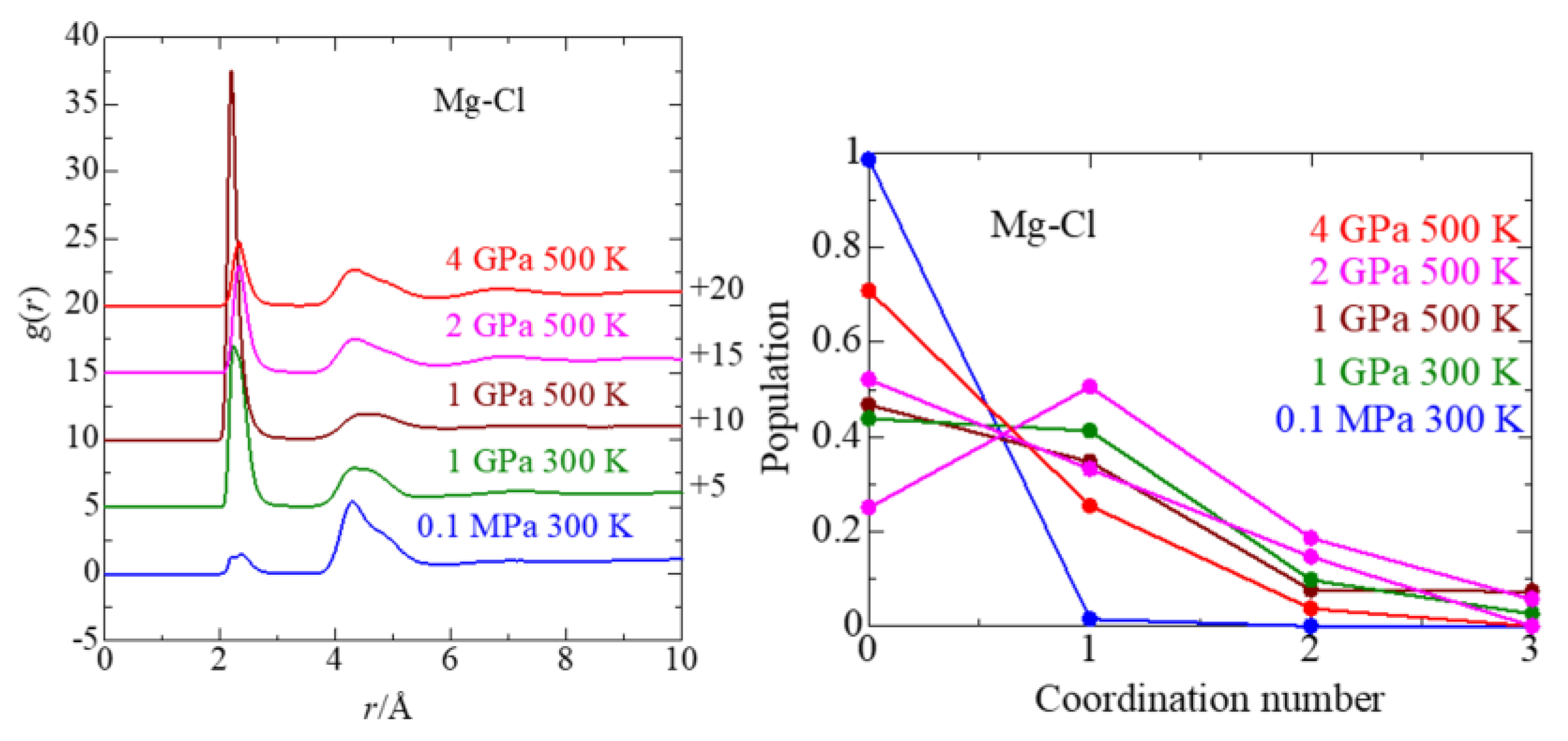
3.3. Cl− Solvation
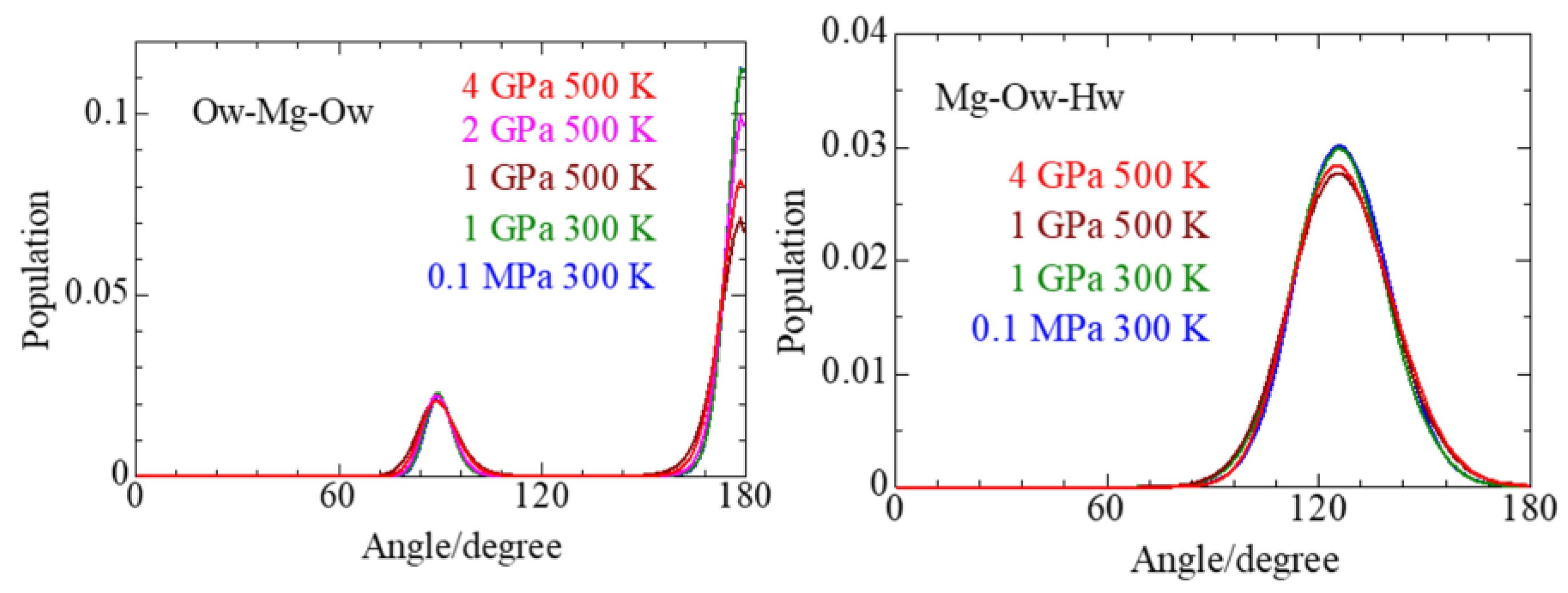
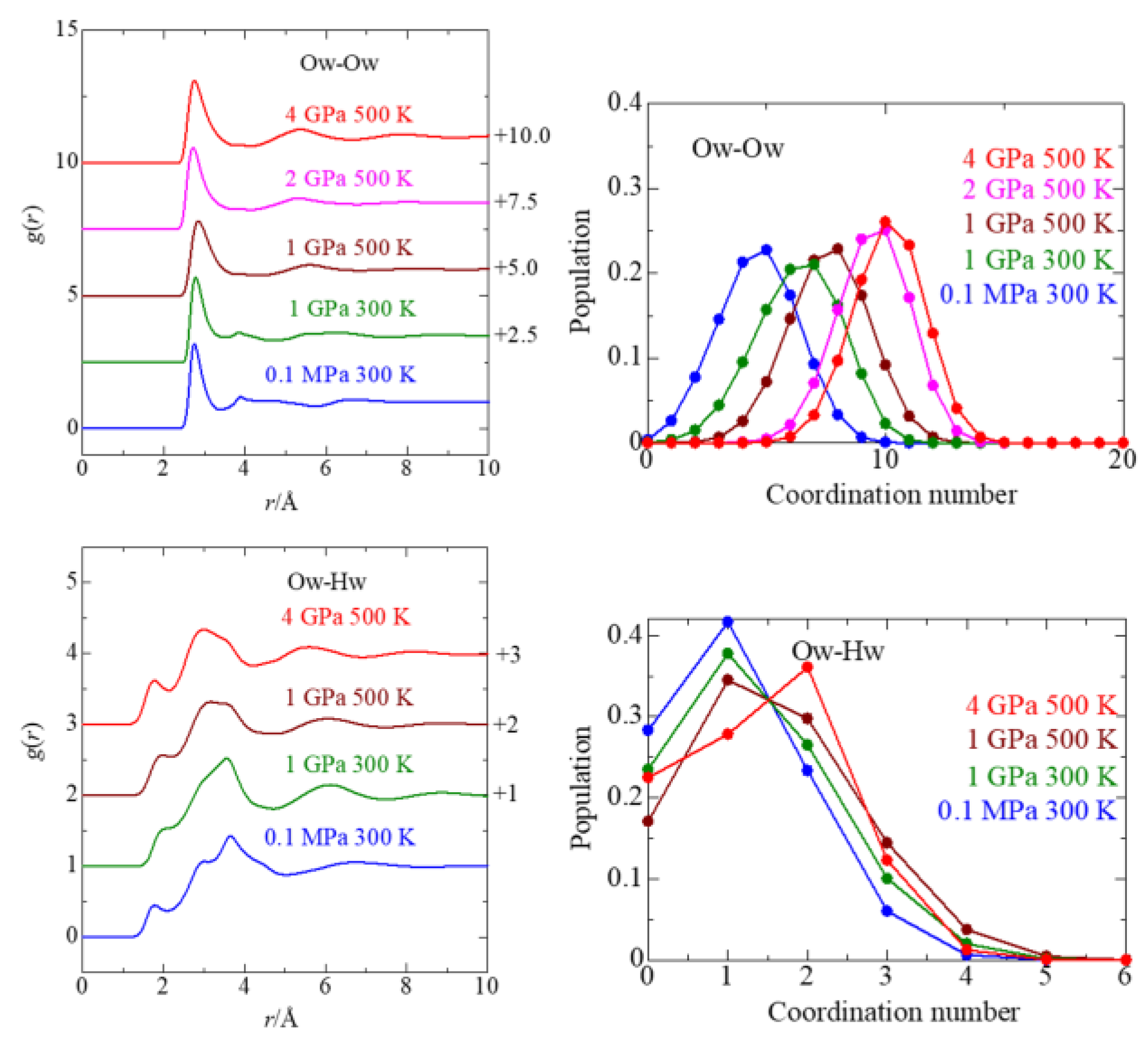
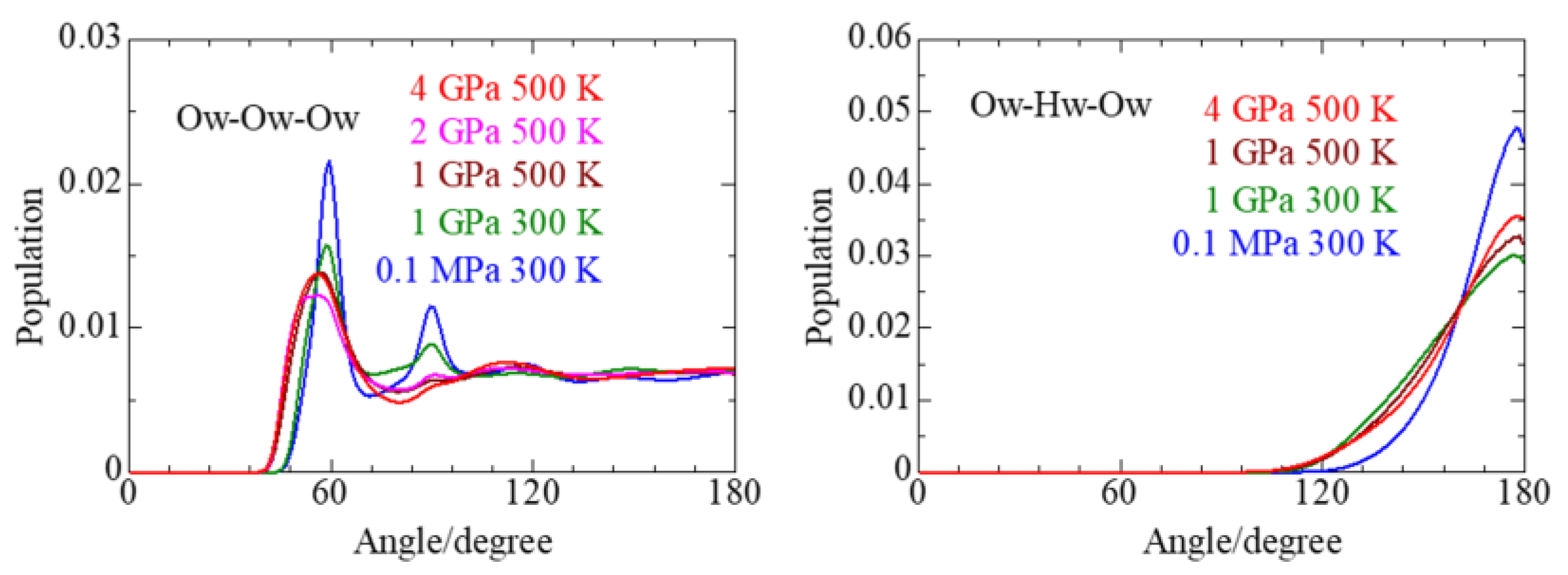
3.4. Solvent Water
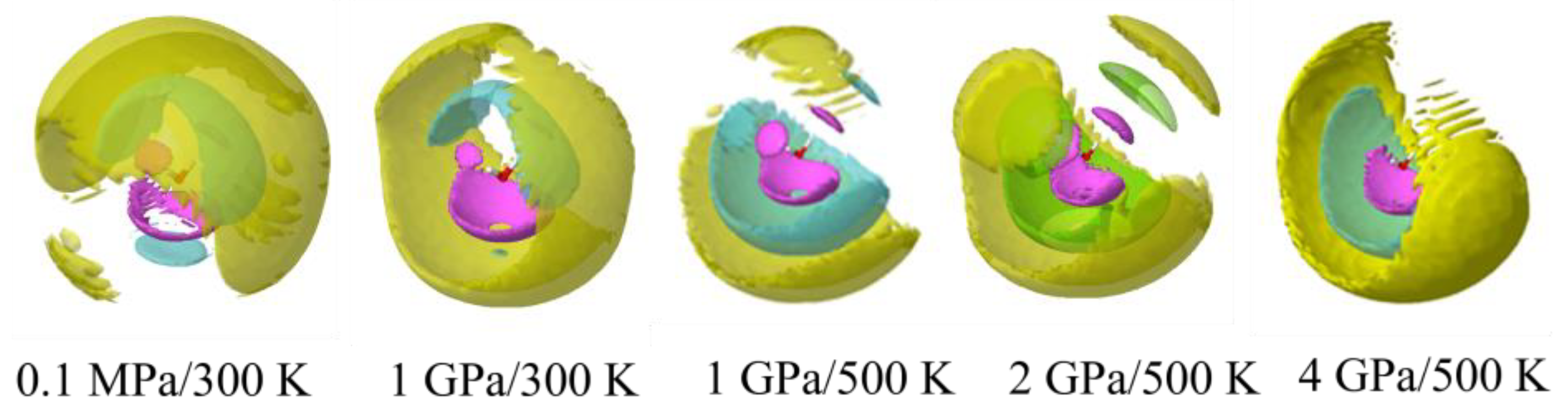
3.5. Ion Association
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saris, N.-E.L.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium, An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-J.; Zhang, Y.-H.; Wei, Z.-F.; Cheng, H.; Li, X.-H. Magnesium sulfate aerosols studied by FTIR spectroscopy: Hygroscopic properties, supersaturated structures, and implications for seawater aerosols. J. Phys. Chem. A 2006, 110, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Hirschmann, M.; Kohlstedt, D. Water in Earth’s Mantle. Phys. Today 2012, 65, 40–45. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Fujimura, K.; Uchi, K.; Yoshida, K.; Katayama, Y. Structure of water from water to 4 GPa revealed by energy-dispersive X-ray diffraction combined with empirical potential structure refinement modeling. J. Mol. Liq. 2012, 176, 44–51. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Fukuyama, N.; Yoshida, K.; Katayama, Y. Ion solvation and water structure in an aqueous sodium chloride solution in the gigapascal pressure range. J. Phys. Chem. Lett. 2021, 12, 250–256. [Google Scholar] [CrossRef]
