Experimental and Computational Study of the Properties of Imidazole Compounds with Branched and Cycloalkyl Substituents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Density Measurements
2.3. Viscosity Measurements
2.4. Simulation Method
3. Results and Discussion
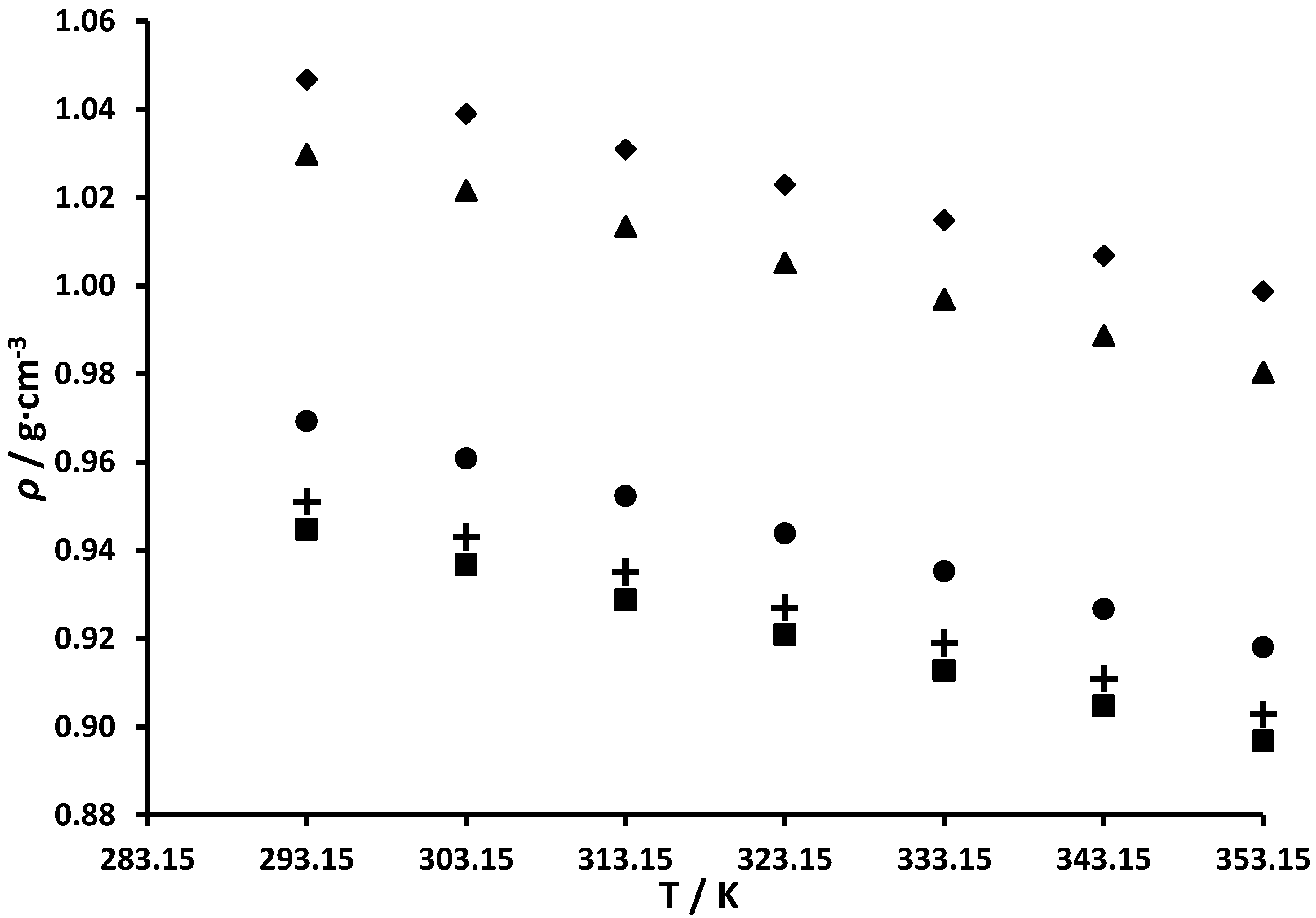
3.1. Densities of Branched and Cycloalkyl Imidazole Compounds
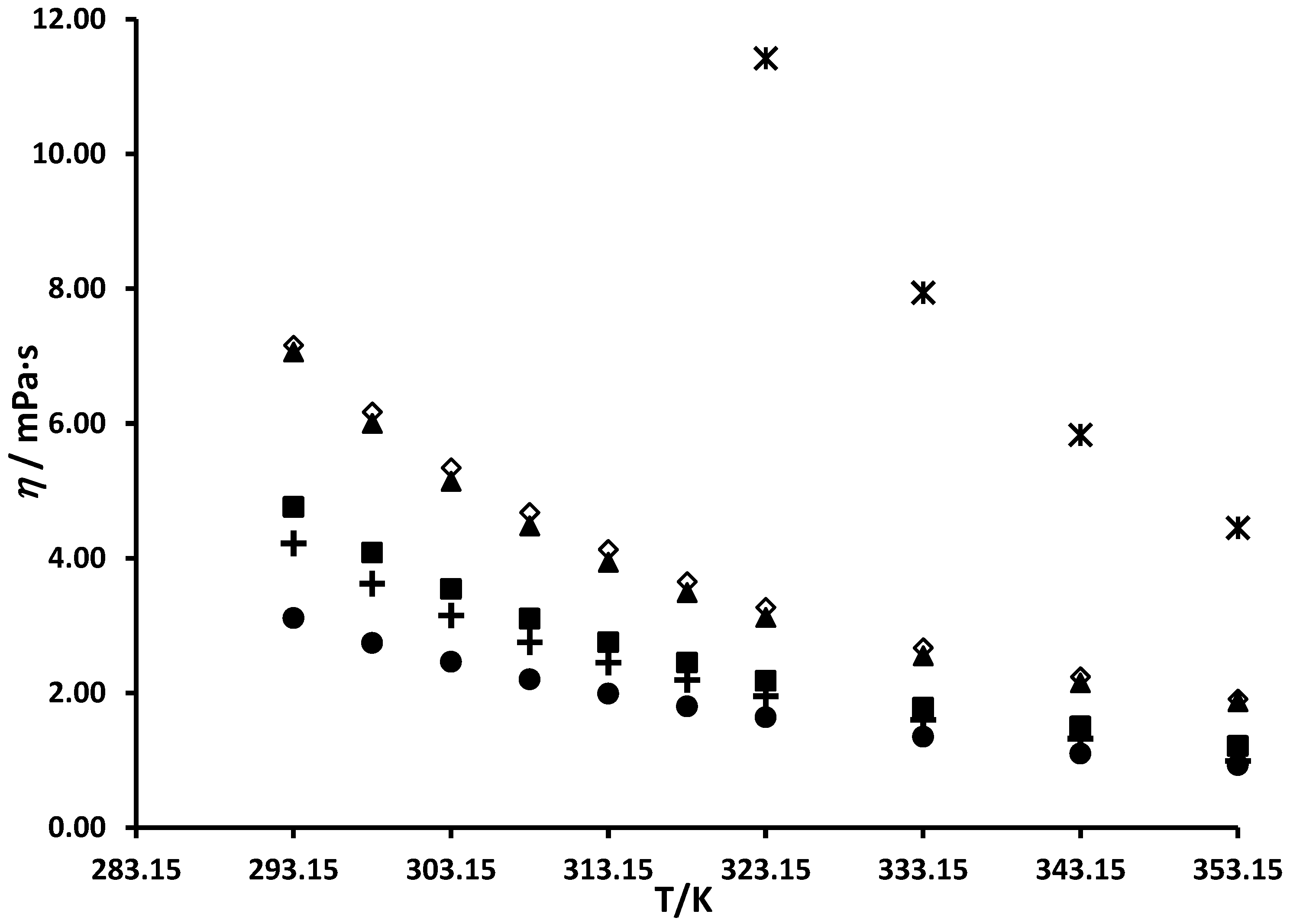
3.2. Viscosity of Branched and Cycloalkyl Imidazole Compounds
3.3. Simulations on Vapor Pressure and Vaporization Enthalpy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, F.; Wang, J.; Xie, F.; Zan, K.; Wang, S.; Wang, S. Applications of ionic liquids in starch chemistry: A review. Green Chem. 2020, 22, 2162–2183. [Google Scholar] [CrossRef]
- Krishnan, A.; Gopinath, K.P.; Vo, D.-V.N.; Malolan, R.; Nagarajan, V.M.; Arun, J. Ionic liquids, deep eutectic solvents and liquid polymers as green solvents in carbon capture technologies: A review. Environ. Chem. Lett. 2020, 18, 2031–2054. [Google Scholar] [CrossRef]
- Bara, J.E.; Finotello, A.; Magee, J.W.; Qian, S.; O’Harra, K.E.; Dennis, G.P.; Noble, R.D. 110th Anniversary: Properties of Imidazolium-Based Ionic Liquids Bearing Both Benzylic and n-Alkyl Substituents. Ind. Eng. Chem. Res. 2019, 58, 17956–17964. [Google Scholar] [CrossRef]
- Liu, X.; O’Harra, K.E.; Bara, J.E.; Turner, C.H. Molecular insight into the anion effect and free volume effect of CO2 solubility in multivalent ionic liquids. Phys. Chem. Chem. Phys. 2020, 22, 20618–20633. [Google Scholar] [CrossRef]
- Atanassova, M. Solvent extraction chemistry in ionic liquids: An overview of f-ions. J. Mol. Liq. 2021, 343, 117530. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Anagnostou, P.; Constantinou, I.; Dakidi, K.; Stalikas, C. Magnetic Ionic Liquids in Sample Preparation: Recent Advances and Future Trends. Separations 2021, 8, 153. [Google Scholar] [CrossRef]
- Correia, D.M.; Fernandes, L.C.; Fernandes, M.M.; Hermenegildo, B.; Meira, R.M.; Ribeiro, C.; Ribeiro, S.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid-Based Materials for Biomedical Applications. Nanomaterials 2021, 11, 2401. [Google Scholar] [CrossRef]
