1. Introduction
Safe and clean water is one of the most basic human needs worldwide [
1]. However, as the world population increases, water pollution is becoming a challenge for wastewater treatment plants (WWTPs), due to the energy demands and the efficient treatment required to clean the effluents.
WWTPs perform at least two types of treatments: primary and secondary. The first one removes big floating objects, while the second treatment, led by microorganisms, eliminates inorganic and organic matter from wastewater. All these processes produce biosolids named sludge that must be treated. Wastewater treatment involves high energy consumption; for instance, in Europe, the overall WWTP’s electric use represents 0.8% of the total energy generated [
2].
Anaerobic digestion of sludges removes pathogens and stabilises biosolids from wastewater treatments and has a very important role in the technical and economic management of WWTPs because it valorises volatile solids, generating methane-rich biogas, a renewable energy source. Anaerobic digestion stands out for its low energy demand and efficiency in processing the sewage sludge in comparison with other commonly used technologies in WWTPs [
3]. Anaerobic digestion is a complex process (
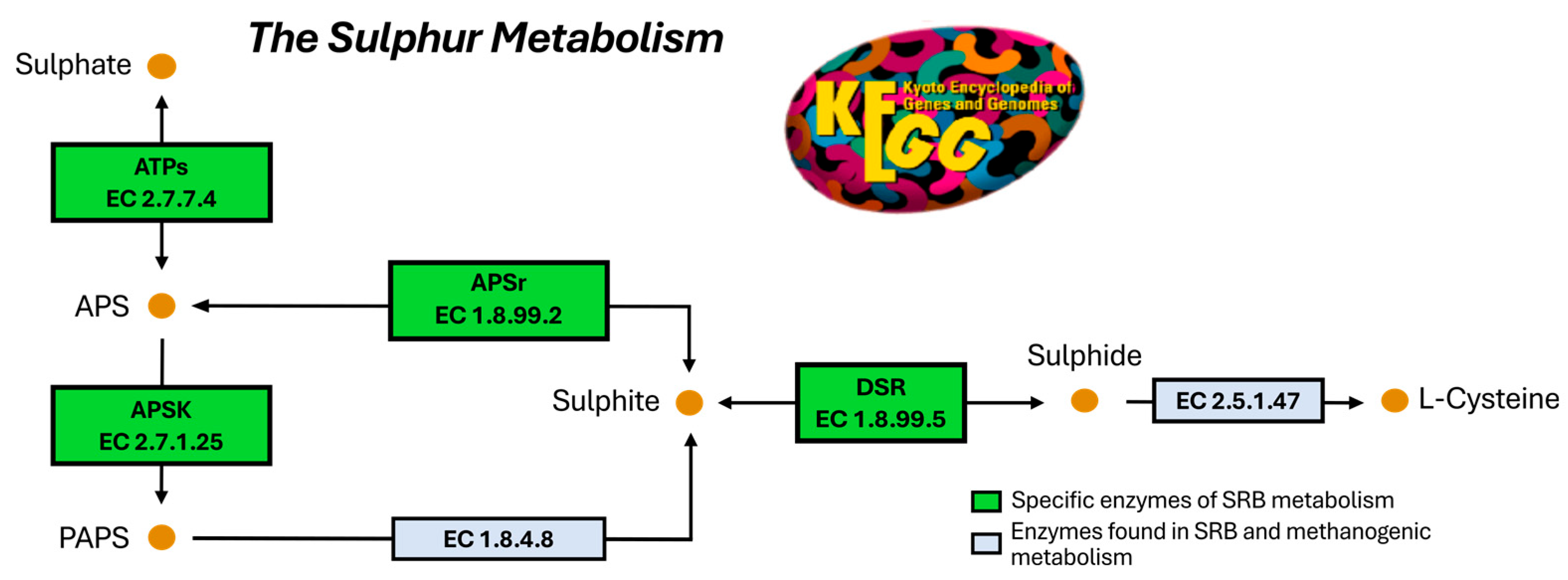
Figure 1A) resulting from a synergistic relationship between hydrolytic, fermentative acidogenic, and acetogenic bacteria and methanogens. Microorganisms collaborate to sequentially process the organic matter into acetate, carbon dioxide, and hydrogen. Then, methanogens take these substrates and generate methane, a potential source of energy [
4].
Generally, domestic wastewaters contain sulphate concentrations below 250 mg/L [
6]; however, in some cases, the levels are higher due to industrial or natural factors, e.g., dyes or pharmaceuticals production, seawater intrusions, or soil composition [
7,
8]. The presence of high levels of sulphate leads to the competence of sulphate-reducing bacteria (SRB) with methanogens for the available substrates [
9]. This phenomenon has been studied in nature [
10,
11], anaerobic tanks [
9], and the human intestine [
12]. In turn, the hydrogen sulphide (H
2S), which is the final product of the sulphate-reducing metabolism, is detrimental to methanogens and other microbial species involved in anaerobic digestion (
Figure 1B) [
9,
13,
14]. This competition eventually results in a lower production of methane and a decrease in the efficiency of the organic processing that takes place in the anaerobic tanks. In addition, the H
2S produced by SRB results in iron and steel corrosion [
15] and represents a potential risk to human health and the environment, causing eye and respiratory irritation and being one of the causes of acid rain [
16].