- Polidori, A.; Rowlands, R.F.; Zeidler, A.; Salanne, M.; Fischer, H.E.; Annighöfer, B.; Klotz, S.; Salmon, P.S. Structure and dynamics of aqueous NaCl solutions at high temperatures and pressures. J. Chem. Phys. 2021, 155, 194506. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yoshida, K.; Machida, S.; Hattori, T. Neutron scattering on an aqueous sodium chloride solution in the gigapascal pressure range. J. Mol. Liq. 2022, 365, 120181. [Google Scholar] [CrossRef]
- Zhang, W.-Q.; Yamaguchi, T.; Fang, C.-H.; Yoshida, K.; Zhou, Y.-Q.; Zhu, F.-Y.; Machida, S.; Hattori, T.; Li, W. Structure of an aqueous RbCl solution in the gigapascal pressure range by neutron diffraction combined with empirical potential structure refinement modeling. J. Mol. Liq. 2022, 348, 118080. [Google Scholar] [CrossRef]
- Takamuku, T.; Yagafarov, O.F.; Katayama, Y.; Umeki, T. Structure of aqueous calcium chloride solutions by energy-dispersive x-ray diffraction under high temperatures and high pressures. Bunseki Kagaku 2015, 64, 203. (In Japanese) [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nishino, M.; Yoshida, K.; Takumi, M.; Nagata, K.; Hattori, T. Ion hydration and association in an aqueous calcium chloride solution in the GPa range. Eur. J. Inorg. Chem. 2019, 2019, 1170–1177. