A Novel Fungicide Consortium: Is It Better for Wheat Production and How Does It Affect the Rhizosphere Microbiome?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Fungicides Used in the Experiment
2.3. Experimental Setup
2.4. Wheat Sampling and Analyses
2.5. Rhizosphere Soil Sampling
2.6. DNA Extraction, Purification and Sequencing
2.7. Bioinformatic Analysis
2.8. Statistical Analysis
3. Results
3.1. Wheat Yield, Grain Quality and Development Parameters as Related to the Treatment with Different Fungicides
3.2. Wheat Yield, Grain Quality and Development Parameters as Related to the Different Modes of Application of the Novel Fungicide
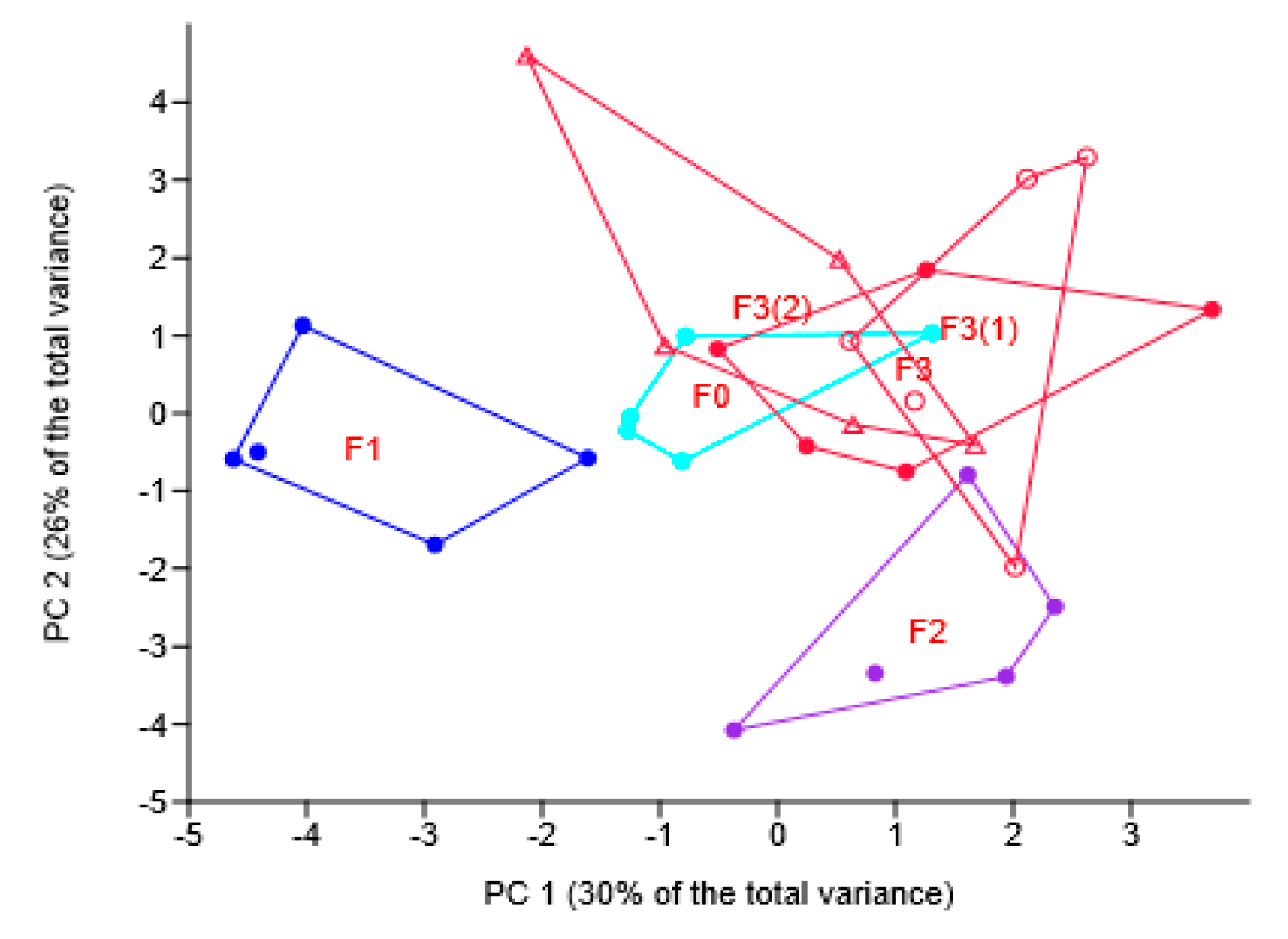
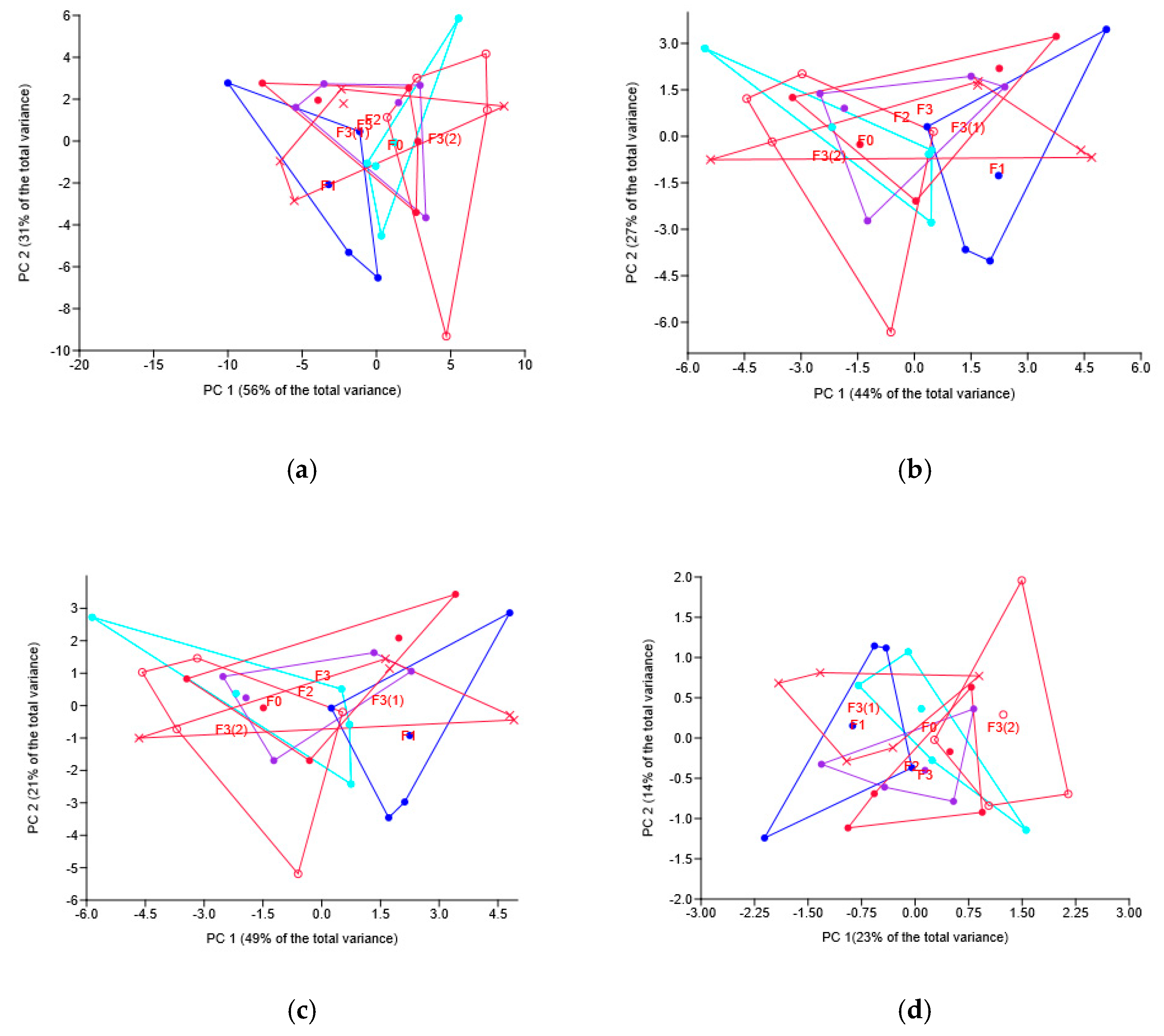
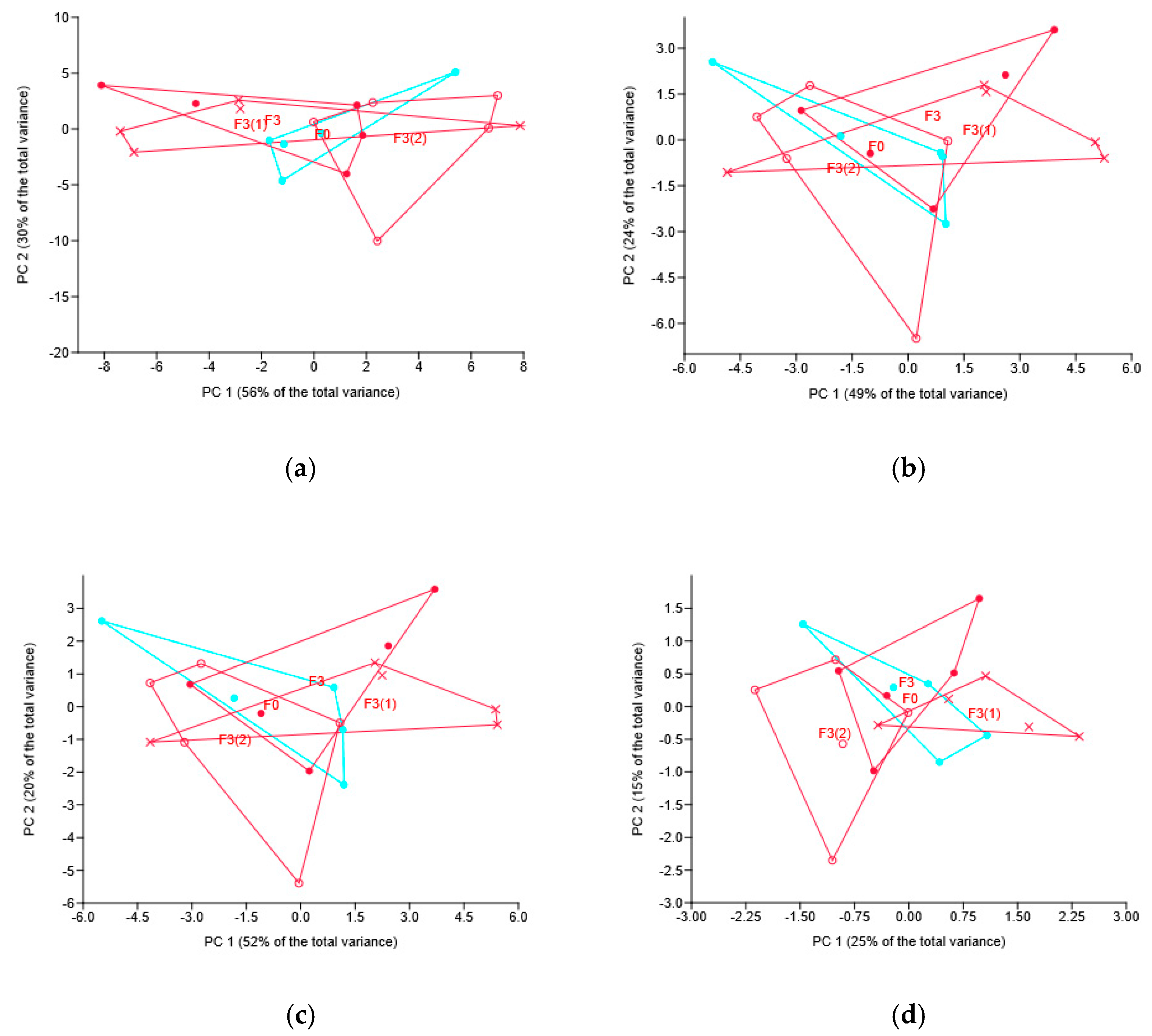
3.3. Soil Mycobiome in Wheat Rhizosphere
3.3.1. Soil Mycobiome as Related to Different Fungicides
3.3.2. Soil Mycobiome as Related to the Different Modes of the Novel Fungicide Application
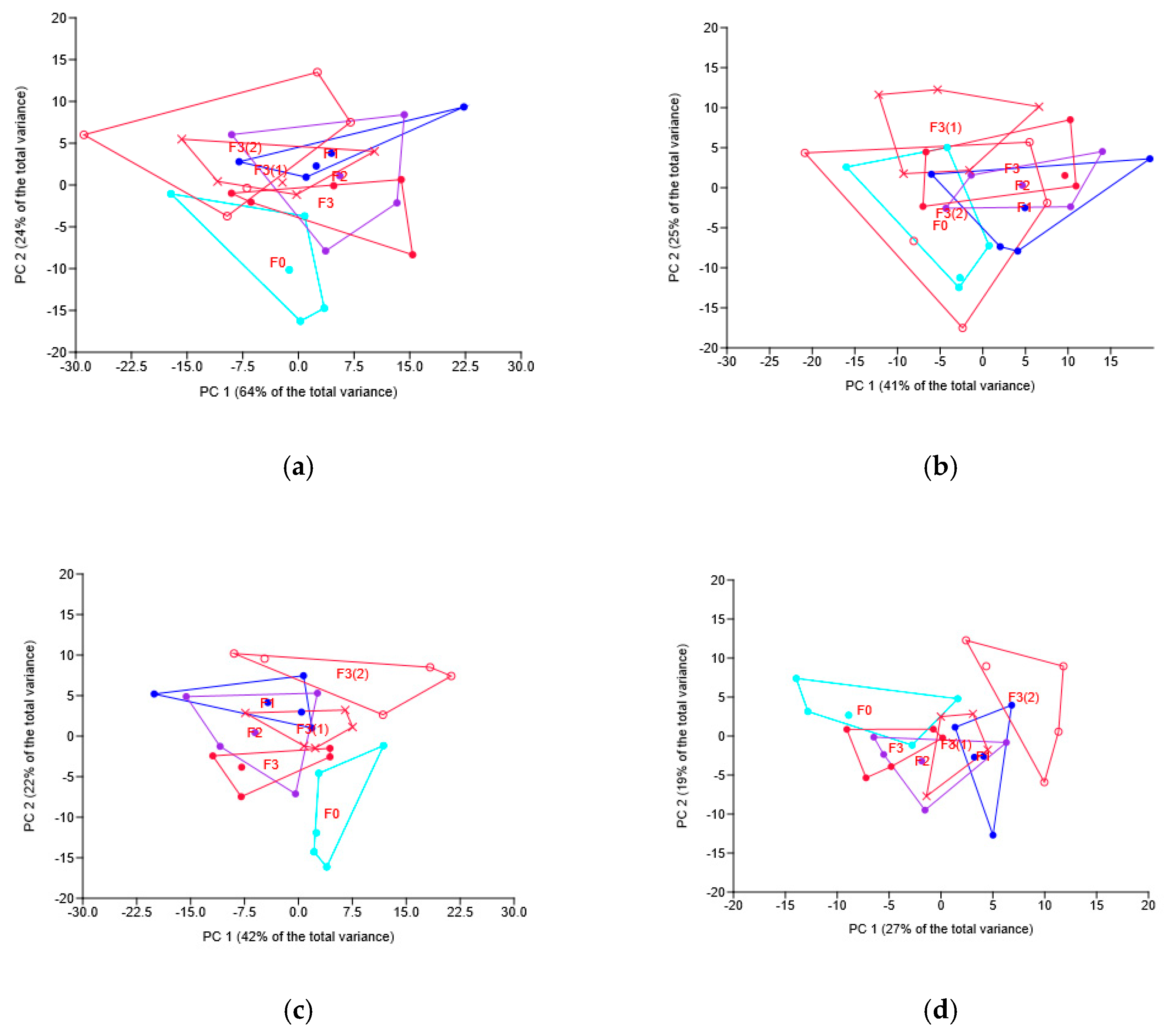
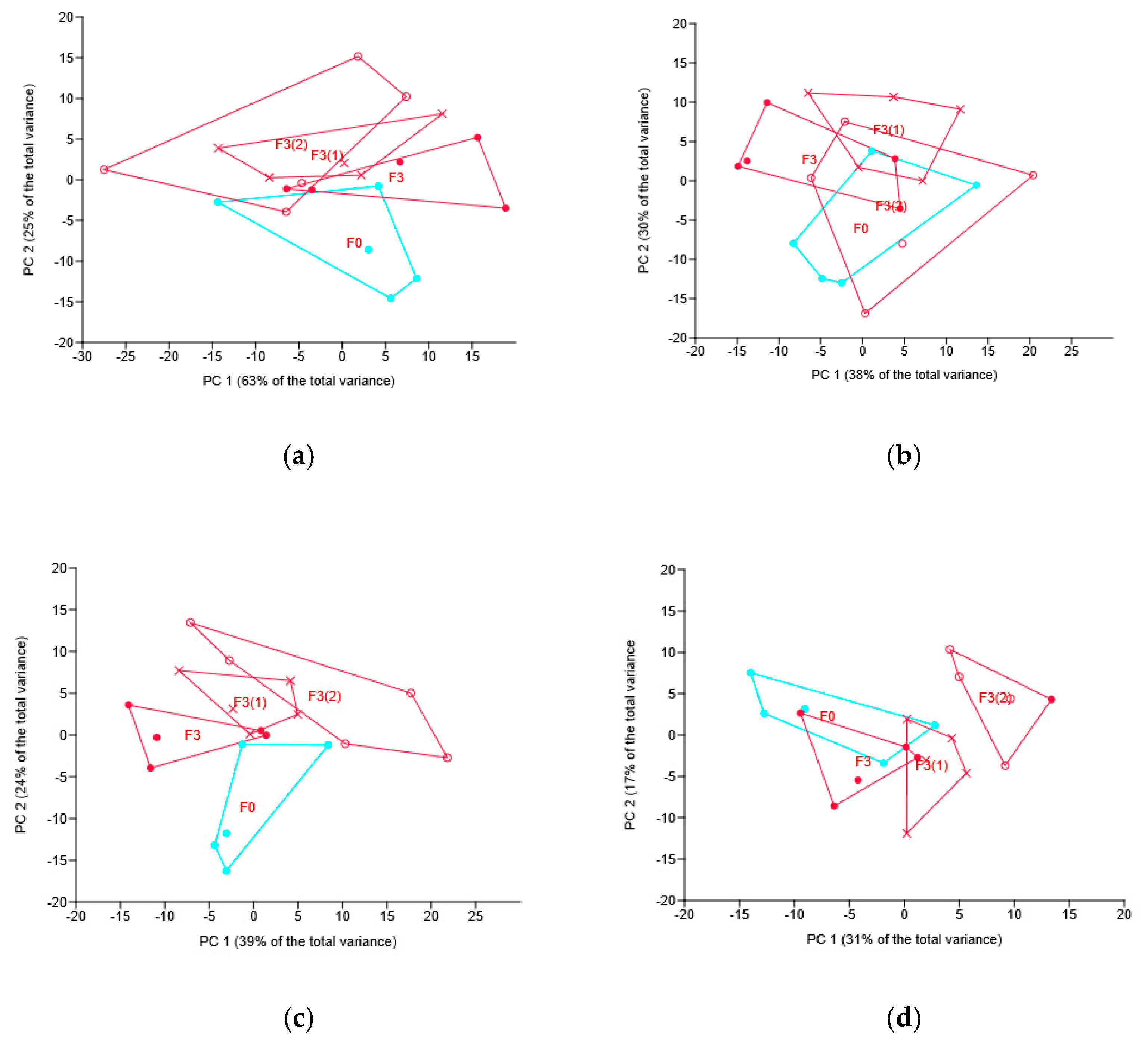
3.4. Soil Bacteriobiome in Wheat Rhizosphere
3.4.1. Soil Bacteriobiome as Related to Different Fungicides
3.4.2. Soil Bacteriobiome as Related to the Different Modes of the Novel Fungicide Application
4. Discussion
4.1. Wheat Yield, Grain Quality and Development Parameters as Related to the Treatment with Different Fungicides
