Liquid-State Volumetric Properties of a Set of Alcohols with Up to Five Carbon Atoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
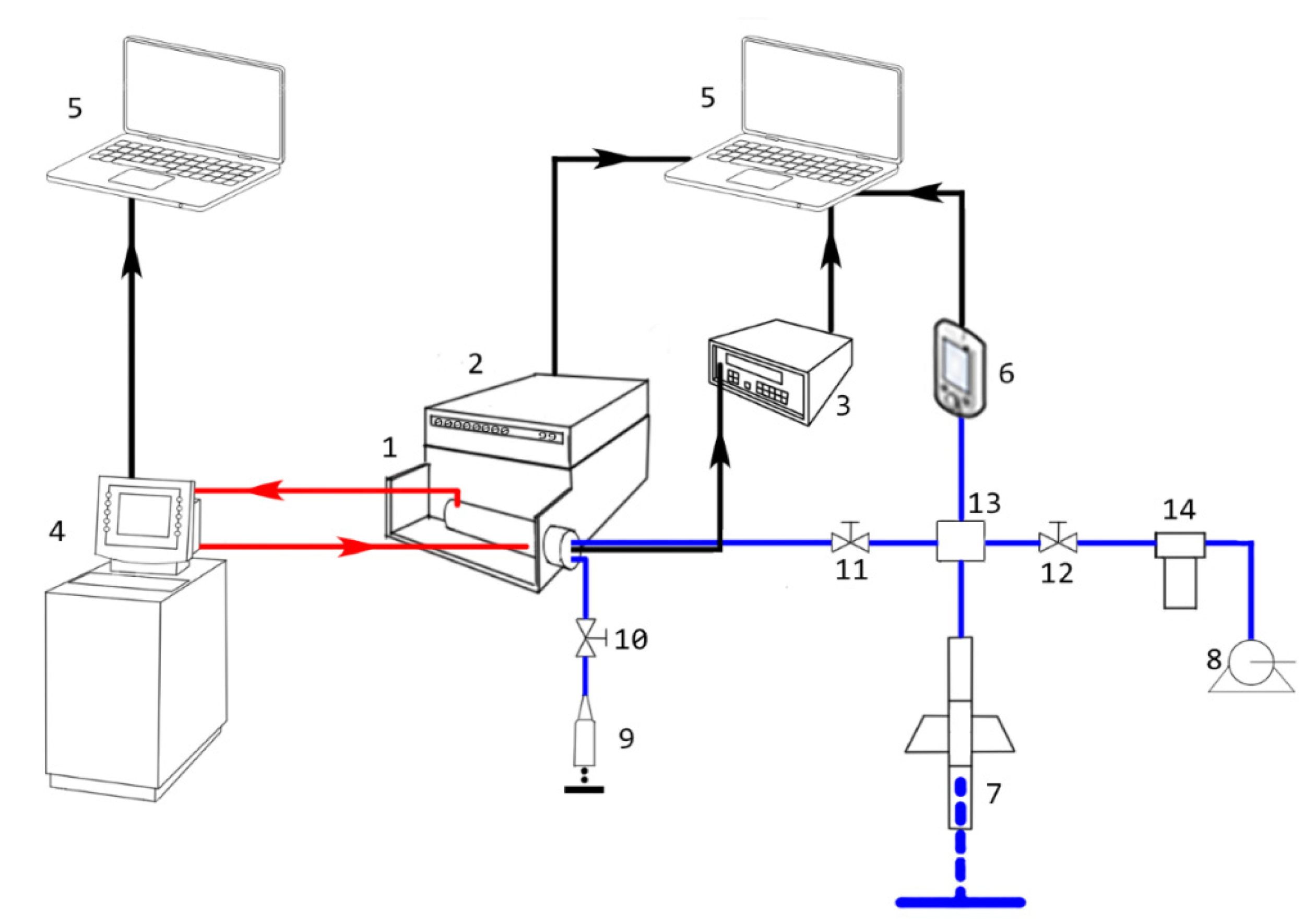
2.2. Apparatus and Experimental Technique
2.2.1. Characterization of Pure Compounds
2.2.2. Measurements of p-rho-T
2.3. Modeling
- i.
- define the first relationship of Equation (7) in the isobaric corresponding to the reference pressure (0.1 MPa);
- ii.
- establish the parameters of the coefficients A(T) and B(T).