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Fukuyama, N.; Yoshida, K.; Katayama, Y. Ion solvation and association and water structure in an aqueous cerium(III) chloride solution in the gigapascal pressure range. Anal. Sci. 2022, 38, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, R.; Licheri, G.; Piccaluga, G.; Pinna, G. X-ray diffraction study of MgCl2 aqueous solution. J. Appl. Crystallogr. 1979, 12, 34–38. [Google Scholar] [CrossRef]
- Persson, I. Structures of hydrated metal ions in the solid state and aqueous solution. Liquids 2022, 2, 210–242. [Google Scholar] [CrossRef]
- Bruni, F.; Imberti, S.; Mancinelli, R.; Ricci, M.A. Aqueous solutions of divalent chlorides; ions hydration shell and water structure. J. Chem. Phys. 2012, 136, 064520. [Google Scholar] [CrossRef]
- Duboué-Dijon, E.; Mason, P.E.; Fischer, H.E.; Jungwirth, P. Hydration and ion pairing in aqueous Mg2+ and Zn2+ solutions; force-field description aided by neutron scattering experiments and ab initio molecular dynamics simulations. J. Phys. Chem. B 2018, 122, 3296–3306. [Google Scholar] [CrossRef]
- Szász, G.I.; Dietz, W.; Heinzinger, K.; Pálinkás, G.; Tadnai, T. On the orientation of water molecules in the hydration shell of the ions in an MgCl2 solution. Chem. Phys. Lett. 1982, 92, 388–392. [Google Scholar] [CrossRef]
