Process Validation of Air-Dried Beef Sticks (Droëwors) to Achieve >5-log Reduction of Salmonella Serovars, Listeria monocytogenes, and E. coli O157:H7 Using Refined Liquid Smoke Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Growth and Storage Conditions, and Inoculum Preparation
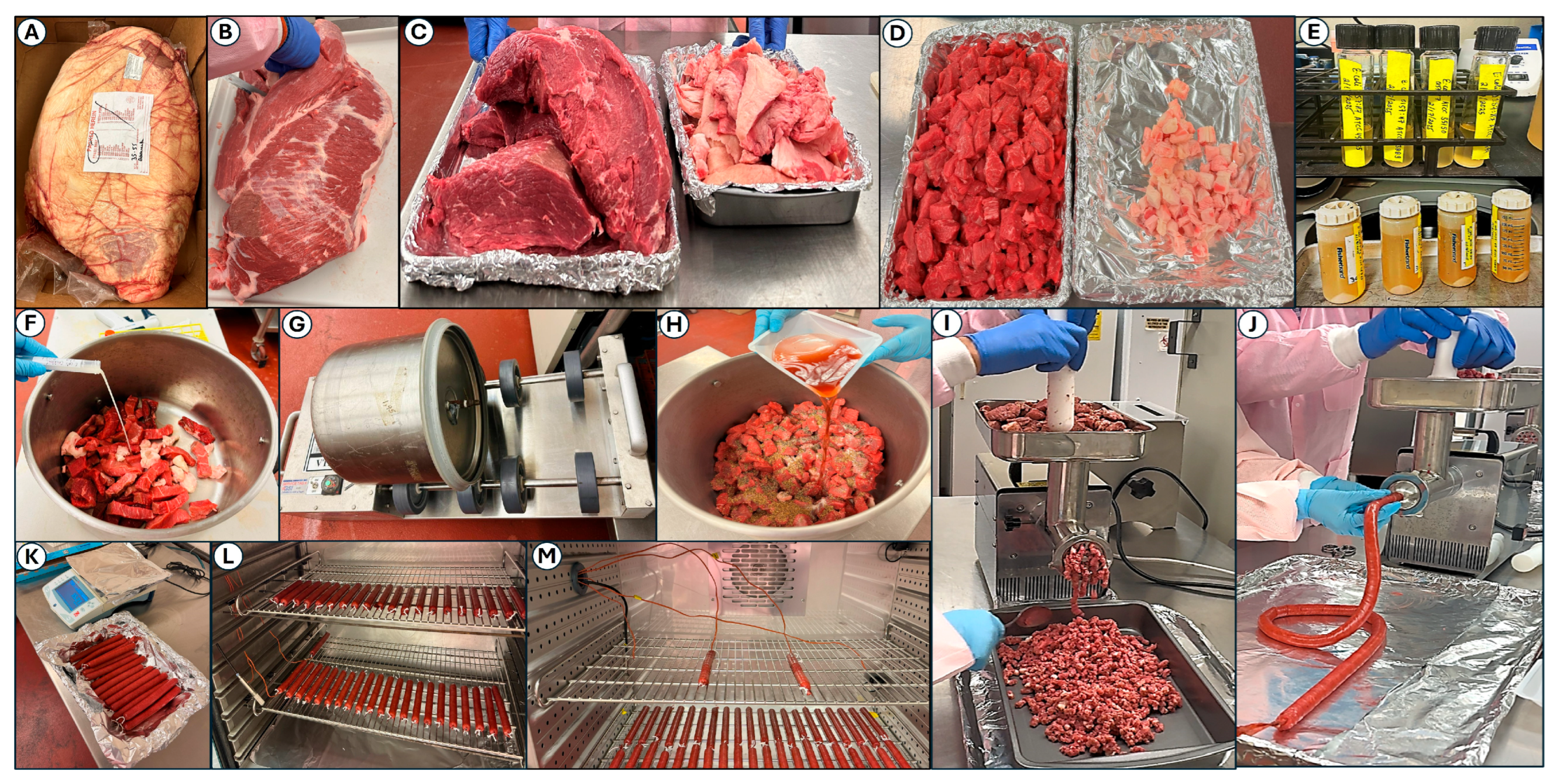
2.2. Beef Preparation, Inoculation, Marinade Seasoning, and Processing
2.2.1. Beef Preparation
2.2.2. Beef Inoculation and Marination
2.3. Droëwors Beef Stick Processing
2.4. Biltong Processing
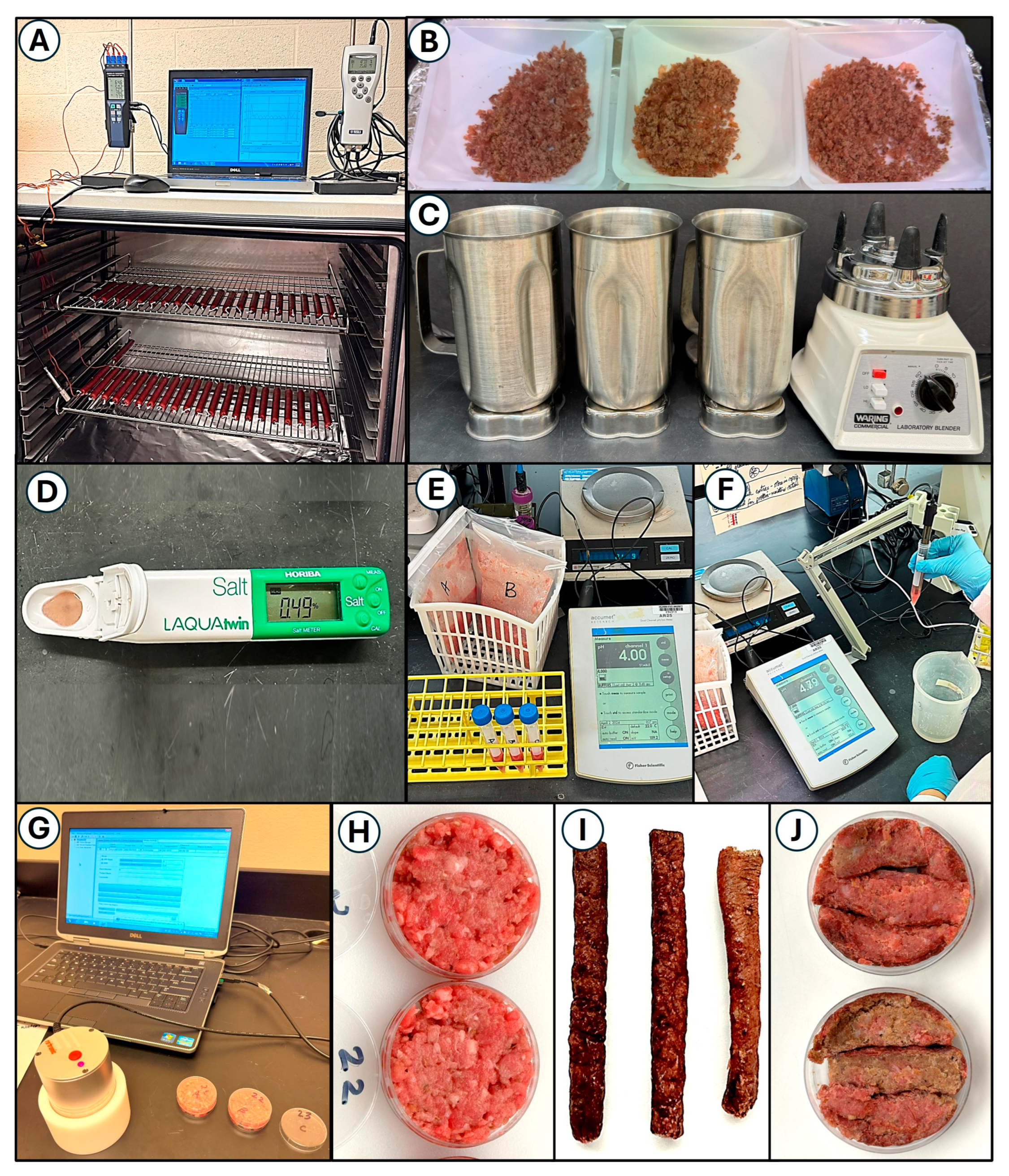
2.5. Quantifying Process Parameters
2.5.1. Temperature and Humidity
2.5.2. Salt
2.5.3. pH
2.5.4. Water Activity
2.5.5. Moisture Loss
2.6. Microbial Sampling
2.7. Statistical Analysis
3. Results and Discussion
3.1. Droëwors: Reduction in Salmonella Serovars Using Pyrolyzed Liquid Smoke Extracts as Flavoring and Antimicrobial
3.2. Droëwors: Reduction in E. coli O157:H7 Using Pyrolyzed Liquid Smoke Extracts as Flavoring and Antimicrobial
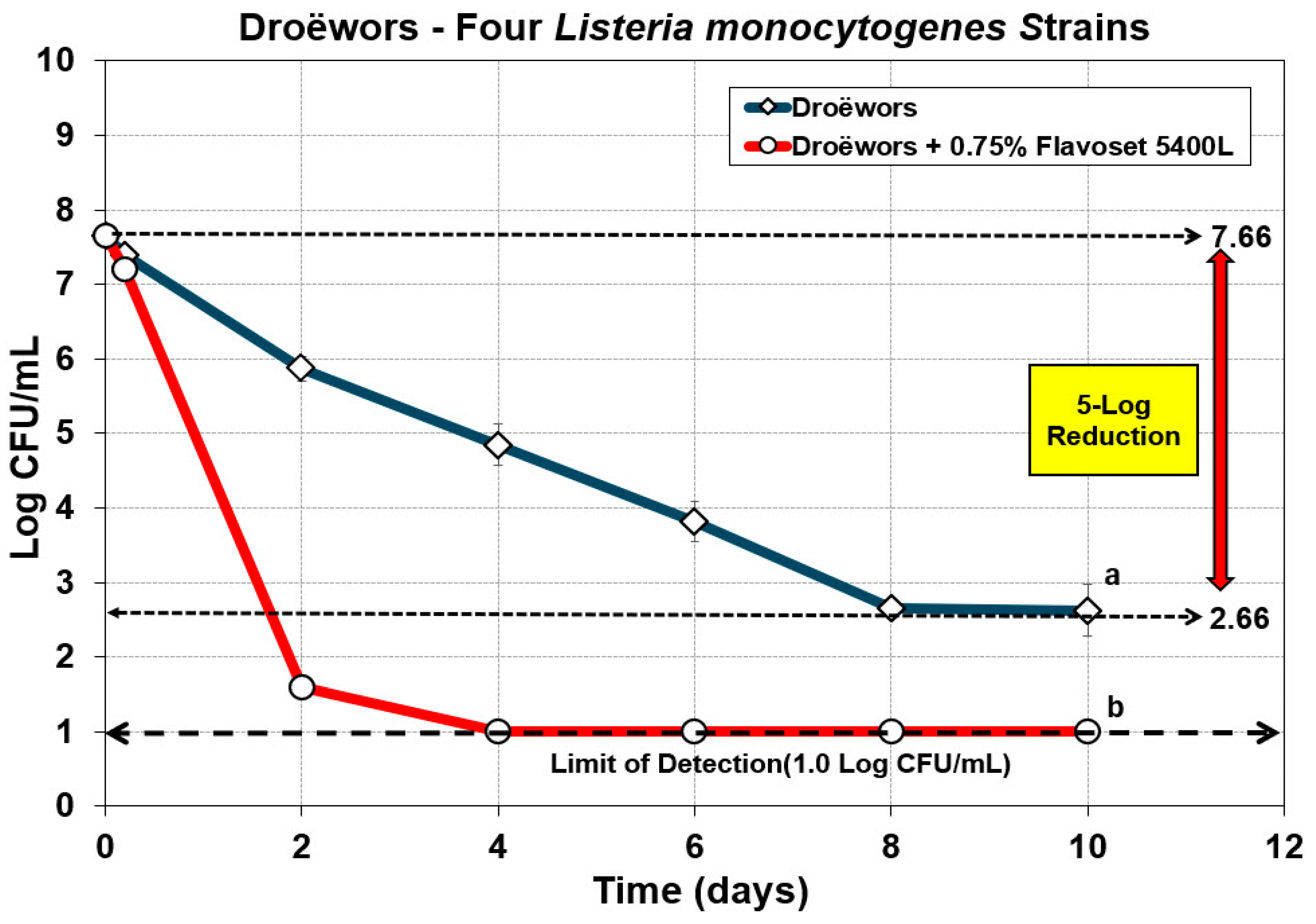
3.3. Droëwors: Reduction in Listeria Monocytogenes Using Pyrolyzed Liquid Smoke Extracts as Flavoring and Antimicrobial
3.4. Biltong: Reduction in Salmonella Serovars Using Pyrolyzed Liquid Smoke Extracts as Flavoring and Antimicrobial
3.5. Droëwors Processing Parameters
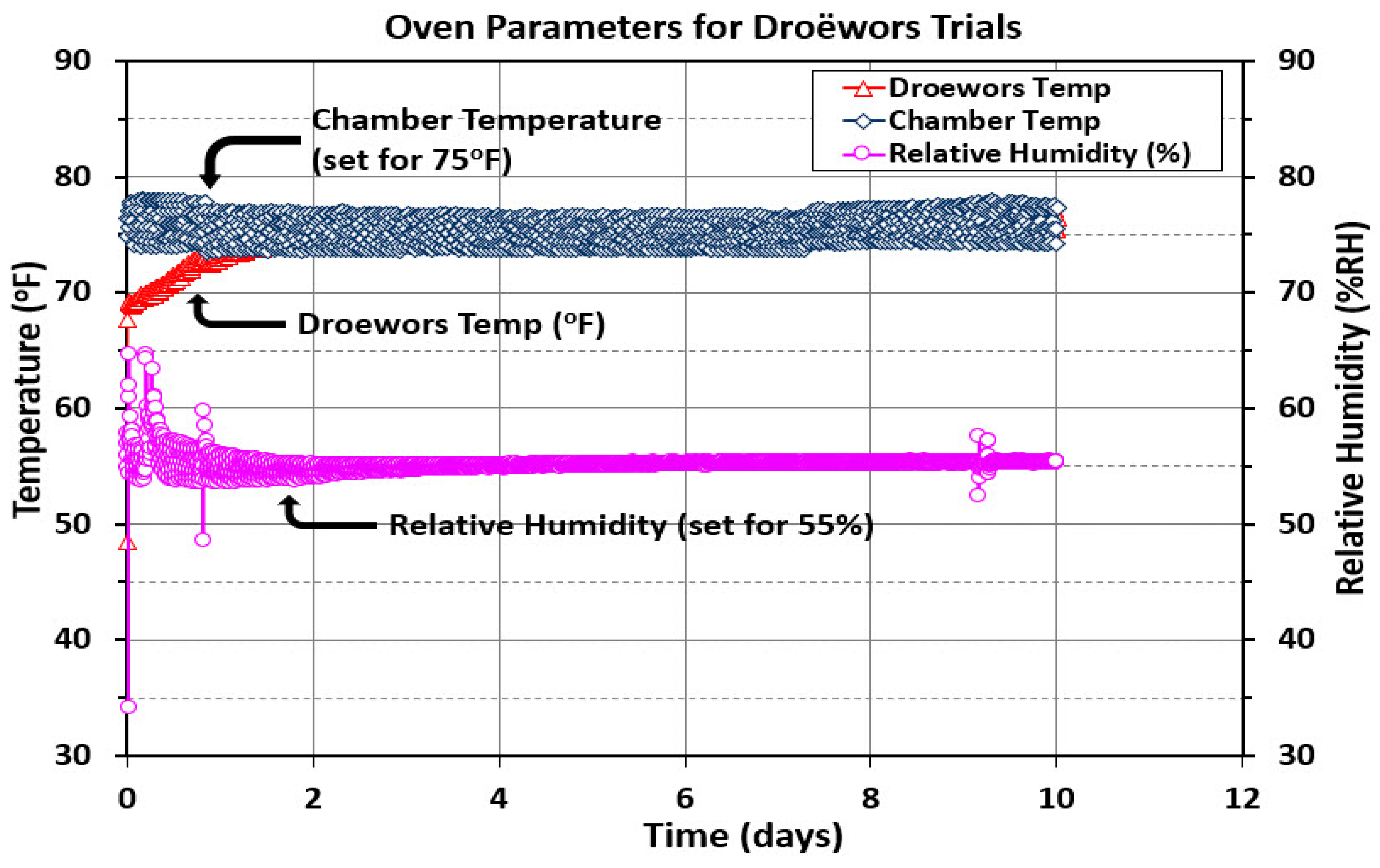
3.5.1. Droëwors Temperature and Relative Humidity Measurements
3.5.2. Comparison of Droëwors Moisture Loss, Salt, and pH Measurements
3.5.3. Comparison of Droëwors Moisture Loss and Water Activity (Aw) Measurements
3.5.4. Liquid Smokes and Their Antimicrobial Activity in Food