4.2. Wheat Yield, Grain Quality and Development Parameters as Related to the Different Modes of Application of the Novel Fungicide
4.3. Soil Microbiome in Wheat Rhizosphere Soil
4.3.1. Soil Mycobiome
4.3.2. Soil Bacteriobiome
4.3.3. General Considerations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skolotneva, E.S.; Leonova, I.N.; Bukatich, E.Y.; Salina, I.A. Methodical approaches to identification of effective wheat genes providing broad-spectrum resistance against fungal diseases. Vavilov J. Genet. Breeding 2017, 21, 862–869. (In Russian) [Google Scholar] [CrossRef]
- Orina, A.S.; Gavrilova, O.P.; Gagkaeva, T.Y.; Gogina, N.N. Contamination of grain in West Siberia by Alternaria fungi and their mycotoxins. Plant Prot. News 2021, 4, 153–162. [Google Scholar] [CrossRef]
- Lipps, S.; Bohn, M.; Rutkoski, J.; Butts-Wilmsmeyer, C.; Mideros, S.; Jamann, T. Comparative Review of Fusarium graminearum Infection in Maize and Wheat: Similarities in Resistance Mechanisms and Future Directions. Molecular plant-microbe interactions. Mol. Plant Microbe Interact. 2025, 38, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Asyakina, L.K.; Serazetdinova, Y.R.; Frolova, A.S.; Fotina, N.V.; Neverova, O.A.; Petrov, A.N. Antagonistic activity of extremophilic bacteria against phytopathogens in agricultural crops. Food Process. Tech. Technol. 2023, 53, 565–575. [Google Scholar] [CrossRef]