- Jiao, D.; King, C.; Grossfield, A.; Darden, T.A.; Ren, P. Simulation of Ca2+ and Mg2+ solvation using polarizable atomic multipole potential. J. Phys. Chem. B 2006, 110, 18553–18559. [Google Scholar] [CrossRef]
- Callahan, K.M.; Casillas-Ituarte, N.N.; Roeselová, M.; Allen, H.C.; Tobias, D.J. Solvation of magnesium dication; molecular dynamics simulation and vibrational spectroscopic study of magnesium chloride in aqueous solution. J. Phys. Chem. A 2010, 114, 5141–5148. [Google Scholar] [CrossRef]
- Mamatkulov, S.; Fyta, M.; Netz, R.R. Force fields for divalent cations based on single-ion and ion-pair properties. J. Chem. Phys. 2013, 138, 024505. [Google Scholar] [CrossRef]
- Mamatkulov, S.; Scwierz, N. Force fields for monovalent and divalent metal cations in TIP3P water based on thermodynamic and kinetic properties. J. Chem. Phys. 2018, 148, 074504. [Google Scholar] [CrossRef]
- Zeron, I.M.; Abascal, J.L.F.; Vega, C. A force field of Li+, Na+, K+, Mg2+, Ca2+, Cl-, and SO42- in aqueous solution based on the TIP4P/2005 water model and scaled charges for the ions. J. Chem. Phys. 2019, 151, 134504. [Google Scholar] [CrossRef]
- Ikeda, T.; Boero, M.; Terakura, K. Hydration properties of magnesium and calcium ions from constrained first-principles molecular dynamics. J. Chem. Phys. 2007, 127, 074503. [Google Scholar] [CrossRef] [PubMed]