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Xu, G.; Hu, S. A Comprehensive Review on the Recent Technological Advancements in the Processing, Safety, and Quality Control of Ready-to-Eat Meals. Processes 2025, 13, 901. [Google Scholar] [CrossRef]
- Mediani, A.; Hamezah, H.S.; Jam, F.A.; Mahadi, N.F.; Chan, S.X.Y.; Rohani, E.R.; Che Lah, N.H.; Azlan, U.K.; Khairul Annuar, N.A.; Azman, N.A.F.; et al. A comprehensive review of drying meat products and the associated effects and changes. Front. Nutr. 2022, 9, 1057366. [Google Scholar] [CrossRef]
- More, A.B.; Chandola, V.; Bhat, S. Biltong Market Research Report 2033; Growth Market Reports: Maharashtra, India; Available online: https://growthmarketreports.com/report/biltong-market (accessed on 24 October 2025).
- Verified Market Research Reports Team. Air-Dried Beef Market Size, Market Growth and Forecast; Verified Market Research Reports: Washington, DC, USA, 2025; Available online: https://www.verifiedmarketreports.com/product/air-dried-beef-market/ (accessed on 26 October 2025).
- USDA-FSIS. FSIS Compliance Guideline for Meat and Poultry Jerky Produced by Small and Very Small Establishments; U.S. Food Safety and Inspection Service: Washington, DC, USA, 2014; pp. pp 1–54. [Google Scholar]
- USDA-FSIS. FSIS Ready-to-Eat Fermented, Salt-Cured, and Dried Products Guideline; U.S. Food Safety and Inspection Service: Washington, DC, USA. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/documents/FSIS-GD-2023-0002.pdf (accessed on 18 April 2025).
- Gavai, K.; Karolenko, C.; Muriana, P.M. Effect of biltong dried beef processing on the reduction of Listeria monocytogenes, E. coli O157:H7, and Staphylococcus aureus, and the contribution of the major marinade components. Microorganisms 2022, 10, 1308. [Google Scholar] [CrossRef]