- The Russian Agricultural Centre for the Kemerovo Region. The survey of the Phytosanitary Status of Agricultural Crops in the Kemerovo Region in 2024 and the Forecast for the Development of Hazardous Objects in 2025. 2025. Available online: https://clck.ru/3PrCVP (accessed on 20 October 2025). (In Russian).
- Fotina, N.V.; Serazetdinova, Y.R.; Kolpakova, D.E.; Asyakina, L.K.; Atuchin, V.V.; Alotaibi, K.M.; Mudgal, G.; Pro-sekov, A.Y. Enhancement of wheat growth by plant growth-stimulating bacteria during phytopathogenic inhibition. Biocatal. Agric. Biotechnol. 2024, 60, 103294. [Google Scholar] [CrossRef]
- Rahman, S.F.S.A.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 262, 102–111. [Google Scholar] [CrossRef]
- Tao, Y.; Jia, C.; Jing, J.; Zhang, J.; Yu, P.; He, M.; Wu, J.; Chen, L.; Zhao, E. Occurrence and dietary risk assessment of 37 pesticides in wheat fields in the suburbs of Beijing, China. Food Chem. 2021, 350, 129245. [Google Scholar] [CrossRef]
- Lu, C.; Yang, Z.; Liu, J.; Liao, Q.; Ling, W.; Waigi, M.G.; Odinga, E.S. Chlorpyrifos inhibits nitrogen fixation in rice-vegetated soil containing Pseudomonas stutzeri A1501. Chemosphere 2020, 256, 127098. [Google Scholar] [CrossRef]
- Serazetdinova, Y.; Chekushkina, D.; Borodina, E.; Kolpakova, D.; Minina, V.; Altshuler, O.; Asyakina, L. Synergistic interaction between Azotobacter and Pseudomonas bacteria in a growth-stimulating consortium. Foods Raw Mater. 2025, 13, 376–393. [Google Scholar] [CrossRef]