- Tommaso, D.D.; de Leeuw, N.H. Structure and dynamics of the hydrated magnesium ion and of the solvated magnesium carbonates; insights from first principles simulations. Phys. Chem. Chem. Phys. 2010, 12, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.A.A.; Sethio, D.; Kraka, E. Assessing the intrinsic strengths of ion-solvent and solvent0-solvent interactions for hydrated Mg2+ clusters. Inorganics 2021, 9, 31. [Google Scholar] [CrossRef]
- Wagner, W.; Pruβ, A. The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use. J. Phys. Chem. Ref. Data 2002, 31, 387. [Google Scholar] [CrossRef]
- Eisenberg, D.; Kauzmann, W. The Structure and Properties of Water; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Tsuji, K.; Yaoita, K.; Imai, M.; Shimomura, O.; Kikegawa, T. Measurements of X-ray Diffraction for Liquid Metals under High Pressure. Rev. Sci. Instrum. 1989, 60, 2425. [Google Scholar] [CrossRef]
- Decker, D.L. High-Pressure Equation of State for NaCl, KCl, and CsCl. J. Appl. Phys. 1971, 42, 3239. [Google Scholar] [CrossRef]
- Datchi, F.; Loubeyre, P.; LeToullec, R. Extended and Accurate Determination of the Melting Curves of Argon, Helium, Ice (H2O), and Hydrogen (H2). Phys. Rev. B 2000, 61, 6535. [Google Scholar] [CrossRef]
- Utsumi, W.; Funakoshi, K.; Katayama, Y.; Yamanaka, M.; Okada, T.; Shimomura, O. High-Pressure Science with a Multi-Anvil Apparatus at SPring-8. J. Phys. Condens. Matter 2002, 14, 10497–10504. [Google Scholar] [CrossRef]