3. Results and Discussion
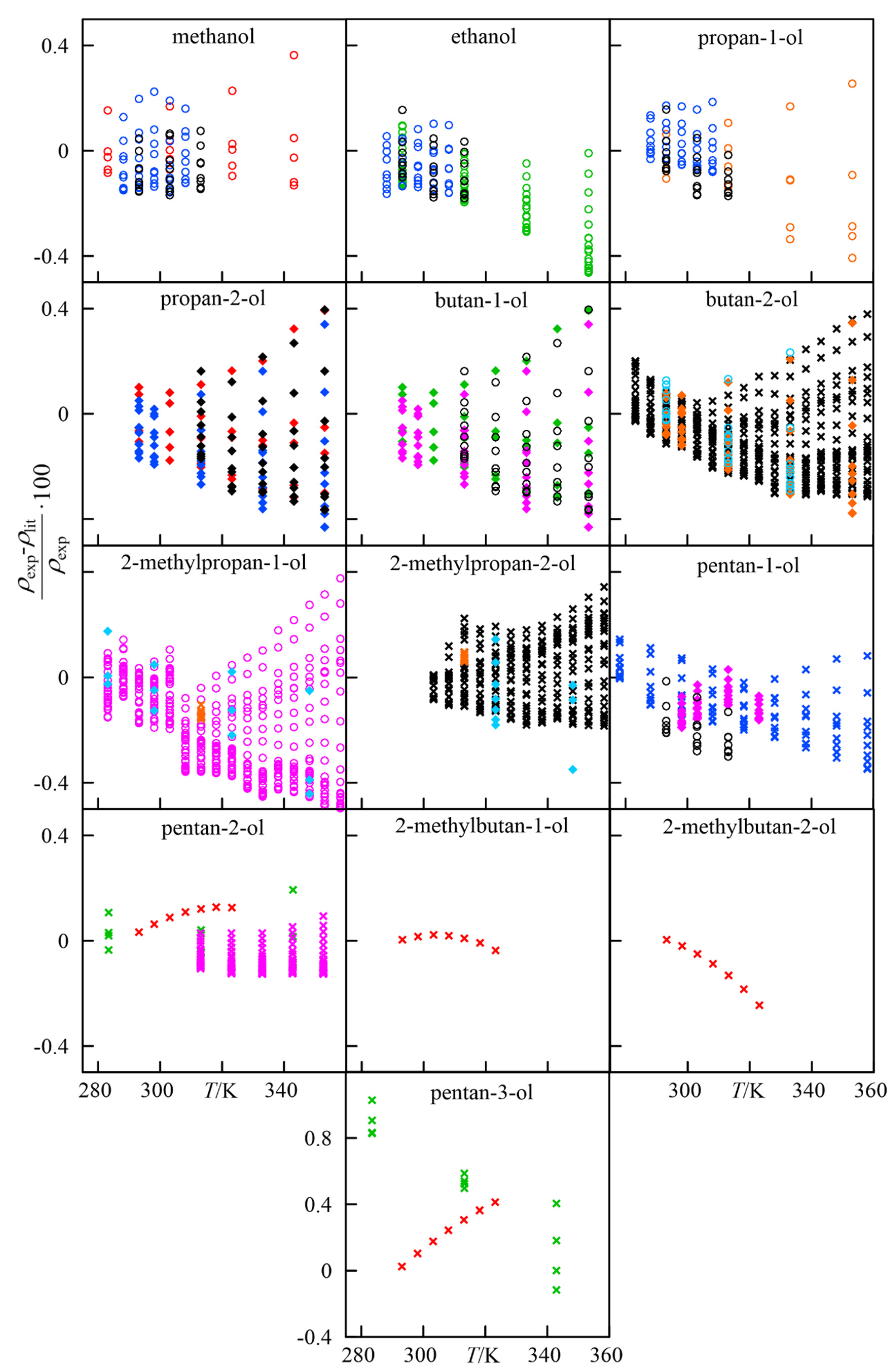
3.1. Comparison to Literature Data
- methanol and ethanol: up to T ≈ 320 K, the literature values and those provided in this work deviate up to ±0.2% for the whole pressure range considered here. At higher temperatures, the deviations increase up to 0.4%. These values are acceptable, confirming the suitability of the experimental method used in our work.
- propan-1-ol, propan-2-ol, butan-1-ol, butan-2-ol, 2-methylpropan-1-ol, pentan-1-ol: a strong increase in the relative deviations between the experiments and literature is observed for these compounds as temperature increases. Nevertheless, the differences are below 0.4%.
- 2-methylpropan-2-ol: experimental data provided for this compound is limited by its solid–liquid saturation curve. It is also observed that the deviations are insensitive to an increase in the temperature, and the deviations are within the interval established by other alcohols.
- pentan-2-ol, pentan-3-ol, 2-methylbutan-1-ol, 2-methylbutan-2-ol, 3-methylbutan-2-ol: literature data for these systems are scarce, therefore no objective comparison of our measurements can be made. At T = 280 K, pentan-3-ol shows the highest deviation observed in this work, ≈1%. No p-v-T data have been found for 3-methylbutan-2-ol, so the information presented here is novel.