- Karolenko, C.E.; Bhusal, A.; Nelson, J.L.; Muriana, P.M. Processing of Biltong (Dried Beef) to Achieve USDA-FSIS 5-log Reduction of Salmonella without a Heat Lethality Step. Microorganisms 2020, 8, 791. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Prieto, M.; Bernardo, A.; Hill, C.; López, M. The acid tolerance response of Salmonella spp.: An adaptive strategy to survive in stressful environments prevailing in foods and the host. Food Res. Intl. 2012, 45, 482–492. [Google Scholar] [CrossRef]
- Gavriil, A.; Giannenas, I.; Skandamis, P.N. A current insight into Salmonella’s inducible acid resistance. Crit. Rev. Food Sci. Nutr. 2025, 65, 3835–3855. [Google Scholar] [CrossRef]
- Leyer, G.J.; Johnson, E.A. Acid adaptation induces cross-protection against environmental stresses in Salmonella typhimurium. Appl. Environ. Microbiol. 1993, 59, 1842–1847. [Google Scholar] [CrossRef]
- Leyer, G.J.; Johnson, E.A. Acid adaptation promotes survival of Salmonella spp. in cheese. Appl. Environ. Microbiol. 1992, 58, 2075–2080. [Google Scholar] [CrossRef]
- Ye, B.; He, S.; Zhou, X.; Cui, Y.; Zhou, M.; Shi, X. Response to acid adaptation in Salmonella enterica Serovar Enteritidis. J. Food Sci. 2019, 84, 599–605. [Google Scholar] [CrossRef]
- National Advisory Committee on Microbiological Criteria for Foods. Parameters for Determining Inoculated Pack/Challenge Study Protocols. J. Food Prot. 2010, 73, 140–203. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Karolenko, C.E.; Wilkinson, J.; Muriana, P.M. Acid Adaptation Leads to Sensitization of Salmonella Challenge Cultures During Processing of Air-Dried Beef (Biltong, Droëwors). Appl. Microbiol. 2025, 5, 106. [Google Scholar] [CrossRef]