- Kaplow, R.; Strong, S.L.; Averbach, B.L. Radial Density Functions for Liquid Mercury and Lead. Phys. Rev. 1965, 138, A1336. [Google Scholar] [CrossRef]
- Sano-Furukawa, A.; Hattori, T.; Arima, H.; Yamada, A.; Tabata, S.; Kondo, M.; Nakamura, A.; Kagi, H.; Yagi, T. Six-axis multi-anvil press for high-pressure, high-temperature neutron diffraction experiments. Rev. Sci. Instrum. 2014, 85, 113905. [Google Scholar] [CrossRef]
- Hattori, T.; Sano-Furukawa, A.; Arima, H.; Komatsu, K.; Yamada, A.; Inamura, Y.; Nakatani, T.; Seto, Y.; Nagai, T.; Tsumi, W.; et al. Design and performance of high-pressure PLANET beamline at pulsed neutron source at J-PARC. Nucl. Instrum. Methods Phys. Res. 2015, A780, 55–67. [Google Scholar] [CrossRef]
- Paalman, H.H.; Pings, C.J. Numerical evaluation of X-ray absorption factors for cylindrical samples and annular sample cells. J. Appl. Phys. 1962, 33, 2635. [Google Scholar] [CrossRef]
- Blech, I.A.; Averbach, B.L. Multiple scattering of neutrons in vanadium and copper. Phys. Rev. 1965, 137, A1113. [Google Scholar] [CrossRef]
- NOVA Group, Institute of Materials Structure Science, Tsukuba, Ibaraki 305-080, Japan. Program nvaSq Manual. Available online: https://research.kek.jp/group/hydrogen/reduction/index.html (accessed on 26 June 2023). (In Japanese).