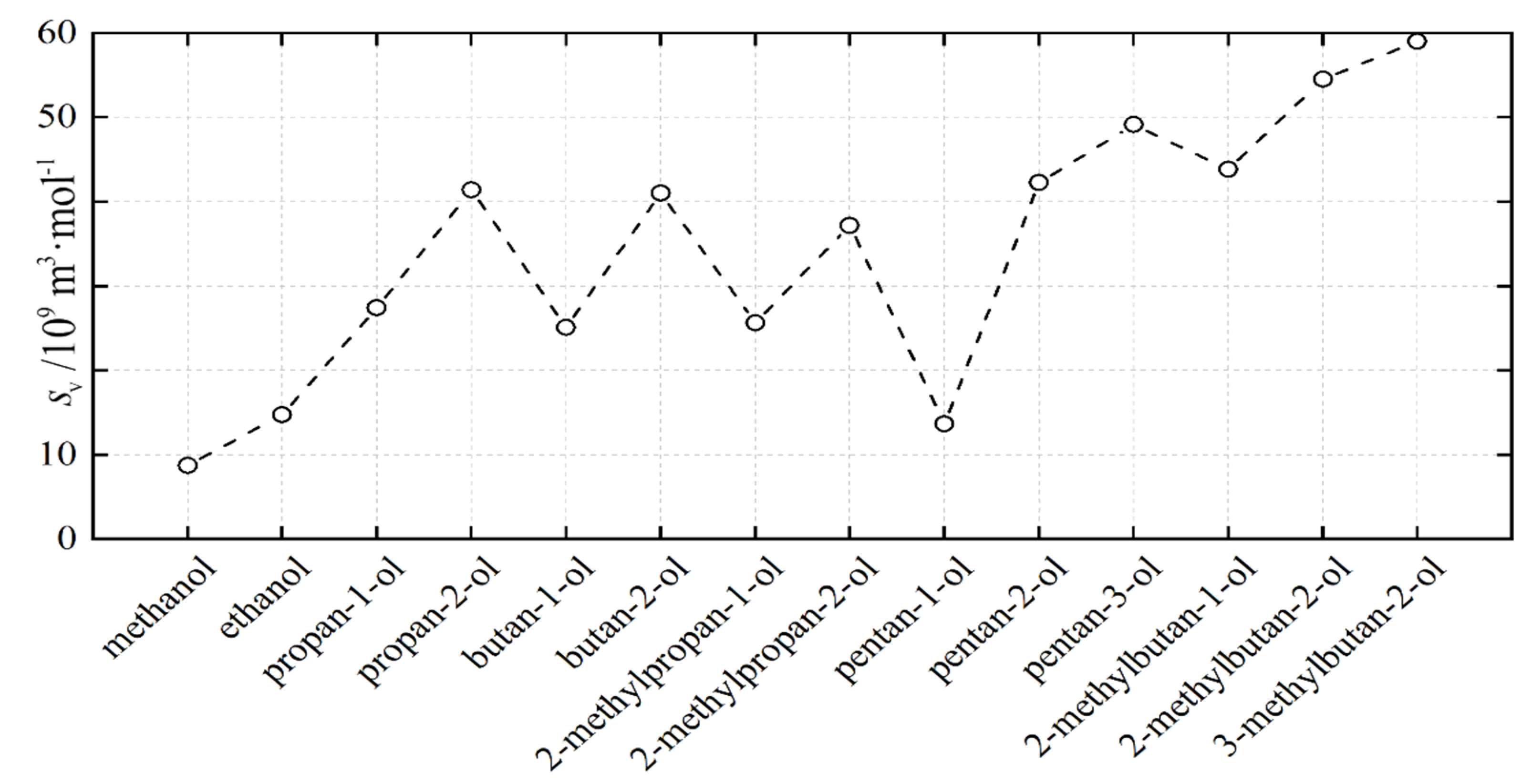
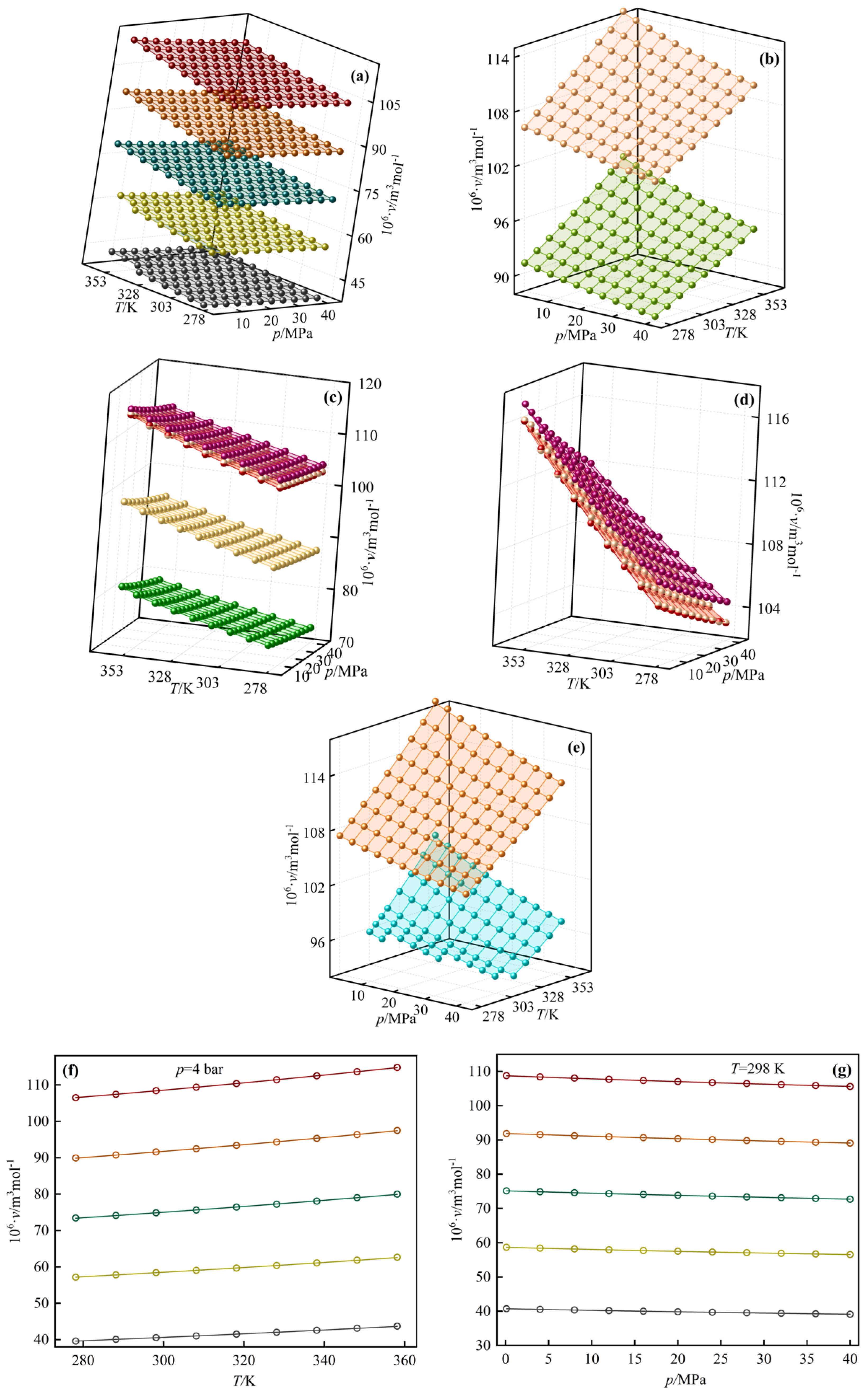
3.2. Modeling Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, B.R.; Saravanan, S. Use of higher alcohol biofuels in diesel engines: A review. Renew. Sustain. Energy Rev. 2016, 60, 84–115. [Google Scholar] [CrossRef]
- Babu, V.B.M.V.; Murthy, M.M.K.M.; Rao, G.A.P. Butanol and pentanol: The promising biofuels for CI engines—A review. Renew. Sustain. Energy Rev. 2017, 78, 1068–1088. [Google Scholar] [CrossRef]
- High Pressure Common Rail—An overview|ScienceDirect Topics, (n.d.). Available online: https://www.sciencedirect.com/topics/engineering/high-pressure-common-rail (accessed on 17 June 2021).
- Scholz, C.W.; Frotscher, O.; Pohl, S.; Span, R.; Richter, M. Measurement and correlation of the (p, ρ, t) behavior of liquid methanol at temperatures from 283.15 to 423.15 k and pressures up to 90 mpa. Ind. Eng. Chem. Res. 2021, 60, 3745–3757. [Google Scholar] [CrossRef]
- Muche, D.N.F.; Olivieri, G.V.; Torres, R.B. Density and Derived Properties of Binary Mixtures Containing{2-(Dimethylamino)ethyl Methacrylate + Alcohols} at Temperatures from T = (293.15 to 313.15) K and Pressures of up to 35 MPa. J. Chem. Eng. Data 2019, 64, 1909–1921. [Google Scholar] [CrossRef]
- Alaoui, F.E.M.; Aguilar, F.; González-Fernández, M.J.; el Amarti, A.; Montero, E.A. Oxygenated Compounds + Hydrocarbon Mixtures in Fuels and Biofuels: Excess Enthalpies of Ternary Mixtures Containing 1-Butoxybutane + Propan-1-ol +1-Hex-1-ene, or Heptane, or 2,2,4-Trimethylpentane at (298.15 and 313.15) K. J. Chem. Eng. Data 2014, 59, 2856–2864. [Google Scholar] [CrossRef]
- Muñoz-Rujas, N.; Aguilar, F.; García-Alonso, J.M.; Montero, E.A. Thermodynamics of binary mixtures 1-ethoxy-1,1,2,2,3,3,4,4,4-nonafluorobutane (HFE-7200) + 2-propanol: High pressure density, speed of sound and derivative properties. J. Chem. Thermodyn. 2019, 131, 630–647. [Google Scholar] [CrossRef]
- Gómez-Álvarez, P.; González-Salgado, D.; Bazile, J.P.; Bessieres, D.; Plantier, F. Excess second-order thermodynamic derivatives of the{2-propanol+water} system from 313.15K to 403.15K up to 140MPa. Experimental and Monte Carlo simulation study. Fluid Phase Equilib. 2013, 358, 7–26. [Google Scholar] [CrossRef]
- Alaoui, F.E.M.; Montero, E.A.; Bazile, J.P.; Aguilar, F.; Boned, C. Liquid density of oxygenated additive 2-propanol at pressures up to 140 MPa and from 293.15 K to 403.15 K. J. Chem. Thermodyn. 2012, 54, 358–365. [Google Scholar] [CrossRef]
- Pimentel-Rodas, A.; Galicia-Luna, L.A.; Castro-Arellano, J.J. Viscosity and Density of n-Alcohols at Temperatures between (298.15 and 323.15) K and Pressures up to 30 MPa. J. Chem. Eng. Data 2019, 64, 324–336. [Google Scholar] [CrossRef]