- Geniselli da Silva, V. Laccases and ionic liquids as an alternative method for lignin depolymerization: A review. Bioresour. Technol. Rep. 2021, 16, 100824. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Abdan, K.; Kaneko, T. A Concise Review on the Physicochemical Properties of Biopolymer Blends Prepared in Ionic Liquids. Molecules 2021, 26, 216. [Google Scholar] [CrossRef]
- Seng, L.K.; Masdar, M.S.; Shyuan, L.K. Ionic Liquid in Phosphoric Acid-Doped Polybenzimidazole (PA-PBI) as Electrolyte Membranes for PEM Fuel Cells: A Review. Membranes 2021, 11, 728. [Google Scholar] [CrossRef]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Song, Y.; Wang, Q.; Zhang, L.; Deng, L. Review of ionic liquids containing, polymer/inorganic hybrid electrolytes for lithium metal batteries. Mater. Des. 2020, 190, 108563. [Google Scholar] [CrossRef]
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Ionic Liquids for Supercapacitive Energy Storage: A Mini-Review. Energy Fuels 2021, 35, 8443–8455. [Google Scholar] [CrossRef]
- Hindman, M.S.; Stanton, A.D.; Irvin, A.C.; Wallace, D.A.; Moon, J.D.; Reclusado, K.R.; Liu, H.; Belmore, K.A.; Liang, Q.; Shannon, M.S.; et al. Synthesis of 1,2-Dialkyl-, 1,4(5)-Dialkyl-, and 1,2,4(5)-Trialkylimidazoles via a One-Pot Method. Ind. Eng. Chem. Res. 2013, 52, 11880–11887. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of Hydrophobic Alkylimidazoles in the Separation of Non-Ferrous Metal Ions across Plasticised Membranes—A Review. Membranes 2020, 10, 331. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Urbaniak, W. Cadmium(II) and lead(II) extraction and transport through polymer inclusion membranes with 1-alkylimidazole. Desalin. Water Treat. 2021, 214, 56–63. [Google Scholar] [CrossRef]
- Shannon, M.S.; Tedstone, J.M.; Danielsen, S.P.O.; Hindman, M.S.; Bara, J.E. Properties and Performance of Ether-Functionalized Imidazoles as Physical Solvents for CO2 Separations. Energy Fuels 2013, 27, 3349–3357. [Google Scholar] [CrossRef]