- Nysanth, N.S.; Divya, S.; Nair, C.B.; Anju, A.B.; Praveena, R.; Anith, K.N. Biological control of foot rot (Phytophthora capsici Leonian) disease in black pepper (Piper nigrum L.) with rhizospheric microorganisms. Rhizosphere 2022, 23, 100578. [Google Scholar] [CrossRef]
- Yusof, N.H.R.; Yusup, S.; Kueh, B.W. B Effectiveness of biopesticides in enhancing paddy growth for yield improvement. Sustain. Chem. Pharm. 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Santos, A.P.; Muratore, L.N.; Solé-Gil, A.; Farías, M.E.; Ferrando, A.; Blázquez, M.A.; Belfiore, C. Extremophilic bacteria restrict the growth of Macrophomina phaseolina by combined secretion of polyamines and lytic enzymes. Biotechnol. Rep. 2021, 32, e00674. [Google Scholar] [CrossRef] [PubMed]
- Asyakina, L.K.; Vorob’eva, E.E.; Proskuryakova, L.A.; Zharko, M.Y. Evaluating extremophilic microorganisms in industrial regions. Foods Raw Mater. 2023, 11, 162–171. [Google Scholar] [CrossRef]
- Asyakina, L.K.; Isachkova, O.A.; Kolpakova, D.E.; Borodina, E.E.; Boger, V.Y.; Prosekov, A.Y. The effect of a microbial consortium on spring barley growth and development in the Kemerovo region, Kuzbass. Grain Econ. Russ. 2024, 16, 104–112. (In Russian) [Google Scholar] [CrossRef]
- Hydromentcenter of Russia. Available online: https://meteoinfo.ru/climatcities?p=1935 (accessed on 26 October 2025). (In Russian).