Co-digestion refers to the simultaneous digestion of two or more substrates to improve the economic viability of anaerobic digestion plants due to increased methane production. In the case of WWTPs, the sludge produced can be co-digested with agri-food waste to increase biogas production, maximising the existing facilities for anaerobic digestion and bringing additional organisational and economic benefits, besides the energy balance, for an environmentally friendly treatment of agri-food waste. This makes good use of existing infrastructures and substantially improves the energy balance of a WWTP, maximising renewable energy production as a step towards energy self-sufficiency and ultimately carbon neutrality [
17]. However, a major challenge lies in the nature of many agri-food wastes, especially those with high protein content, as they often contain elevated concentrations of sulphates. This can promote sulphate-reducing metabolic pathways, increasing the activity of SRB, which compete with methanogens and produce H
2S. Therefore, it is crucial for the proper management of co-digestion to monitor and properly manage sulphate levels in the digestion and co-digestion processes [
18,
19].
In addition, considering the global status of the climate crisis, novel approaches to optimise and improve energy efficiency are key in the endeavours to reduce industrial carbon footprints [
20,
21]. Circular economy strategies can align economic growth with the goal of reducing dependence on non-sustainable energy sources [
22]. WWTPs play a central role in harnessing energy resources from wastewater, contributing to the efficient design of circular cities that could eventually mitigate the effects of climate change [
21]. For instance, the recovery of chemical, hydraulic, and thermal energies from wastewater improves energy efficiency and reduces the environmental impacts caused by conventional treatment systems. Depending on the level of recovery, it could just supply energy to the WWTPs themselves or even the neighbourhood. However, the lack of public policies related to energy efficiency and government support is hindering this transformation [
23]. Additionally, processed sludge offers a more sustainable way to produce fertilisers [
24]. These examples represent some of the achievable measures in circular cities.
Prior to this work, some studies have focused on the issues caused by SRB such as oil, gas, and iron corrosion, resulting in emissions of H
2S [
15,
25]. This gas, along with other sulphur species, is noxious to the environment and to human health [
16]. Nevertheless, they remain key players in biogeochemical and biotechnological processes, for instance, the biomining of heavy metals or the bioremediation of sulphate-rich wastewaters [
4,
26]. Conventional SRB inhibitors, such as formaldehyde or antibiotics, could result in a bigger environmental issue due to the potential of SRB to develop resistance to these compounds. Alternative strategies have aimed to limit sulphate bioavailability by adding metallic salts, particularly iron salts. This is the solution traditionally used, as metallic salts were once relatively cheap; however, their cost is increasing and they have the disadvantage of adding metals to the digested sludge, which could affect the viability of their application as agricultural fertiliser. They are also hazardous substances due to their corrosiveness, which pose a risk to people during transport and handling. Other metallic salts, such as zinc and copper sulphide, have shown potent inhibitory activities against SRB [
27]; however, they might affect the methanogenic archaea growth [
28,
29] or even disrupt the sludge structure and affect other phases of the consortium [
30]. Additionally, molybdenum is an essential trace metallic atom present in different SRB enzymes. However, at high concentrations it is toxic to these bacteria, due to the inhibition of key proteins of their metabolism [
31]. Therefore, the use of specific inhibitors is an optimal and efficient approach to tackle SRB growth in WWTPs. Exploration of endogenous ligands and their derivatives, as previously studied, can be a valid approach to identify novel inhibitors [
32,
33].