- Barbosa, G.D.; Bara, J.E.; Weinman, S.T.; Turner, C.H. Molecular aspects of temperature swing solvent extraction for brine desalination using imidazole-based solvents. Chem. Eng. Sci. 2022, 247, 116866. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Shen, C.; Wang, Z. Towards understanding the effect of electrostatic interactions on the density of ionic liquids. Fluid Phase Equilibria 2009, 279, 87–91. [Google Scholar] [CrossRef]
- Bara, J.E.; Moon, J.D.; Reclusado, K.R.; Whitley, J.W. COSMOTherm as a Tool for Estimating the Thermophysical Properties of Alkylimidazoles as Solvents for CO2 Separations. Ind. Eng. Chem. Res. 2013, 52, 5498–5506. [Google Scholar] [CrossRef]
- Shannon, M.S.; Hindman, M.S.; Danielsen, S.P.O.; Tedstone, J.M.; Gilmore, R.D.; Bara, J.E. Properties of alkylbenzimidazoles for CO2 and SO2 capture and comparisons to ionic liquids. Sci. China Chem. 2012, 55, 1638–1647. [Google Scholar] [CrossRef]
- Shannon, M.S.; Bara, J.E. Properties of Alkylimidazoles as Solvents for CO2 Capture and Comparisons to Imidazolium-Based Ionic Liquids. Ind. Eng. Chem. Res. 2011, 50, 8665–8677. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Portnova, S.V.; Verevkin, S.P.; Skrzypczak, A.; Schubert, T. Building blocks for ionic liquids: Vapor pressures and vaporization enthalpies of 1-(n-alkyl)-imidazoles. J. Chem. Thermodyn. 2011, 43, 1500–1505. [Google Scholar] [CrossRef]
- Garist, I.V.; Verevkin, S.P.; Bara, J.E.; Hindman, M.S.; Danielsen, S.P.O. Building Blocks for Ionic Liquids: Vapor Pressures and Vaporization Enthalpies of 1-(n-Alkyl)-benzimidazoles. J. Chem. Eng. Data 2012, 57, 1803–1809. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Portnova, S.V.; Verevkin, S.P.; Skrzypczak, A. Building Blocks for Ionic Liquids: A Study of Alkyl Chain Length Dependence of Vaporization Enthalpies of 1-(n-Alkyl)-2-methylimidazoles. J. Chem. Eng. Data 2011, 56, 3532–3540. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitsau, D.H.; Emel’yanenko, V.N.; Paulechka, Y.U.; Blokhin, A.V.; Bazyleva, A.B.; Kabo, G.J. Thermodynamics of Ionic Liquids Precursors: 1-Methylimidazole. J. Phys. Chem. B 2011, 115, 4404–4411. [Google Scholar] [CrossRef]
- Turner, C.H.; Cooper, A.; Zhang, Z.; Shannon, M.S.; Bara, J.E. Molecular Simulation of the Thermophysical Properties of N-Functionalized Alkylimidazoles. J. Phys. Chem. B 2012, 116, 6529–6535. [Google Scholar] [CrossRef] [PubMed]