- Fotina, N.V. The Search, Study of Properties and Practical Application of Rhizobacteria in Regulating the Biotic Stress of Wheat: Specialty 4.3. Ph.D. Thesis, Kemerovo State University, Kemerovo, Russia, 2025; 168p. (In Russian). [Google Scholar]
- Serazetdinova, Y.R.; Borodina, E.E.; Fotina, N.V.; Naik, A.; Mudgal, G.; Asyakina, L.K. Rhizobia as complex biofertilizers for wheat: Biological nitrogen fixation and plant growth promotion. Foods Raw Mater. 2026, 14, 214–227. [Google Scholar] [CrossRef]
- Rosstandart. Agricultural Seeds. Methods for Determination of Disease Infestation: GOST 12044-93; Federal Agency on Technical Regulating and Metrology: Moscow, Russia, 1995; 59p. [Google Scholar]
- Barillot, C.D.C.; Sarde, C.-O.; Bert, V.; Tarnaud, E.; Cochet, N. A standardized method for the sampling of rhizosphere and rhizoplane soil bacteria associated to a herbaceous root system. Ann. Microbiol. 2013, 63, 471–476. [Google Scholar] [CrossRef]
- Herlemann, D.P.; Labrenz, M.; Jurgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef]
- Toju, H.; Tanabe, A.S.; Yamamoto, S.; Sato, H. High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS ONE 2012, 7, e40863. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 4 November 2025).
- Hughes, J.B.; Hellmann, J.J. The Application of Rarefaction Techniques to Molecular Inventories of Microbial Diversity. Methods Enzymol. 2005, 397, 292–308. [Google Scholar] [CrossRef]
- Minutillo, S.A.; Ruano-Rosa, D.; Abdelfattah, A.; Schena, L.; Malacrinò, A. The Fungal Microbiome of Wheat Flour Includes Potential Mycotoxin Producers. Foods 2022, 25, 676. [Google Scholar] [CrossRef]
- Kriuchkova, L.O. Micromycetes associated with wheat diseases in different regions of Ukraine. Mikrobiol. Zhurnal 2013, 75, 59–68. [Google Scholar] [PubMed]
- Naumova, N.B.; Belanov, I.P.; Alikina, T.Y.; Kabilov, M.R. Undisturbed Soil Pedon under Birch Forest: Characterization of Microbiome in Genetic Horizons. Soil. Syst. 2021, 5, 14. [Google Scholar] [CrossRef]
- Ji, C.; Huang, J.; Yu, H.; Tian, Y.; Rao, X.; Zhang, X. Do the Reclaimed Fungal Communities Succeed Toward the Original Structure in Eco-Fragile Regions of Coal Mining Disturbances? A Case Study in North China Loess-Aeolian Sand Area. Front. Microbiol. 2022, 13, 770715. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Yang, P.; Zhao, Y.; Fang, J.; Yang, T.; Yang, R. Isolation and identification of Alternaria alstroemeriae causing postharvest black rot in citrus and its control using curcumin-loaded nanoliposomes. Front. Microbiol. 2025, 16, 1555774. [Google Scholar] [CrossRef]
- Skiada, A.; Pavleas, I.; Drogari-Apiranthitou, M. Epidemiological Trends of Mucormycosis in Europe, Comparison with Other Continents. Mycopathologia 2024, 189, 100. [Google Scholar] [CrossRef] [PubMed]
- Moghdam, Y.; Arghavan, B.; Kermani, F.; Jeddi, S.A.; Khojasteh, S.; Shokohi, T.; Aslani, N.; Ebrahimi, A.; Javidnia, J. A fatal post-COVID-19 sino-orbital mucormycosis in an adult patient with diabetes mellitus: A case report and review of the literature. J. Infect. Dev. Ctries. 2025, 19, 661–668. [Google Scholar] [CrossRef]
- Benbrik, B.; Reid, T.E.; Nkir, D.; Chaouki, H.; Aallam, Y.; Clark, I.M.; Mauchline, T.H.; Harris, J.; Pawlett, M.; Barakat, A.; et al. Unlocking the agro-physiological potential of wheat rhizoplane fungi under low P conditions using a niche-conserved consortium approach. J. Exp. Bot. 2025, 76, 2320–2337. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.S.; Fathey, H.A.; Mohamed, A.H.; Ibrahim, A.A.; Abdel-Haleem, M. Mitigating drought stress and enhancing maize resistance through biopriming with Rhizopus arrhizus: Insights into Morpho-Biochemical and molecular adjustments. BMC Plant Biol. 2025, 25, 779. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Zhang, H.; Jia, H.; Zhao, Y.; Li, J.; Feng, X.; Tang, B.; Zhao, B.; Liu, Y. Residual Dynamics of Chlorantraniliprole and Fludioxonil in Soil and Their Effects on the Microbiome. J. Xenobiot. 2025, 15, 4. [Google Scholar] [CrossRef]