- Sears, V.F. Neutron scattering lengths and cross sections. Neutron News 1992, 3, 26. [Google Scholar] [CrossRef]
- Granada, J.R.; Gillette, V.H.; Mayer, R.E. Neutron cross sections and thermalization parameters using a synthetic scattering function. II: Applications to H2O, D2O, and C6H6. Phys. Rev. A 1987, 36, 5594. [Google Scholar] [CrossRef] [PubMed]
- Kameda, Y.; Sasaki, M.; Usuki, T.; Othomo, T.; Itoh, K.; Suzuya, K.; Fukunaga, T. Inelasticity effects on neutron scattering intensities of the null-H2O. J. Neutron Res. 2003, 11, 153–163. [Google Scholar] [CrossRef]
- OriginPro. The Ultimate Software for Graphing and Analysis; OriginLab Corp.: Northampton, MA, USA, 2022. [Google Scholar]
- Druchok, M.; Holovko, M. Molecular dynamics study of ion hydration under pressure. J. Mol. Liq. 2011, 159, 24–30. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The Missing Term in Effective Pair Potentials. J. Phys. Chem. 1987, 91, 6269. [Google Scholar] [CrossRef]
- Soper, A.K. Computer simulation as a tool for the interpretation of total scattering data from glasses and liquids. Mol. Simul. 2012, 38, 1171–1185. [Google Scholar] [CrossRef]
- Soper, A.K. Empirical potential Monte Carlo simulation of fluid structure. Chem. Phys. 1996, 202, 295–306. [Google Scholar] [CrossRef]
- Soper, A.K. Partial structure factors from disordered materials diffraction data: An approach using empirical potential structure refinement. Phys. Rev. B 2005, 72, 104204. [Google Scholar] [CrossRef]
- Amann-Winkel, K.; Bellissent-Funel, M.-C.; Bove, L.E.; Loerting, T.; Nilsson, A.; Paciaroni, A.; Schlesinger, D.; Skinner, L. X-ray and Neutron Scattering of Water. Chem. Rev. 2016, 116, 7570–7589. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halide and chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
| Abbreviation | c/mol dm–3 | dX/g cm–3 | dN/g cm–3 | ρS/atoms Å–3 | PX/GPa | PN/GPa | TX/K | TN/K | Method |
|---|---|---|---|---|---|---|---|---|---|
| 0.1 MPa 300 K | 2.024 | 1.113 | 1.204 | 0.09577 | 1.0 × 10−4 | 1.0 × 10−4 | 300 | 298 | X, N |
| 1 GPa 300 K | 2.397 | 1.377 | 1.426 | 0.1134 | 1.0 | 0.65 | 300 | 298 | X, N |
| 1 GPa 500 K | 2.125 | 1.269 | 1.264 | 0.1005 | 1.0 | 0.63 | 500 | 523 | X, N |
| 2 GPa 500 K | 2.020 | 1.396 | ⋯ | 0.1386 | 2.0 | ⋯ | 500 | ⋯ | X |
| 4 GPa 500 K | 2.439 | 1.459 | 1.451 | 0.1388 | 3.8 | 4.5 | 500 | 523 | X, N |
| Atoms | ε/kJ mol–1 | Atomic Mass | Coulomb Charge/e | Refs. | |
|---|---|---|---|---|---|
| Mg2+ | 0.0040 | 2.63 | 24.31 | 2 | [20] |
| Cl− | 0.4187 | 4.40 | 35 | −1 | [41] |
| Ow | 0.65 | 3.16 | 16.00 | −0.8476 | [42] |
| Hw | 0.00 | 0.00 | 2.00 | 0.4238 | [42] |
| P, T | Parameter | Mg–Ow(I) | Mg–Hw(I) | Mg–Ow(II) | Cl–Ow(I) | Cl–Hw(I) | Cl–Ow(II) | Mg–Cl(I) | Mg–Cl(II) |
|---|---|---|---|---|---|---|---|---|---|