- Breidt, F.J.; Andress, E.L.; Ingham, B. Recommendations for Designing and Conducting Cold-fill Hold Challenge Studies for Acidified Food Products. Food Prot. Trends 2018, 38, 322–328. [Google Scholar]
- USDA-FSIS. Availability of FSIS Ready-to-Eat Fermented, Salt-Cured, and Dried Products Guideline. Fed. Regist. 2023, 88, 29188–29189. [Google Scholar]
- USDA-FSIS. Safe and Suitable Ingredients Used in the Production of Meat, Poultry, and Egg Products; FSIS Directive 7120.1, rev. 44; U.S. Department of Agriculture, Food Safety and Inspection Service: Washington, DC, USA, 2018. [Google Scholar]
- Juneja, V.K.; Hwang, C.A.; Friedman, M. Thermal inactivation and postthermal treatment growth during storage of multiple Salmonella serotypes in ground beef as affected by sodium lactate and oregano oil. J. Food Sci. 2010, 75, M1–M6. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.E.; Smith, J.V.; Broadbent, J.R. Efficacy of washing meat surfaces with 2% levulinic, acetic, or lactic acid for pathogen decontamination and residual growth inhibition. Meat Sci. 2011, 88, 256–260. [Google Scholar] [CrossRef]
- Juneja, V.K.; Eblen, B.S.; Marks, H.M. Modeling non-linear survival curves to calculate thermal inactivation of Salmonella in poultry of different fat levels. Int. J. Food Microbiol. 2001, 70, 37–51. [Google Scholar] [CrossRef]
- Juneja, V.K.; Yadav, A.S.; Hwang, C.A.; Sheen, S.; Mukhopadhyay, S.; Friedman, M. Kinetics of thermal destruction of Salmonella in ground chicken containing trans-cinnamaldehyde and carvacrol. J. Food Prot. 2012, 75, 289–296. [Google Scholar] [CrossRef]