- Ali, A.A.; Alfalahi, A.O.; Hassan, A.K.; Khalofah, A.; Mena, E.; Dababat, A.A.; Mokrini, F. Investigating the efficacy of bioactive compounds from selected plant extracts against Gibberella fujikuroi species complex associated with damping off disease in sweet corn. Sci. Rep. 2025, 15, 21712. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella Species as the Plant Growth-Promoting Fungi Present in the Agricultural Soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Naumova, N.; Barsukov, P.; Baturina, O.; Rusalimova, O.; Kabilov, M. West-Siberian Chernozem: How Vegetation and Tillage Shape Its Bacteriobiome. Microorganisms 2023, 11, 2431. [Google Scholar] [CrossRef]
- Domnariu, H.; Trippe, K.M.; Botez, F.; Partal, E.; Postolache, C. Long-term impact of tillage on microbial communities of an Eastern European Chernozem. Sci. Rep. 2025, 15, 642. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Response of wheat barley during germination to seed osmopriming at different water potential. J. Agron. Crop Sci. 1998, 181, 229–235. [Google Scholar] [CrossRef]
- Mim, T.F.; Anwar, P.; Ahmed, M.; Sriti, N.; Moni, E.H.; Hasan, A.K.; Yeasmin, S. Competence of different priming agents for increasing seed germination, seedling growth and vigor of wheat. Fund. Appl. Agric. 2021, 6, 444–445. [Google Scholar] [CrossRef]
- Khaeim, H.; Kende, Z.; Jolánkai, M.; Kovács, G.P.; Gyuricza, C.; Tarnawa, Á. Impact of Temperature and Water on Seed Germination and Seedling Growth of Maize (Zea mays L.). Agronomy 2022, 12, 397. [Google Scholar] [CrossRef]
- Li, D.; Kang, X.; Chu, L.; Wang, Y.; Song, X.; Zhao, X.; Cao, X. Algicidal mechanism of Raoultella ornithinolytica against Microcystis aeruginosa: Antioxidant response, photosynthetic system damage and microcystin degradation. Environ. Pollut. 2021, 287, 117644. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.I.; Castro, P.M. Diversity and characterization of culturable bacterial endophytes from Zea mays and their potential as plant growth-promoting agents in metal-degraded soils. Environ. Sci. Pollut. Res. Int. 2014, 21, 14110–14123. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.M.H.; Vilas-Boas, Â.; Sousa, C.A.; Soares, H.M.V.M.; Soares, E.V. Comparison of five bacterial strains producing siderophores with ability to chelate iron under alkaline conditions. AMB Express 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Shin, G.Y.; Stice, S.; Bown, J.L.; Coutinho, T.; Metcalf, W.W.; Gitaitis, R.; Kvitko, B.; Dutta, B. A Novel Biosynthetic Gene Cluster Across the Pantoea Species Complex Is Important for Pathogenicity in Onion. Mol. Plant Microbe Interact. 2023, 36, 176–188. [Google Scholar] [CrossRef] [PubMed]
| Indicators | Raoultella ornithinolytica | Pantoea allii_1 | Pantoea allii_2 | Novel Fungicide |
|---|---|---|---|---|
| Indolyl-3-acetic acid 1, mg/mL | 8.64 | 8.59 | 9.12 | 10.2 |
| Gibberellic acid, μg/mL | 368 | 346 | 258 | 842 |
| Antagonistic activity against Fusarium graminearum F-877, mm | 76 | 72 | 90 | 91 |
| Antagonistic activity against Bipolaris sorokiniana F-529, mm | 23 | 20 | 95 | 95 |
| Antagonistic activity against Botrytis cinerea F-1006, mm | 68 | 45 | 73 | 82 |
| Parameter | F0 | F1 | F2 | F3 | F3(1) | F3(2) |
|---|---|---|---|---|---|---|
| Yield, t/ha | 1.70 a 1 | 1.68 a | 2.05 b | 2.24 b | 2.15 b | 1.99 b |
| Grain density, g/L | 717 | 734 | 709 | 721 | 717 | 728 |
| Grain quality characteristics | ||||||
| Protein, % | 15.9 a | 15.5 a | 17.3 b | 15.8 a | 15.9 a | 15.6 a |
| Water, % | 9.5 a | 9.5 a | 10.1 b | 9.9 ab | 9.9 ab | 9.6 ab |
| Gluten, | 41.0 b | 38.2 a | 42.5 c | 40.4 b | 40.9 b | 40.0 ab |
| Ash, % | 1.84 b | 1.73 a | 1.82 b | 1.80 ab | 1.81 ab | 1.81 ab |
| Fiber, % | 2.32 ab | 2.24 a | 2.35 b | 2.29 ab | 2.33 ab | 2.32 ab |
| Starch, % | 54.1 bc | 55.3 c | 51.7 a | 53.2 ab | 53.4 ab | 54.6 b |
| Wheat growth and development characteristics | ||||||
| Number of plants per 1 m2 | 453 b | 323 a | 391 ab | 520 c | 563 c | 515 c |
| Number of stems per 1 m2 | 476 bc | 335 a | 404 ab | 539 c | 566 c | 526 c |
| Number of stems with ears, per 1 m2 | 428 b | 294 a | 368 ab | 477 c | 505 c | 477 c |
| Total phytomass, g/m2 | 475 ab | 415 a | 537 bc | 611 bc | 645 c | 542 bc |
| Plant height, cm | 65 a | 69 ab | 71 b | 68 ab | 69 ab | 68 ab |
| Ear length, cm | 6.3 a | 6.4 ab | 6.8 b | 6.5 ab | 6.4 ab | 6.2 a |
| Number of grains in an ear, pcs. | 19 a | 20 ab | 22 b | 21 ab | 18 a | 19 a |
| Taxon | Treatments | |||||
|---|---|---|---|---|---|---|
| F0 | F1 | F2 | F3 | F3(1) | F3(2) | |