- Lenarcik, B.; Ojczenasz, P. The influence of the size and position of the alkyl groups in alkylimidazole molecules on their acid-base properties. J. Heterocycl. Chem. 2002, 39, 287–290. [Google Scholar] [CrossRef]
- Bara, J.E. Versatile and Scalable Method for Producing N-Functionalized Imidazoles. Ind. Eng. Chem. Res. 2011, 50, 13614–13619. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Zaitseva, K.V.; Stanton, A.D.; Hindman, M.S.; Irvin, A.C.; Bara, J.E. Building Blocks for Ionic Liquids: Vapor Pressures and Vaporization Enthalpies of N-Functionalized Imidazoles with Branched and Cycloalkyl Substituents. Ind. Eng. Chem. Res. 2015, 54, 9850–9856. [Google Scholar] [CrossRef]
- BIOVIA COSMOtherm, Dassault Systèmes. Available online: https://www.3ds.com/products-services/biovia/products/molecular-modeling-simulation/solvation-chemistry/biovia-cosmotherm/ (accessed on 30 January 2022).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Eckert, F.; Klamt, A. Fast solvent screening via quantum chemistry: COSMO-RS approach. AIChE J. 2002, 48, 369–385. [Google Scholar] [CrossRef] [Green Version]
- Klamt, A.; Eckert, F. COSMO-RS: A novel and efficient method for the a priori prediction of thermophysical data of liquids. Fluid Phase Equilibria 2000, 172, 43–72. [Google Scholar] [CrossRef] [Green Version]
- Shannon, M.S.; Tedstone, J.M.; Danielsen, S.P.O.; Hindman, M.S.; Irvin, A.C.; Bara, J.E. Free Volume as the Basis of Gas Solubility and Selectivity in Imidazolium-Based Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 5565–5576. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Wang, W. Screening of ionic liquids to capture CO2 by COSMO-RS and experiments. AIChE J. 2008, 54, 2717–2728. [Google Scholar] [CrossRef]
- Sumon, K.Z.; Henni, A. Ionic liquids for CO2 capture using COSMO-RS: Effect of structure, properties and molecular interactions on solubility and selectivity. Fluid Phase Equilibria 2011, 310, 39–55. [Google Scholar] [CrossRef]
- Andrade, E.N.D.C. The Viscosity of Liquids. Nature 1930, 125, 309–310. [Google Scholar] [CrossRef]