- Fang, D.; Meng, X.; Wu, J. Compressed Liquid Densities of Binary Mixtures of 1-Butanol and Diethylene Glycol Dimethyl Ether from (283 to 363) K at Pressures up to 100 MPa. J. Chem. Eng. Data 2017, 62, 2937–2943. [Google Scholar] [CrossRef]
- Torín-Ollarves, G.; Martín, C.; Segovia, J. Thermophysical properties of 1,2,4-trimethylbenzene in admixtures with 1-butanol or 2-butanol at high pressures. J. Chem. Thermodyn. 2017, 111, 41–51. [Google Scholar] [CrossRef]
- Bravo-Sánchez, M.G.; Guerrero-Zárate, D.; Iglesias-Silva, G.A.; Estrada-Baltazar, A.; Bouchot, C. P-ρ-T Data for 2-Butanol and tert-Butanol from 283.15 to 363.15 K and 303.15 to 363.15 K at Pressures up to 66 MPa. J. Chem. Eng. Data 2016, 61, 1555–1565. [Google Scholar] [CrossRef]
- Dakkach, M.; Aguilar, F.; Alaoui, F.E.M.; Montero, E.A. Liquid density of oxygenated additives to biofuels: 2-Butanol at pressures up to 140 MPa and temperatures from (293.15 to 393.27) K. J. Chem. Thermodyn. 2015, 89, 278–285. [Google Scholar] [CrossRef]
- Iglesias-Silva, G.A.; Bravo-Sánchez, M.; Estrada-Baltazar, A.; Bouchot, C.; Hall, K.R. P-ρ-T Data for 1-Butanol and Isobutyl Alcohol from (283.15 to 363.15) K at Pressures up to 66 MPa. J. Chem. Eng. Data 2015, 60, 1076–1090. [Google Scholar] [CrossRef]
- Kodama, D.; Kato, M.; Kaneko, T. Volumetric behavior of carbon dioxide+2-methyl-1-propanol and carbon dioxide+2-methyl-2-propanol mixtures at 313.15K. Fluid Phase Equilib. 2013, 357, 57–63. [Google Scholar] [CrossRef]
- Kubota, H.; Tanaka, Y.; Makita, T. Volumetric behavior of pure alcohols and their water mixtures under high pressure. Int. J. Thermophys. 1987, 8, 47–70. [Google Scholar] [CrossRef]
- Dávila, M.J.; Alcalde, R.; Atilhan, M.; Aparicio, S. PρT measurements and derived properties of liquid 1-alkanols. J. Chem. Thermodyn. 2012, 47, 241–259. [Google Scholar] [CrossRef]
- Sommer, D.; Kleinrahm, R.; Span, R.; Wagner, W. Measurement and correlation of the (p, ρ, T) relation of liquid cyclohexane, toluene, and ethanol in the temperature range from 233.15 K to 473.15 K at pressures up to 30 MPa for use as density reference liquids. J. Chem. Thermodyn. 2011, 43, 117–132. [Google Scholar] [CrossRef]
- Olivieri, G.V.; Torres, R.B. Thermodynamic and spectroscopic study of binary mixtures containing {dimethyl carbonate (DMC) + alcohols} at T = (288.15–308.15) K and p = (0.1–40) MPa: Experimental study and modelling. J. Chem. Thermodyn. 2019, 133, 229–260. [Google Scholar] [CrossRef]