- Karolenko, C.E.; Bhusal, A.; Gautam, D.; Muriana, P.M. Selenite cystine agar for enumeration of inoculated Salmonella serovars recovered from stressful conditions during antimicrobial validation studies. Microorganisms 2020, 8, 338. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.D.; Cutter, C.N. Effects of acid adaptation of Escherichia coli O157:H7 on efficacy of acetic acid spray washes to decontaminate beef carcass tissue. Appl. Environ. Microbiol. 2000, 66, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- USDA-AMS. Institutional Meat Purchase Specifications; Fresh beef series 100; Agricultural Marketing Service: Washington, DC, USA, 2014; pp. 1–71. [Google Scholar]
- Karolenko, C.; Muriana, P.M. Quantification of process lethality (5-log reduction) of Salmonella and salt concentration during sodium replacement in biltong marinade. Foods 2020, 9, 1570. [Google Scholar] [CrossRef]
- West, R.M. Best practice in statistics: The use of log transformation. Ann. Clin. Biochem. 2022, 59, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Martos, P. Log Transformation and the Effect on Estimation, Implication, and Interpretation of Mean and Measurement Uncertainty in Microbial Enumeration. J. AOAC Int. 2018, 102, 233–238. [Google Scholar] [CrossRef]
- Lombard, B.; Cornu, M.; Lahellec, C.; Feinberg, M.H. Experimental Evaluation of Different Precision Criteria Applicable to Microbiological Counting Methods. J. AOAC Int. 2019, 88, 830–841. [Google Scholar] [CrossRef]
- Richardson, B.A.; Overbaugh, J. Basic Statistical Considerations in Virological Experiments. J. Virol. 2005, 79, 669–676. [Google Scholar] [CrossRef]
- Su, Y.; Liu, A.; Zhu, M.-J. Mapping the landscape of listeriosis outbreaks (1998–2023): Trends, challenges, and regulatory responses in the United States. Trends Food Sci. Technol. 2024, 154, 104750. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Mishra, S.; Tuglo, L.S.; Sarangi, A.K.; Kandi, V.; AL Ibrahim, A.A.; Alsaif, H.A.; Rabaan, A.A.; Zahan, M.K.-E. Recurring food source-based Listeria outbreaks in the United States: An unsolved puzzle of concern? Health Sci. Rep. 2024, 7, e1863. [Google Scholar] [CrossRef] [PubMed]