- Qian, S.; Liu, X.; Dennis, G.P.; Turner, C.H.; Bara, J.E. Properties of symmetric 1,3-diethers based on glycerol skeletons for CO2 absorption. Fluid Phase Equilibria 2020, 521, 112718. [Google Scholar] [CrossRef]
- Qian, S.; Liu, X.; Turner, C.H.; Bara, J.E. Synthesis and properties of symmetric glycerol-derived 1,2,3-triethers and 1,3-diether-2-ketones for CO2 absorption. Chem. Eng. Sci. 2022, 248, 117150. [Google Scholar] [CrossRef]
- Qian, S.; Liu, X.; Turner, C.H.; Bara, J.E. Glycerol-derived solvents containing two or three distinct functional groups enabled by trifluoroethyl glycidyl ether. AIChE J. 2022, e17533. [Google Scholar] [CrossRef]
- Seeton, C.J. Viscosity–temperature correlation for liquids. Tribol. Lett. 2006, 22, 67–78. [Google Scholar] [CrossRef]
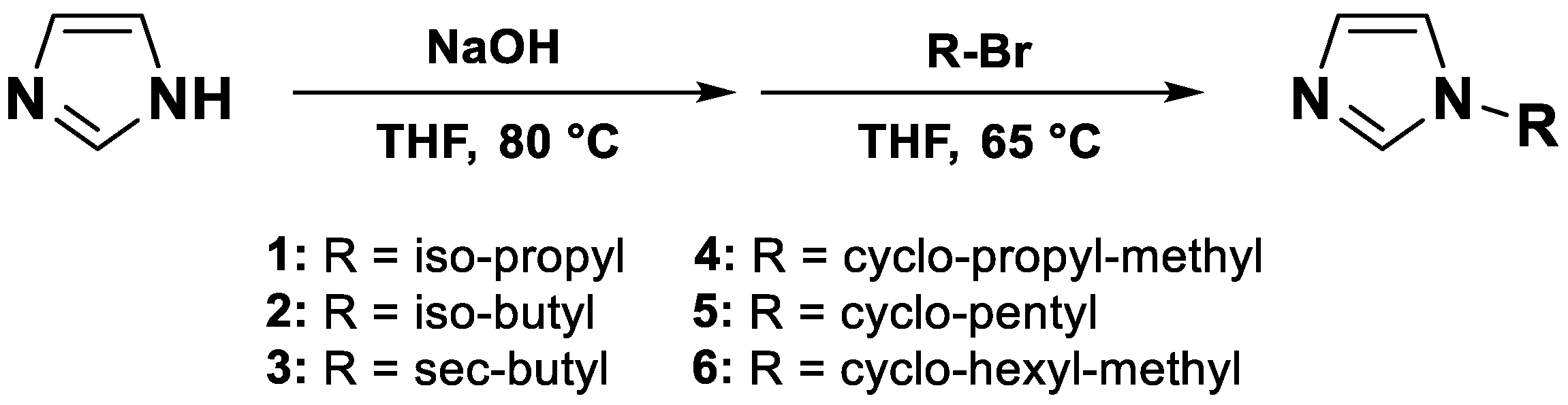
| Compound # | Name/CAS # | Formula | Structure |
|---|---|---|---|
| 1 | N-isopropylimidazole 4532-96-1 | C6H10N2 |  |
| 2 | N-isobutylimidazole 16245-89-9 | C7H12N2 |  |
| 3 | N-sec-butylimidazole 20075-29-0 | C7H12N2 |  |
| 4 | N-cyclopropylmethylimidazole 717908-74-2 | C7H10N2 | 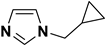 |
| 5 | N-cyclopentylimidazole 71614-58-9 | C8H12N2 |  |
| 6 | N-cyclohexylmethylimidazole 71621-00-6 | C10H16N2 |  |
| (1) N-isopropylimidazole | (2) N-isobutylimidazole | ||||||
| 293.15 | 0.96927 | 0.96079 | 0.88 | 293.15 | 0.94471 | 0.93562 | 0.96 |
| 303.15 | 0.96079 | 0.95101 | 1.02 | 303.15 | 0.93675 | 0.92597 | 1.15 |
| 313.15 | 0.95231 | 0.94132 | 1.15 | 313.15 | 0.92879 | 0.91643 | 1.33 |
| 323.15 | 0.94379 | 0.93172 | 1.28 | 323.15 | 0.92081 | 0.90698 | 1.50 |
| 333.15 | 0.93525 | 0.92222 | 1.39 | 333.15 | 0.91280 | 0.89763 | 1.66 |
| 343.15 | 0.92667 | 0.91280 | 1.50 | 343.15 | 0.90476 | 0.88838 | 1.81 |
| 353.15 | 0.91804 | 0.90348 | 1.59 | 353.15 | 0.89678 | 0.87923 | 1.96 |
| (3) N-sec-butylimidazole | (4) N-cyclopropylmethylimidazole | ||||||
| 293.15 | 0.95106 | 0.93546 | 1.64 | 293.15 | 1.02976 | 1.03326 | −0.34 |
| 303.15 | 0.94302 | 0.92579 | 1.83 | 303.15 | 1.02154 | 1.02327 | −0.17 |
| 313.15 | 0.93502 | 0.91623 | 2.01 | 313.15 | 1.01335 | 1.01332 | 0.00 |