- Wappmann, S.; Karger, N.; Lüdemann, H.D. pVT Data of Liquid 1-, 2-, and 3-Pentanol from 10 to 200 MPa and from 233 to 433 K. J. Chem. Eng. Data 1995, 40, 233–236. [Google Scholar] [CrossRef]
- Zúñiga-Moreno, A.; Galicia-Luna, L.A. Compressed Liquid Densities of 1-Pentanol and 2-Pentanol from 313 to 363 K at Pressures to 25 MPa. Int. J. Thermophys. 2007, 28, 146–162. [Google Scholar] [CrossRef]
- Bennett, R.G.; Hall, G.H.; Calderwood, J.H. The pressure dependence of the static permittivity of pentanol isomers. J. Phys. D Appl. Phys. 1973, 6, 781–789. [Google Scholar] [CrossRef]
- Tait, P.G. Report on Some of the Physical Properties of Fresh Water and of Sea Water; Thomson, C.W., Murray, J., Eds.; Johnson Reprint Corporation: New York, NY, USA, 1888; Volume 2. [Google Scholar]
- Aminabhavi, T.M.; Aralaguppi, M.I.; Harogoppad, S.B.; Balundgi, R.H. Densities, Viscosities, Refractive Indices, and Speeds of Sound for Methyl Acetoacetate + Aliphatic Alcohols (C1–C8). J. Chem. Eng. Data 1993, 38, 31–39. [Google Scholar] [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K.; Weissberger, A. Organic Solvents: Physical Properties and Methods of Purification, 4th ed.; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Canosa, J.; Rodríguez, A.; Orge, B.; Iglesias, M.; Tojo, J. Densities, Refractive Indices, and Derived Excess Properties of the System Methyl Acetate + Methanol + 2-Butanol at 298.15 K. J. Chem. Eng. Data 1997, 42, 1121–1125. [Google Scholar] [CrossRef]
- Fuangfoo, S.; Viswanath, D.S. Densities and viscosities of 2-methoxy-2-methylpropane + 2-methyl-2-propanol at 303.15 and 313.15 K. J. Chem. Eng. Data 1993, 38, 404–406. [Google Scholar] [CrossRef]
- Wilhoit, R.; Zwolinski, B. Physical and Thermodynamic Properties of Aliphatic Alcohols. J. Phys. Chem. Ref. Data 1973, 2 (Suppl. 1). Available online: https://apps.dtic.mil/sti/citations/ADD095038 (accessed on 10 October 2021).
- Ortega, J.; Matos, J.S. Estimation of the isobaric expansivities from several equations of molar refraction for some pure organic compounds. Mater. Chem. Phys. 1986, 15, 415–425. [Google Scholar] [CrossRef]
- Lagourette, B.; Boned, C.; Saint-Guirons, H.; Xans, P.; Zhou, H. Densimeter calibration method versus temperature and pressure. Meas. Sci. Technol. 1992, 3, 699–703. [Google Scholar] [CrossRef]
- Kell, G.S.; Whalley, E. Reanalysis of the density of liquid water in the range 0–150 °C and 0–1 kbar. J. Chem. Phys. 1975, 62, 3496–3503. [Google Scholar] [CrossRef]
- Lagarias, J.C.; Reeds, J.A.; Wright, M.H.; Wright, P.E. Convergence Properties of the Nelder--Mead Simplex Method in Low Dimensions. SIAM J. Optim. 1998, 9, 112–147. [Google Scholar] [CrossRef] [Green Version]