| 0.1 MPa/ 300 K | r/Å | 1.92 | 2.58 | 3.78 | 3.18 | 2.32 | 5.76 | 2.37 | 4.32 |
| C.N. | 6.0 ± 0.2 | 12.0 ± 0.4 | 8.2 ± 1.2 | 6.7 ± 1.1 | 0.01 ± 0.12 | ||||
| rmax/Å | 2.40 | 3.15 | 4.05 | 3.00 | 3.21 | ||||
| 1 GPa/ 300 K | r/Å | 1.92 | 2.58 | 4.53 | 3.14 | 2.24 | 4.41, 5.75 | 2.33 | 4.57 |
| C.N. | 5.0 ± 1.3 | 10.2 ± 2.6 | 8.2 ± 1.8 | 6.2 ± 2.0 | 0.78 ± 0.90 | ||||
| rmax/Å | 2.37 | 3.09 | 3.87 | 2.91 | 3.15 | ||||
| 1 GPa/ 500 K | r/Å | 1.92 | 2.61 | 4.14 | 3.21 | 2.31 | 5.86 | 2.19 | 4.50 |
| C.N. | 4.2 ± 2.0 | 8.6 ± 4.0 | 8.4 ± 2.2 | 4.0 ± 2.0 | 1.12 ± 1.27 | ||||
| rmax/Å | 2.58 | 3.18 | 4.17 | 2.88 | 2.85 | ||||
| 2 GPa/ | r/Å | 1.92 | ⋯ | 4.03 | 3.12 | ⋯ | 5.70 | 2.35 | 4.34 |
| 500 K | C.N. | 5.3 ± 0.9 | ⋯ | 14.2 ± 1.5 | ⋯ | 0.65 ± 0.75 | |||
| rmax/Å | 2.32 | ⋯ | 4.19 | ⋯ | 3.00 | ||||
| 4 GPa/500 K | r/Å | 1.93 | 2.61 | 3.99 | 3.14 | 2.18 | 5.73 | 2.36 | 4.34 |
| C.N. | 5.6 ± 0.7 | 11.4 ± 1.2 | 14.0 ± 1.4 | 6.2 ± 1.6 | 0.33 ± 0.54 | ||||
| rmax/Å | 2.31 | 3.07 | 4.15 | 2.72 | 3.30 |
| P, T | Parameters | Ow–Ow(I) | Ow–Hw(I) | Ow–Ow(II) | Ow–Ow(III) |
|---|---|---|---|---|---|
| 0.1 MPa/ 300 K | r/Å | 2.76 | 1.80 | 3.91–4.80 | 6.63 |
| C.N. | 4.6 ± 1.7 | 1.1 ± 0.90 | |||
| rmax/Å | 3.39 | 2.04 | |||
| 1 GPa/ 300 K | r/Å | 2.79 | 2.07 | 3.84 | 6.09 |
| C.N. | 6.3 ± 1.8 | 1.3 ± 1.0 | |||
| rmax/Å | 1.8 | 2.13 | |||
| 1 GPa/ 500 K | r/Å | 2.85 | 1.98 | 5.61 | 8.22 |
| C.N. | 7.6 ± 1.7 | 1.5 ± 1.1 | |||
| rmax/Å | 3.78 | 2.22 | |||
| 2 GPa/ | r/Å | 2.71 | 5.31 | 7.99 | |
| 500 K | C.N. | 9.5 ± 1.5 | |||
| rmax/Å | 3.63 | ||||
| 4 GPa/ 500 K | r/Å | 2.74 | 1.74 | 5.38 | 7.83 |
| C.N. | 8.4 ± 1.6 | 0.88 ± 0.84 | |||
| rmax/Å | 3.69 | 1.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, T.; Fukuyama, N.; Yoshida, K.; Katayama, Y.; Machida, S.; Hattori, T. An X-ray and Neutron Scattering Study of Aqueous MgCl2 Solution in the Gigapascal Pressure Range. Liquids 2023, 3, 288-302. https://doi.org/10.3390/liquids3030019
Yamaguchi T, Fukuyama N, Yoshida K, Katayama Y, Machida S, Hattori T. An X-ray and Neutron Scattering Study of Aqueous MgCl2 Solution in the Gigapascal Pressure Range. Liquids. 2023; 3(3):288-302. https://doi.org/10.3390/liquids3030019
Chicago/Turabian StyleYamaguchi, Toshio, Nami Fukuyama, Koji Yoshida, Yoshinori Katayama, Shinichi Machida, and Takanori Hattori. 2023. "An X-ray and Neutron Scattering Study of Aqueous MgCl2 Solution in the Gigapascal Pressure Range" Liquids 3, no. 3: 288-302. https://doi.org/10.3390/liquids3030019
APA StyleYamaguchi, T., Fukuyama, N., Yoshida, K., Katayama, Y., Machida, S., & Hattori, T. (2023). An X-ray and Neutron Scattering Study of Aqueous MgCl2 Solution in the Gigapascal Pressure Range. Liquids, 3(3), 288-302. https://doi.org/10.3390/liquids3030019