| 323.15 | 0.92698 | 0.90676 | 2.18 | 323.15 | 1.00513 | 1.00344 | 0.17 |
| 333.15 | 0.91895 | 0.89740 | 2.35 | 333.15 | 0.99690 | 0.99361 | 0.33 |
| 343.15 | 0.91091 | 0.88813 | 2.50 | 343.15 | 0.98866 | 0.98385 | 0.49 |
| 353.15 | 0.90280 | 0.87897 | 2.64 | 353.15 | 0.98035 | 0.97415 | 0.63 |
| (5) N-cyclopentylimidazole | |||||||
| 293.15 | 1.04677 | 1.05621 | −0.90 | ||||
| 303.15 | 1.03893 | 1.04605 | −0.68 | ||||
| 313.15 | 1.03091 | 1.03594 | −0.49 | ||||
| 323.15 | 1.02286 | 1.02588 | −0.30 | ||||
| 333.15 | 1.01481 | 1.01588 | −0.10 | ||||
| 343.15 | 1.00677 | 1.00593 | 0.08 | ||||
| 353.15 | 0.99869 | 0.99604 | 0.26 | ||||
| Compound | R2 | Maximum Absolute Residual | RSS | ||
|---|---|---|---|---|---|
| 1 | 8.5354 × 10−4 | 1.220 | 1.0000 | 0.8 × 10−4 | 2.030 × 10−8 |
| 2 | 7.9914 × 10−4 | 1.179 | 1.0000 | 0.4 × 10−4 | 0.503 × 10−8 |
| 3 | 8.0382 × 10−4 | 1.187 | 1.0000 | 0.5 × 10−4 | 0.486 × 10−8 |
| 4 | 8.2300 × 10−4 | 1.271 | 1.0000 | 0.6 × 10−4 | 0.749 × 10−8 |
| 5 | 8.0236 × 10−4 | 1.282 | 1.0000 | 1.2 × 10−4 | 2.825 × 10−8 |
| Compound | Maximum Absolute Residual | RSS | ||||
|---|---|---|---|---|---|---|
| 1 | 0.9438 | −0.8535 × 10−3 | −1.502 × 10−7 | 323.15 | 0.1 × 10−4 | 0.011 × 10−8 |
| 2 | 0.8968 | −0.8027 × 10−3 | −0.610 × 10−7 | 353.15 | 0.3 × 10−4 | 0.133 × 10−8 |
| 3 | 0.9670 | −0.7964 × 10−3 | −0.730 × 10−7 | 273.15 | 0.3 × 10−4 | 0.169 × 10−8 |
| 4 | 1.0379 | −0.8145 × 10−3 | −1.053 × 10−7 | 283.15 | 0.3 × 10−4 | 0.165 × 10−8 |
| 5 | 0.9987 | −0.8116 × 10−3 | −1.554 × 10−7 | 353.15 | 0.6 × 10−4 | 0.813 × 10−8 |
| (1) N-isopropylimidazole | (2) N-isobutylimidazole | ||||||
| 293.15 | 3.11 | 3.10 | 0.45 | 293.15 | 4.76 | 3.70 | 22.28 |
| 298.15 | 2.74 | 2.79 | −1.71 | 298.15 | 4.08 | 3.31 | 18.84 |
| 303.15 | 2.46 | 2.52 | −2.33 | 303.15 | 3.54 | 2.97 | 15.97 |
| 308.15 | 2.20 | 2.28 | −3.70 | 308.15 | 3.10 | 2.68 | 13.50 |
| 313.15 | 1.99 | 2.07 | −4.22 | 313.15 | 2.75 | 2.43 | 11.80 |
| 318.15 | 1.80 | 1.89 | −5.07 | 318.15 | 2.45 | 2.20 | 10.18 |
| 323.15 | 1.64 | 1.73 | −5.45 | 323.15 | 2.18 | 2.00 | 8.13 |
| 333.15 | 1.35 | 1.46 | −7.99 | 333.15 | 1.78 | 1.67 | 6.02 |