- Ihmels, E.C.; Gmehling, J. Liquid Densities and Excess Volumes of Diisopropyl Ether (DIPE) + 1-Butanol Mixtures from 273 to 473 K and Pressures up to 35 MPa. J. Chem. Eng. Data 2002, 47, 1314–1319. [Google Scholar] [CrossRef]
- Taravillo, M.; Pérez, F.J.; Núñez, J.; Cáceres, M.; Baonza, V.G. Thermodynamic Properties of Compressed Liquid Methanol in the Vicinity of the Freezing Line. J. Chem. Eng. Data 2007, 52, 481–486. [Google Scholar] [CrossRef]
- Navia, P.; Troncoso, J.; Romaní, L. Isobaric thermal expansivity behaviour against temperature and pressure of associating fluids. J. Chem. Thermodyn. 2010, 42, 23–27. [Google Scholar] [CrossRef]
- Navia, P.; Troncoso, J.; Romaní, L. New calibration methodology for calorimetric determination of isobaric thermal expansivity of liquids as a function of temperature and pressure. J. Chem. Thermodyn. 2008, 40, 1607–1611. [Google Scholar] [CrossRef]
- Peña, M.D.; Tardajos, G. Isothermal compressibilities of n-1-alcohols from methanol to 1-dodecanol at 298.15, 308.15, 318.15, and 333.15 K. J. Chem. Thermodyn. 1979, 11, 441–445. [Google Scholar] [CrossRef]
- Alavianmehr, M.M.; Hemmati, N.; Ghodrati, H. Excess molar volumes, excess thermal expansion coefficients and isentropic compressibility deviations for binary mixtures of benzyl alcohol + (1-butanol, 2-butanol, 2-methyl-1-butanol and tert -butanol) at T = (298.15 − 328.15) K and ambient pressure. Phys. Chem. Liq. 2017, 55, 85–99. [Google Scholar] [CrossRef]
- Sahli, B.P.; Gager, H.; Richard, A.J. Ultracentrifugal studies of the isothermal compressibilities of organic alcohols and alkanes. Correlation with surface tension. J. Chem. Thermodyn. 1976, 8, 179–188. [Google Scholar] [CrossRef]
- Egorov, G.I.; Makarov, D.M. Compressibility coefficients of water-2-propanol mixtures over the temperature and pressure ranges 278–323.15 K and 1–1000 bar. Russ. J. Phys. Chem. A 2008, 82, 1037–1041. [Google Scholar] [CrossRef]
- Egorov, G.I.; Makarov, D.M.; Kolker, A.M. Liquid phase PVTx properties of (water+tert-butanol) binary mixtures at temperatures from 278.15 to 323.15 K and pressures from 0.1 to 100 MPa. J. Chem. Thermodyn. 2013, 61, 161–168. [Google Scholar] [CrossRef]
| Alcohol | No. of References | ΔT/K/p/MPa | Selected References |
|---|---|---|---|
| methanol | 45 | 283–423/40 | [4,5,13] |
| ethanol | 43 | 233–473/40 | [5,12,13] |
| propan-1-ol | 27 | 288–313/40 | [5,13,14] |
| propan-2-ol | 21 | 293–403/140 | [15,16,17] |
| butan-1-ol | 28 | 293–363/100 | [5,18,19] |
| butan-2-ol | 8 | 283–393/140 | [6,7,20] |
| 2-methylpropan-1-ol | 7 | 283–363/66 | [8,9,10] |