| Phyla | ||||||
| Ascomycota | 50.9 ab 1 | 49.3 ab | 47.4 a | 47.9 a | 54.1 ab | 59.4 b |
| Zygomycota | 12.6 a | 24.2 b | 23.2 b | 20.3 ab | 17.3 ab | 18.5 ab |
| Mucoromycota | 18.3 b | 9.1 a | 11.2 a | 13.6 ab | 8.7 a | 8.2 a |
| Basidiomycota | 7.5 bc | 4.1 a | 5.2 a | 5.4 ab | 9.3 c | 5.3 ab |
| Chytridiomycota | 2.4 ab | 2.3 ab | 2.5 ab | 3.1 b | 1.9 a | 2.8 ab |
| un. 2 Fungi | 7.7 ab | 10.0 b | 8.7 ab | 9.0 ab | 8.0 ab | 5.4 a |
| Classes | ||||||
| Sordariomycetes | 27.1 b | 29.6 bc | 26.2 ab | 20.6 a | 22.3 a | 33.7 c |
| Mucoromycotina_is 3 | 12.5 a | 24.0 b | 23.1 b | 20.3 ab | 17.2 ab | 18.5 ab |
| Dothideomycetes | 17.0 ab | 12.7 a | 14.8 a | 15.8 a | 23.9 c | 18.4 abc |
| Mucoromycetes | 18.3 b | 9.1 a | 11.2 a | 13.6 ab | 8.7 a | 8.2 a |
| Agaricomycetes | 4.6 b | 1.5 a | 1.3 a | 2.0 ab | 5.4 b | 2.0 a |
| Leotiomycetes | 2.4 a | 4.0 ab | 3.9 ab | 4.8 b | 3.6 ab | 3.4 ab |
| Eurotiomycetes | 2.2 4 | 1.4 | 1.8 | 4.6 | 2.2 | 1.4 |
| Tremellomycetes | 1.4 a | 1.2 a | 1.9 ab | 1.9 ab | 2.3 b | 2.1 b |
| un. Ascomycota | 1.6 b | 1.1 a | 1.1 a | 1.5 ab | 1.4 a | 2.1 b |
| Spizellomycetes | 1.1 b | 0.9 ab | 0.5 a | 0.9 ab | 0.7 a | 1.3 b |
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Taxon | F0 | F1 | F2 | F3 | F3(1) | F3(2) |
| Families | ||||||
| Mortierellaceae | 12.2 a 1 | 24.2 b | 24.0 b | 20.4 ab | 16.7 ab | 16.3 ab |
| Rhizopodaceae | 18.1 b | 8.9 a | 11.0 a | 13.3 ab | 8.6 a | 8.0 a |
| Nectriaceae | 10.9 b | 15.2 b | 10.9 ab | 8.8 a | 12.0 ab | 23.6 c |
| Pleosporaceae | 7.1 a | 5.9 a | 6.6 a | 7.6 a | 12.9 b | 8.4 a |
| Hypocreales_is 3 | 6.8 b | 2.3 a | 2.1 a | 1.4 a | 2.0 a | 2.2 a |
| un. 2 Pleosporales | 4.8 b | 2.5 ab | 1.8 a | 2.6 ab | 4.3 ab | 3.3 ab |
| Helotiales_is | 1.5 a | 2.8 b | 2.6 ab | 3.5 b | 2.7 b | 1.4 a |
| Chaetomiaceae | 1.8 a | 3.2 b | 2.5 ab | 1.8 a | 1.0 a | 1.5 a |
| Trichocomaceae | 1.5 4 | 0.7 | 1.0 | 4.0 | 1.5 | 0.9 |
| un. Hypocreales | 1.0 a | 1.9 b | 2.0 b | 1.9 b | 1.5 ab | 1.6 ab |
| Hypocreaceae | 1.1 ab | 1.5 ab | 1.2 ab | 1.7 b | 1.2 ab | 0.9 a |
| Mycosphaerellaceae | 1.1 | 0.5 | 0.9 | 0.4 | 1.4 | 2.3 |
| un. Ascomycota | 1.6 ab | 1.1 a | 1.1 a | 1.5 ab | 1.4 a | 2.1 b |
| Sordariomycetes_is | 1.3 b | 0.7 ab | 1.0 ab | 1.3 ab | 0.5 ab | 0.3 a |
| Lasiosphaeriaceae | 0.9 ab | 1.2 b | 1.2 ab | 0.7 a | 1.0 ab | 0.7 a |
| un. Agaricales | 2.0 | 0.0 | 0.0 | 0.1 | 0.9 | 0.0 |
| Lophiostomataceae | 0.0 a | 0.2 a | 1.7 b | 0.7 ab | 1.0 ab | 0.3 a |
| OTUs | ||||||
| Rhizopus arrhizus | 18.1 b | 8.9 a | 11.0 a | 13.3 ab | 8.6 a | 8.0 a |
| Gibberella sp. | 7.1 a | 10.0 a | 7.1 a | 5.8 a | 8.8 a | 17.1 b |
| Mortierella sp. | 5.1 a | 13.1 b | 6.4 a | 5.7 a | 5.9 a | 9.8 ab |
| Alternaria sp. | 4.2 bc | 2.9 ab | 2.1 a | 2.8 ab | 5.8 c | 4.1 bc |
| Mortierella elongata | 2.6 a | 3.7 ab | 7.3 b | 4.5 ab | 5.3 ab | 1.9 a |
| Stachybotrys bisbyi | 3.6 b | 1.4 a | 0.9 a | 0.4 a | 0.7 a | 1.0 a |
| un. Pleosporales | 3.1 b | 1.2 a | 0.7 a | 1.4 a | 1.9 ab | 0.5 a |
| Talaromyces sp. | 1.1 | 0.3 | 0.4 | 2.7 | 0.9 | 0.3 |
| un. Pleosporaceae | 0.1 a | 0.8 ab | 1.8 bc | 2.7 c | 1.6 bc | 2.0 bc |
| Chaetomium sp. | 1.6 a | 2.9 c | 2.2 b | 1.5 ab | 0.8 a | 1.0 a |
| Edenia gomezpompae | 1.7 a | 1.1 a | 1.7 a | 1.2 a | 3.1 b | 1.3 a |
| un. Hypocreales | 0.6 a | 1.3 b | 1.6 b | 1.6 b | 1.3 ab | 1.0 ab |
| Mortierella hyalina | 0.7 a | 3.1 b | 1.9 ab | 1.8 ab | 1.5 ab | 1.4 ab |
| Myrmecridium schulzeri | 1.3 b | 0.7 ab | 1.0 ab | 1.3 b | 1.0 ab | 0.6 a |
| Tetracladium sp_WMM_2012d | 0.4 a | 1.6 b | 1.3 b | 1.8 b | 1.7 b | 0.5 a |
| Davidiella sp. | 1.7 | 1.3 | 1.2 | 1.1 | 1.4 | 2.3 |
| Myrothecium verrucaria | 2.3 | 0.6 | 0.8 | 0.7 | 0.5 | 0.5 |
| Fusarium oxysporum | 0.9 ab | 1.3 c | 0.9 abc | 0.6 a | 0.5 a | 1.1 bc |
| Mortierella alpina | 0.9 a | 1.3 a | 3.0 b | 1.3 a | 1.2 a | 0.7 a |
| Mortierella alpina | 1.9 | 1.5 | 2.5 | 3.1 | 1.1 | 1.7 |
| Gibberella sp. | 1.0 abc | 1.4 c | 0.7 a | 0.7 a | 0.8 ab | 1.2 bc |
| Mortierella_elongata | 0.2 a | 0.3 a | 0.6 ab | 2.9 b | 0.7 ab | 0.2 a |
| Treatments | ||||||
|---|---|---|---|---|---|---|
| Taxon | F0 | F1 | F2 | F3 | F3(1) | F3(2) |
| Phyla | ||||||
| Pseudomonadota | 27.5 a 1 | 25.8 a | 27.4 a | 26.7 a | 26.9 a | 30.7 b |
| Acidobacteriota | 21.9 2 | 24.3 | 23.2 | 23.3 | 24.3 | 20.4 |