| 343.15 | 1.10 | 1.24 | −12.84 | 343.15 | 1.50 | 1.41 | 5.87 |
| 353.15 | 0.93 | 1.07 | −14.68 | 353.15 | 1.21 | 1.20 | 0.56 |
| (3) N-sec-butylimidazole | (4) N-cyclopropylmethylimidazole | ||||||
| 293.15 | 4.22 | 3.65 | 13.48 | 293.15 | 7.06 | 4.92 | 30.32 |
| 298.15 | 3.62 | 3.27 | 9.69 | 298.15 | 6.00 | 4.36 | 27.28 |
| 303.15 | 3.15 | 2.94 | 6.73 | 303.15 | 5.14 | 3.89 | 24.41 |
| 308.15 | 2.75 | 2.65 | 3.65 | 308.15 | 4.48 | 3.47 | 22.48 |
| 313.15 | 2.45 | 2.40 | 2.15 | 313.15 | 3.94 | 3.12 | 20.92 |
| 318.15 | 2.19 | 2.18 | 0.64 | 318.15 | 3.49 | 2.80 | 19.64 |
| 323.15 | 1.95 | 1.98 | −1.59 | 323.15 | 3.12 | 2.53 | 18.83 |
| 333.15 | 1.60 | 1.66 | −3.49 | 333.15 | 2.55 | 2.08 | 18.26 |
| 343.15 | 1.32 | 1.40 | −5.95 | 343.15 | 2.15 | 1.74 | 19.29 |
| 353.15 | 0.99 | 1.19 | −20.46 | 353.15 | 1.87 | 1.46 | 21.95 |
| (5) N-cyclopentylimidazole | (6) N-cyclohexylmethylimidazole | ||||||
| 293.15 | 7.16 | 6.50 | 9.21 | 293.15 | N/A (Solid) | ||
| 298.15 | 6.17 | 5.72 | 7.37 | 298.15 | N/A (Solid) | ||
| 303.15 | 5.34 | 5.05 | 5.51 | 303.15 | N/A (Solid) | ||
| 308.15 | 4.68 | 4.47 | 4.42 | 308.15 | N/A (Solid) | ||
| 313.15 | 4.13 | 3.98 | 3.62 | 313.15 | N/A (Solid) | ||
| 318.15 | 3.65 | 3.56 | 2.60 | 318.15 | N/A (Solid) | ||
| 323.15 | 3.27 | 3.19 | 2.55 | 323.15 | 11.42 | 4.72 | 58.69 |
| 333.15 | 2.67 | 2.59 | 3.18 | 333.15 | 7.94 | 3.73 | 52.97 |
| 343.15 | 2.24 | 2.12 | 5.22 | 343.15 | 5.83 | 3.00 | 48.60 |
| 353.15 | 1.91 | 1.76 | 7.69 | 353.15 | 4.45 | 2.43 | 45.29 |
| Compound | R2 | Maximum Absolute Residual | RSS | ||
|---|---|---|---|---|---|
| 1 | 0.2670 × 10−2 | 2.070 × 103 | 0.9994 | 0.03 | 0.0020 |
| 2 | 0.1687 × 10−2 | 2.320 × 103 | 0.9985 | 0.14 | 0.0277 |
| 3 | 0.1161 × 10−2 | 2.399 × 103 | 0.9972 | 0.06 | 0.0136 |
| 4 | 0.2619 × 10−2 | 2.230 × 103 | 0.9937 | 0.37 | 0.2178 |
| 5 | 0.2838 × 10−2 | 2.286 × 103 | 0.9971 | 0.24 | 0.1010 |
| 6 | 0.0172 × 10−2 | 3.584 × 103 | 0.9984 | 0.16 | 0.0517 |
| Compound | Maximum Absolute Residual | RSS | |||
|---|---|---|---|---|---|
| 1 | 0.0438 × 10−1 | 1.786 × 103 | 21.01 | 0.02 | 0.069 × 10−5 |
| 2 | 0.3290 × 10−1 | 0.806 × 103 | 131.06 | 0.03 | 0.166 × 10−5 |