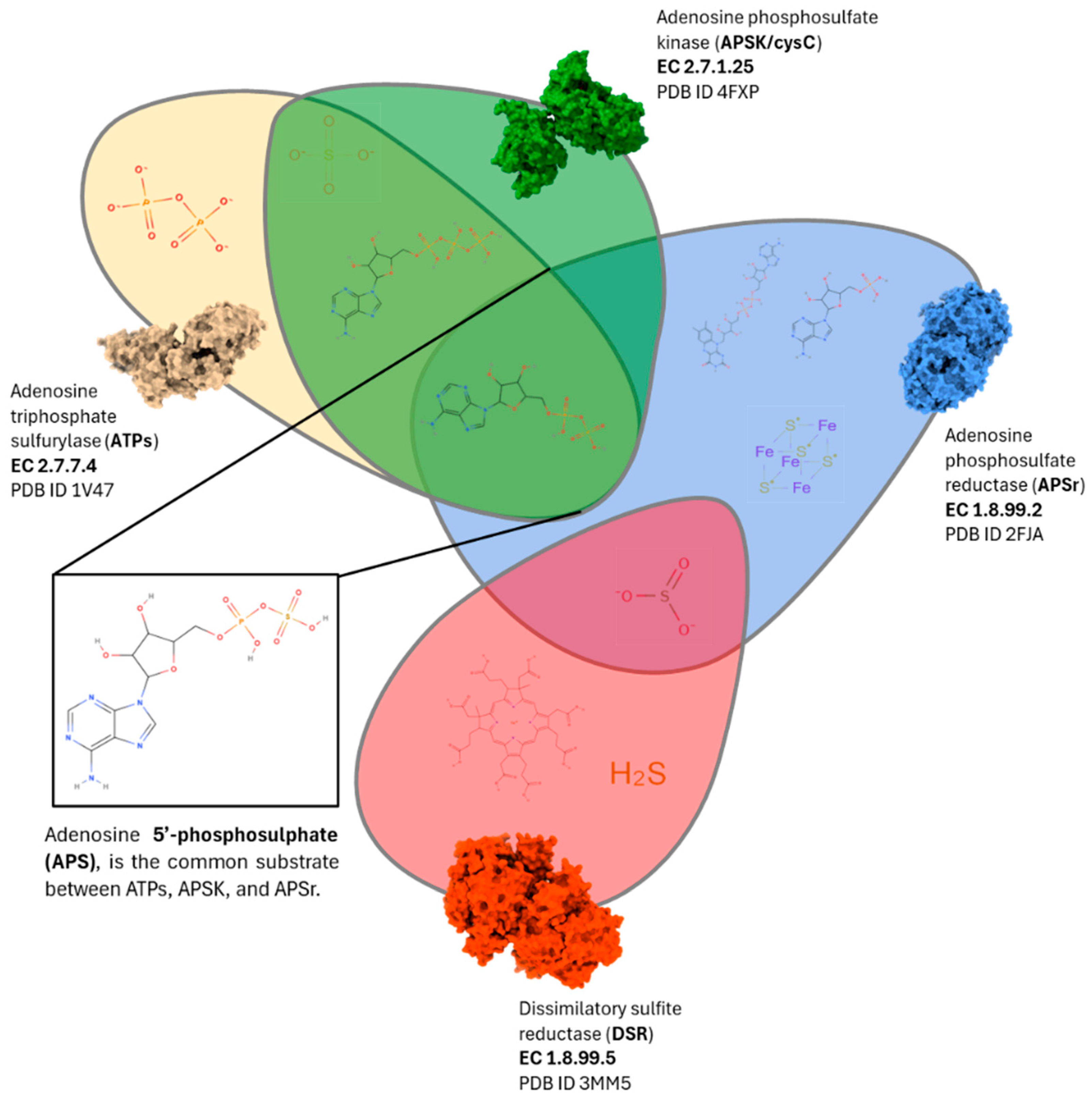
It has been described that SRB metabolism can be inhibited using chemical analogues of the endogenous ligands, such as sulphate, adenosine-5′-phosphosulphate (APS), and sulphite, which compete with the enzymatic activities, decreasing SRB growth [
34]. Moreover, novel compounds with anti-SRB activities have been characterised, such as the synthetic diquaternary Schiff bases [
35], but also, some natural products have an anti-SRB effect, as shown in the case of the lemongrass essential oil [
36] or antimicrobial substances generated by fungi [
37].
In summary, the endeavours in the field of SRB inhibition have focused solely on studying SRB without considering their effect on the methanogenic population and the quality of the sludge. Our aim is to consider the identification of SRB inhibitors that do not affect other populations present in the sludge. In this context, identifying novel bioactive compounds is a slow and expensive process that requires a huge experimentation effort in conventional laboratories. Computer-aided drug design methodologies are able to accelerate the discovery process, saving time and money by reducing experimentation on inactive compounds [
38]. Quantitative Structure–Activity Relationship (QSAR) is a widely used computational technique that allows to predict biological activities from a set of calculated descriptors that encode the chemical structures of the molecules. Therefore, QSARs need a training set of molecules with experimental data of the studied activity as well as the corresponding molecular structures. After cleaning and processing, the model is generated, choosing an algorithm that will fit the data. Once the model is generated, it can be applied to another set of molecules with unknown biological activities [
38,
39,
40]. The potential of this technique has enabled the development of diverse models generated to identify novel antimicrobials, many of which were built from relevant specific chemical families [
41,
42], even peptides [
43,
44,
45].