| Bacteroidota | 13.5 b | 9.5 a | 12.9 b | 12.5 b | 11.7 b | 14.0 b |
| Actinomycetota | 12.3 | 12.5 | 11.0 | 10.9 | 11.3 | 12.0 |
| Verrucomicrobiota | 6.5 b | 7.1 b | 6.4 b | 7.0 b | 7.3 b | 4.9 a |
| Gemmatimonadota | 3.4 | 3.5 | 3.4 | 3.3 | 3.1 | 3.6 |
| Bacillota | 1.9 | 2.4 | 2.1 | 2.2 | 2.1 | 2.2 |
| Chloroflexota | 1.6 ab | 2.2 b | 1.6 ab | 1.7 ab | 1.4 a | 1.2 a |
| Planctomycetota | 1.3 | 1.3 | 1.0 | 1.3 | 1.1 | 1.0 |
| un. 3 Bacteria | 6.8 a | 7.9 b | 7.1 a | 6.7 a | 6.8 a | 5.9 a |
| Classes | ||||||
| Alphaproteobacteria | 16.7 a | 15.7 a | 16.3 a | 16.7 a | 15.7 a | 19.2 b |
| Chitinophagia | 10.0 b | 7.2 a | 9.7 b | 9.4 ab | 8.7 ab | 10.0 b |
| Acidobacteria_Gp6 | 7.1 a | 8.2 a | 7.7 a | 8.0 a | 9.7 b | 6.7 a |
| Actinobacteria | 6.8 | 6.2 | 5.5 | 5.7 | 5.7 | 6.1 |
| Spartobacteria | 5.3 | 5.8 | 5.3 | 5.8 | 5.9 | 4.0 |
| Betaproteobacteria | 5.1 ab | 4.6 a | 4.9 ab | 4.6 a | 4.9 ab | 5.6 b |
| Acidobacteria_Gp4 | 3.9 a | 5.3 b | 4.6 b | 4.1 ab | 4.1 ab | 3.2 a |
| Thermoleophilia | 3.6 | 4.3 | 3.7 | 3.6 | 3.9 | 4.2 |
| Blastocatellia | 3.6 | 3.5 | 4.0 | 4.6 | 3.5 | 3.8 |
| Gammaproteobacteria | 3.1 | 3.0 | 3.5 | 3.1 | 3.5 | 3.4 |
| Gemmatimonadia | 2.8 | 2.8 | 2.9 | 2.8 | 2.6 | 3.1 |
| Acidobacteria_Gp3 | 3.1 | 2.5 | 2.7 | 2.5 | 2.8 | 2.7 |
| Bacilli | 1.7 | 2.0 | 1.9 | 2.0 | 1.9 | 1.9 |
| Deltaproteobacteria | 1.8 ab | 1.7 ab | 1.7 ab | 1.5 a | 2.0 b | 1.8 ab |
| Acidobacteria_Gp16 | 1.3 | 1.6 | 1.4 | 1.4 | 1.5 | 1.3 |
| Phycisphaerae | 1.2 | 1.2 | 0.9 | 1.2 | 1.1 | 0.9 |
| Cytophagia | 1.1 b | 0.6 a | 0.9 ab | 1.0 ab | 1.1 b | 1.1 b |
| Treatments | ||||||
|---|---|---|---|---|---|---|
| Taxon | F0 | F1 | F2 | F3 | F3(1) | F3(2) |
| Families | ||||||
| Chitinophagaceae | 10.0 b 1 | 7.2 a | 9.7 b | 9.4 ab | 8.7 ab | 10.0 b |
| un. 2 Acidobacteria_Gp6 | 7.1 a | 8.2 ab | 7.6 ab | 8.0 ab | 9.6 b | 6.7 a |
| Spartobacteria_is 3 | 5.2 ab | 5.7 ab | 5.2 ab | 5.7 ab | 5.8 b | 3.9 a |
| Sphingomonadaceae | 4.5 ab | 4.1 a | 4.9 ab | 5.1 b | 3.6 a | 5.5 b |
| un. Acidobacteria_Gp4 | 3.3 a | 4.7 c | 4.0 bc | 3.6 ab | 3.5 ab | 2.7 a |
| Blastocatellaceae | 3.3 | 3.2 | 3.7 | 4.3 | 3.1 | 3.5 |
| un. Acidobacteria_Gp3 | 3.1 | 2.5 | 2.7 | 2.5 | 2.8 | 2.7 |
| Gemmatimonadaceae | 2.8 | 2.8 | 2.9 | 2.8 | 2.6 | 3.1 |
| un. Hyphomicrobiales | 2.7 | 3.0 | 2.9 | 2.7 | 3.0 | 3.0 |
| Gaiellaceae | 2.4 | 2.8 | 2.4 | 2.3 | 2.5 | 2.8 |
| Bradyrhizobiaceae | 2.3 ab | 2.3 ab | 2.3 ab | 2.1 a | 2.3 ab | 2.7 b |
| un. Betaproteobacteria | 2.0 | 1.8 | 1.9 | 1.6 | 1.8 | 1.8 |
| OTUs | ||||||
| Bradyrhizobium sp. | 2.1 ab | 2.1 ab | 2.2 ab | 2.0 a | 2.1 ab | 2.5 b |
| Sphingomonas sp. | 2.9 b | 2.8 ab | 3.3 bc | 3.5 c | 2.1 a | 3.6 c |
| Pseudarthrobacter sp. | 1.8 b | 1.6 ab | 1.2 a | 1.5 ab | 1.2 a | 1.3 ab |
| un. Acidobacteria_Gp4 | 2.1 a | 3.0 b | 2.6 b | 2.2 ab | 2.1 a | 1.7 a |
| Stenotrophobacter sp. | 0.8 | 0.7 | 0.9 | 1.0 | 0.7 | 0.9 |
| un. Spartobacteria_gis | 1.4 ab | 1.7 ab | 1.5 ab | 1.7 ab | 1.9 b | 1.1 a |
| Hyphomicrobiales | 1.2 | 1.3 | 1.2 | 1.1 | 1.3 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asyakina, L.; Barsukov, P.; Serazetdinova, Y.; Baturina, O.; Fotina, N.; Prosekov, A.; Kabilov, M. A Novel Fungicide Consortium: Is It Better for Wheat Production and How Does It Affect the Rhizosphere Microbiome? Appl. Microbiol. 2025, 5, 142. https://doi.org/10.3390/applmicrobiol5040142
Asyakina L, Barsukov P, Serazetdinova Y, Baturina O, Fotina N, Prosekov A, Kabilov M. A Novel Fungicide Consortium: Is It Better for Wheat Production and How Does It Affect the Rhizosphere Microbiome? Applied Microbiology. 2025; 5(4):142. https://doi.org/10.3390/applmicrobiol5040142
Chicago/Turabian StyleAsyakina, Lyudmila, Pavel Barsukov, Yuliya Serazetdinova, Olga Baturina, Natalya Fotina, Alexander Prosekov, and Marsel Kabilov. 2025. "A Novel Fungicide Consortium: Is It Better for Wheat Production and How Does It Affect the Rhizosphere Microbiome?" Applied Microbiology 5, no. 4: 142. https://doi.org/10.3390/applmicrobiol5040142
APA StyleAsyakina, L., Barsukov, P., Serazetdinova, Y., Baturina, O., Fotina, N., Prosekov, A., & Kabilov, M. (2025). A Novel Fungicide Consortium: Is It Better for Wheat Production and How Does It Affect the Rhizosphere Microbiome? Applied Microbiology, 5(4), 142. https://doi.org/10.3390/applmicrobiol5040142