| 3 | 0.0470 × 10−1 | 1.590 × 103 | 59.11 | 0.06 | 2.522 × 10−5 |
| 4 | 1.7126 × 10−1 | 0.397 × 103 | 186.34 | 0.02 | 0.018 × 10−5 |
| 5 | 0.8963 × 10−1 | 0.605 × 103 | 155.13 | 0.02 | 0.018 × 10−5 |
| 6 | 0.8476 × 10−1 | 0.617 × 103 | 197.37 | 0.01 | 0.005 × 10−5 |
| Compound | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 283.4 | 5.04 | 5.23 | −3.74 | 59.39 | 54.61 | 8.04 |
| 289.4 | 8.51 | 8.45 | 0.74 | 58.98 | 54.43 | 7.72 | |
| 2 | 289.6 | 4.42 | 4.37 | 1.07 | 62.81 | 56.97 | 9.30 |
| 292.6 | 5.85 | 5.57 | 4.75 | 62.57 | 56.88 | 9.10 | |
| 3 | 295.7 | 7.11 | 6.84 | 3.78 | 64.78 | 56.81 | 12.31 |
| 298.4 | 8.99 | 8.43 | 6.22 | 64.57 | 56.72 | 12.61 | |
| 4 | 303.0 | 3.70 | 7.25 | −95.88 | 59.49 | 57.46 | 3.42 |
| 305.4 | 4.54 | 8.67 | −90.95 | 59.32 | 57.38 | 3.27 | |
| 5 | 305.0 | 2.42 | 2.16 | 10.79 | 70.1 | 61.53 | 12.23 |
| 308.0 | 3.19 | 2.73 | 14.29 | 69.87 | 61.43 | 12.08 | |
| 6 | 323.1 | 2.55 | 1.77 | 30.69 | 75.41 | 67.15 | 10.96 |
| 326.0 | 3.24 | 2.21 | 31.88 | 75.16 | 67.04 | 10.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, S.; Mileski, P.; Irvin, A.C.; Soyemi, A.; Szilvási, T.; Bara, J.E. Experimental and Computational Study of the Properties of Imidazole Compounds with Branched and Cycloalkyl Substituents. Liquids 2022, 2, 14-25. https://doi.org/10.3390/liquids2010002
Qian S, Mileski P, Irvin AC, Soyemi A, Szilvási T, Bara JE. Experimental and Computational Study of the Properties of Imidazole Compounds with Branched and Cycloalkyl Substituents. Liquids. 2022; 2(1):14-25. https://doi.org/10.3390/liquids2010002
Chicago/Turabian StyleQian, Shuai, Patrick Mileski, Adam C. Irvin, Ademola Soyemi, Tibor Szilvási, and Jason E. Bara. 2022. "Experimental and Computational Study of the Properties of Imidazole Compounds with Branched and Cycloalkyl Substituents" Liquids 2, no. 1: 14-25. https://doi.org/10.3390/liquids2010002
APA StyleQian, S., Mileski, P., Irvin, A. C., Soyemi, A., Szilvási, T., & Bara, J. E. (2022). Experimental and Computational Study of the Properties of Imidazole Compounds with Branched and Cycloalkyl Substituents. Liquids, 2(1), 14-25. https://doi.org/10.3390/liquids2010002