| 2-methylpropan-2-ol | 3 | 303–363/66 | [6,9,10] |
| pentan-1-ol | 14 | 293–323/35 | [5,11,18] |
| pentan-2-ol | 3 | 233–433/100 | [21,22,23] |
| pentan-3-ol | 2 | 233–433/100 | [21,23] |
| 2-methylbutan-1-ol | 1 | 293–323/400 | [23] |
| 2-methylbutan-2-ol | 1 | 293–323/400 | [23] |
| 3-methylbutan-2-ol | 0 | - | - |
| a0 | a1 | b0 | b1 | b2 |
|---|---|---|---|---|
| 5.7258 × 104 | −2.2876 × 101 | −6.7907 × 103 | 2.8953 × 10−1 | −2.1857 × 10−4 |
| Ref. | Type of Ref | Alcohols * | p or T Averaged | T-Range K | p-Range MPa |
|---|---|---|---|---|---|
| [26] | α | ●,■,🞜,✚,🞫,▽,▲,▼,✱ | T | 273–313 | 0.1 |
| [35] | α | ● | None | 278–300 | 0.6–45 |
| [36] | α | ●,■,🞜,▽,▲,▼ | None | 278–328 | 5–45 |
| [37] | α | 🞜 | None | 278–328 | 5–45 |
| [38] | β | ●,■,🞜,▽ | None | 298–333 | 0.1 |
| [39] | α | 🞜,✚,△,🞱 | None | 298–328 | 0.1 |
| [40] | β | ●,■,◯,☐,✚,🞫,▽,▲,▼,🞱,✱,🞽 | None | 293,298 | 0.1 |
| [41] | β | ☐ | p | 278–323 | 0.1–[10–100] |
| [42] | β | △ | p | 323 | 0.1–[10–100] |
| 105 × v0 | 1010 × v1 | 1011 × v2 | 106 × A0 | 108 × A1 | 1011 × A2 | 10−4 × B0 | 10−2 × B1 | 100 × B2 | 109 × sv | |
|---|---|---|---|---|---|---|---|---|---|---|
| methanol | 32.866 | 37.288 | 76.018 | −16.611 | 10.286 | −19.777 | 43.734 | −22.164 | 32.938 | 8.75 |
| ethanol | 48.783 | 13.636 | 10.671 | −40.286 | 25.316 | −44.825 | 71.715 | −39.966 | 61.146 | 14.8 |
| propan-1-ol | 62.470 | 5.4.825 | 12.419 | −23.618 | 11.888 | −21.057 | 90.632 | −46.439 | 64.448 | 27.4 |
| propan-2-ol | 63.633 | 91.502 | 14.736 | −18.870 | 13.270 | −29.532 | 46.334 | −25.666 | 40.837 | 41.4 |
| butan-1-ol | 78.391 | −78.209 | 15.459 | −23.165 | 10.210 | −17.778 | 87.189 | −43.003 | 57.707 | 25.1 |
| butan-2-ol | 76.892 | −10.920 | 17.811 | −52.048 | 31.730 | −54.940 | 104.11 | −58.654 | 87.161 | 41.0 |
| 2-methylpropa-1-ol | 79.101 | −17.516 | 16.101 | −48.429 | 27.575 | −45.900 | 94.187 | −50.772 | 72.759 | 25.7 |
| 2-methylpropan-2-ol | 75.148 | −27.973 | 23.040 | 34.296 | −16.367 | −49.358 | 44.287 | −22.237 | 30.621 | 37.2 |
| pentan-1ol | 94.496 | −39.692 | 17.359 | −53.125 | 28.621 | −45.914 | 86.932 | −43.902 | 59.740 | 13.7 |
| pentan-2-ol | 92.731 | −47.508 | 20.494 | −64.773 | 23.970 | −9.411 | 52.288 | −25.572 | 35.112 | 42.3 |
| pentan-3-ol | 90.103 | −25.972 | 21.102 | 53.000 | −33.857 | 45.474 | −48.323 | 97.447 | −18.894 | 49.1 |
| 2-methylbutan-1-ol | 91.903 | 29.320 | 17.217 | −122.59 | 65.366 | −94.969 | 240.78 | −130.16 | 18.111 | 43.8 |
| 2-methylbutan-2-ol | 91.025 | −55.381 | 22.791 | −193.32 | 128.08 | −223.73 | 156.09 | −95.595 | 15.575 | 54.5 |
| 3-methylbutan-2-ol | 91.082 | −47.755 | 20.931 | −171.27 | 117.06 | −215.51 | 148.87 | −90.316 | 15.234 | 59.0 |