- Gedela, S.; Escoubas, J.R.; Muriana, P.M. Effect of inhibitory liquid smoke fractions on Listeria monocytogenes during long-term storage of frankfurters. J. Food Prot. 2007, 70, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Gedela, S.; Gamble, R.K.; Macwana, S.; Escoubas, J.R.; Muriana, P.M. Effect of inhibitory extracts derived from liquid smoke combined with postprocess pasteurization for control of Listeria monocytogenes on ready-to-eat meats. J. Food Prot. 2007, 70, 2749–2756. [Google Scholar] [CrossRef]
- Sperber, W.H. Influence of Water Activity on Foodborne Bacteria—A Review1. J. Food Prot. 1983, 46, 142–150. [Google Scholar] [CrossRef]
- Durham, T.R. Salt, Smoke, and History. Gastronomica 2001, 1, 78–82. [Google Scholar] [CrossRef]
- Simon, R.; de la Calle, B.; Palme, S.; Meier, D.; Anklam, E. Composition and analysis of liquid smoke flavouring primary products. J. Sep. Sci. 2005, 28, 871–882. [Google Scholar] [CrossRef]
- Montazeri, N.; Oliveira, A.C.M.; Himelbloom, B.H.; Leigh, M.B.; Crapo, C.A. Chemical characterization of commercial liquid smoke products. Food Sci. Nutr. 2013, 1, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Lingbeck, J.M.; Cordero, P.; O’Bryan, C.A.; Johnson, M.G.; Ricke, S.C.; Crandall, P.G. Functionality of liquid smoke as an all-natural antimicrobial in food preservation. Meat Sci. 2014, 97, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.M.; O’Bryan, C.A.; Lary, R.Y.; Griffis, C.L.; Vaughn, K.L.S.; Marcy, J.A.; Ricke, S.C.; Crandall, P.G. Spray application of liquid smoke to reduce or eliminate Listeria monocytogenes surface inoculated on frankfurters. Meat Sci. 2010, 85, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, N.; Himelbloom, B.H.; Oliveira, A.C.M.; Leigh, M.B.; Crapo, C.A. Refined Liquid Smoke: A Potential Antilisterial Additive to Cold-Smoked Sockeye Salmon (Oncorhynchus nerka). J. Food Prot. 2013, 76, 812–819. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adhikari, P.; Muriana, P.M. Process Validation of Air-Dried Beef Sticks (Droëwors) to Achieve >5-log Reduction of Salmonella Serovars, Listeria monocytogenes, and E. coli O157:H7 Using Refined Liquid Smoke Extracts. Appl. Microbiol. 2025, 5, 145. https://doi.org/10.3390/applmicrobiol5040145
Adhikari P, Muriana PM. Process Validation of Air-Dried Beef Sticks (Droëwors) to Achieve >5-log Reduction of Salmonella Serovars, Listeria monocytogenes, and E. coli O157:H7 Using Refined Liquid Smoke Extracts. Applied Microbiology. 2025; 5(4):145. https://doi.org/10.3390/applmicrobiol5040145
Chicago/Turabian StyleAdhikari, Pratikchhya, and Peter M. Muriana. 2025. "Process Validation of Air-Dried Beef Sticks (Droëwors) to Achieve >5-log Reduction of Salmonella Serovars, Listeria monocytogenes, and E. coli O157:H7 Using Refined Liquid Smoke Extracts" Applied Microbiology 5, no. 4: 145. https://doi.org/10.3390/applmicrobiol5040145
APA StyleAdhikari, P., & Muriana, P. M. (2025). Process Validation of Air-Dried Beef Sticks (Droëwors) to Achieve >5-log Reduction of Salmonella Serovars, Listeria monocytogenes, and E. coli O157:H7 Using Refined Liquid Smoke Extracts. Applied Microbiology, 5(4), 145. https://doi.org/10.3390/applmicrobiol5040145