| α | β | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound/Ref. | [35] | [36] | [37] | [39] | [26] | [38] | [40] | [41] * | [42] * |
| methanol | 0.81 | 0.66 | 0.92 | 0.82 | |||||
| ethanol | 2.43 | 0.46 | 0.82 | 0.45 | |||||
| propan-1-ol | 13.71 | 9.05 | 2.34 | 5.65 | |||||
| propan-2-ol | 1.73 | 3.98 | 2.06 | 64.04 | |||||
| butan-1-ol | 4.72 | 4.47 | 2.74 | 4.69 | |||||
| butan-2-ol | 23.52 | 1.74 | |||||||
| 2-methylpropa-1-ol | 4.08 | 5.46 | |||||||
| 2-methylpropan-2-ol | 10.62 | ||||||||
| pentan-1ol | 10.89 | 1.84 | 1.17 | 1.88 | |||||
| pentan-2-ol | 17.12 | 9.14 | 1.19 | ||||||
| pentan-3-ol | 18.02 | 4.75 | 27.99 | 0.53 | |||||
| 2-methylbutan-1-ol | 10.25 | ||||||||
| 2-methylbutan-2-ol | 11.69 | 7.17 | |||||||
| 3-methylbutan-2-ol | 11.68 | ||||||||
| MARD Tait Equation (6) | MARD Equation (10) | ΔMARD * | |||
|---|---|---|---|---|---|
| methanol | 10.68 | 9.926 | 0.01 | 0.19 | 12.38 |
| ethanol | 9.828 | 9.122 | 0.02 | 0.20 | 10.93 |
| propan-1-ol | 9.266 | 8.088 | 0.03 | 0.19 | 5.71 |
| propan-2-ol | 9.794 | 9.059 | 0.04 | 0.23 | 5.04 |
| butan-1-ol | 8.771 | 7.631 | 0.02 | 0.18 | 7.31 |
| butan-2-ol | 9.812 | 7.942 | 0.03 | 0.23 | 6.16 |
| 2-methylpropa-1-ol | 8.858 | 8.108 | 0.02 | 0.20 | 9.05 |
| 2-methylpropan-2-ol | 12.22 | 11.48 | 0.03 | 0.18 | 5.75 |
| pentan-1ol | 8.249 | 7.216 | 0.01 | 0.16 | 15.03 |
| pentan-2-ol | 9.228 | 7.675 | 0.03 | 0.21 | 6.51 |
| pentan-3-ol | 9.725 | 7.393 | 0.03 | 0.23 | 5.96 |
| 2-methylbutan-1-ol | 8.740 | 7.216 | 0.03 | 0.25 | 8.10 |
| 2-methylbutan-2-ol | 10.78 | 8.343 | 0.03 | 0.21 | 6.00 |
| 3-methylbutan-2-ol | 10.00 | 7.573 | 0.03 | 0.24 | 6.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo, B.; Yánez, J.A.; Ortega, J.; Sosa, A.; Fernández, L. Liquid-State Volumetric Properties of a Set of Alcohols with Up to Five Carbon Atoms. Liquids 2022, 2, 1-13. https://doi.org/10.3390/liquids2010001
Lorenzo B, Yánez JA, Ortega J, Sosa A, Fernández L. Liquid-State Volumetric Properties of a Set of Alcohols with Up to Five Carbon Atoms. Liquids. 2022; 2(1):1-13. https://doi.org/10.3390/liquids2010001
Chicago/Turabian StyleLorenzo, Beatriz, José Aythami Yánez, Juan Ortega, Adriel Sosa, and Luis Fernández. 2022. "Liquid-State Volumetric Properties of a Set of Alcohols with Up to Five Carbon Atoms" Liquids 2, no. 1: 1-13. https://doi.org/10.3390/liquids2010001
APA StyleLorenzo, B., Yánez, J. A., Ortega, J., Sosa, A., & Fernández, L. (2022). Liquid-State Volumetric Properties of a Set of Alcohols with Up to Five Carbon Atoms. Liquids, 2(1), 1-13. https://doi.org/10.3390/liquids2010001