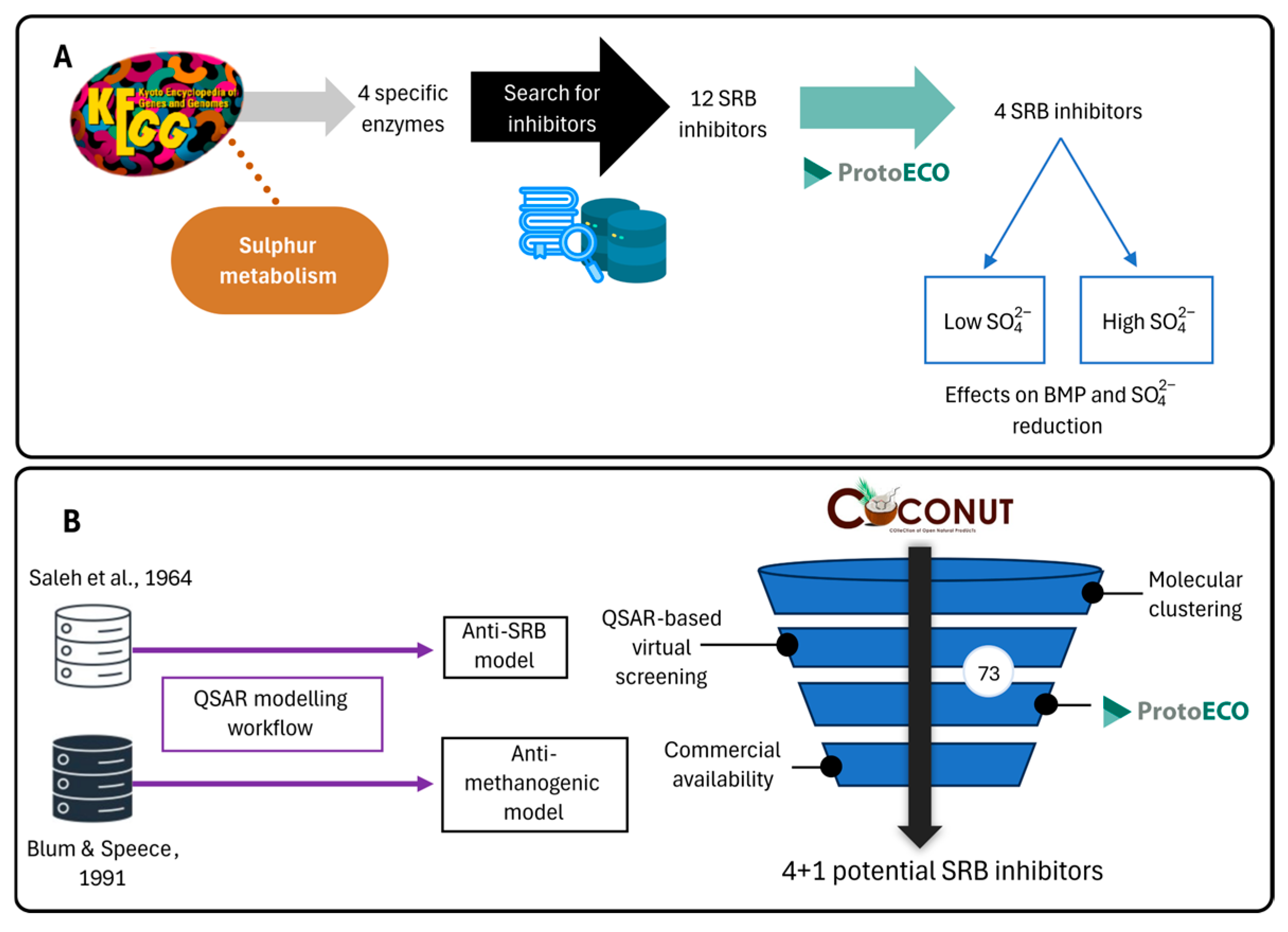
Herein, the use of computational approaches (a database search-driven approach and a virtual screening based on two QSAR models) to study potential SRB inhibitors to overcome the methanogenic competence in anaerobic digestors was proposed.
4. Discussion
Microbial methane production in WWTPs is a promising methodology for green bio-gas production. However, this production could be affected by the competitive relationship existing between methanogenic archaea and sulphate-reducing bacteria, reducing the efficiency of anaerobic digestion and producing a dangerous and corrosive gas. As a possible strategy, inhibition of SRB can help to favour methane production. This inhibition reduces the competition for substrates such as acetate or hydrogen, potentially recovering the methanogenic activity, yet it also may disrupt the redox balance and the syntrophic interactions. The outcome depends on how the inhibitors affect the SRB population and the rest of microbial species in the anaerobic system. It is also essential to consider the accumulation of toxic intermediates. In microbial syntrophic relationships, community changes and environmental perturbations can lead to compensating mechanisms. These mechanisms can inhibit or promote certain metabolic pathways [
91]. Therefore, whole-picture approaches which consider all the species in the consortium should be taken and are key for understanding the complexity of these systems.
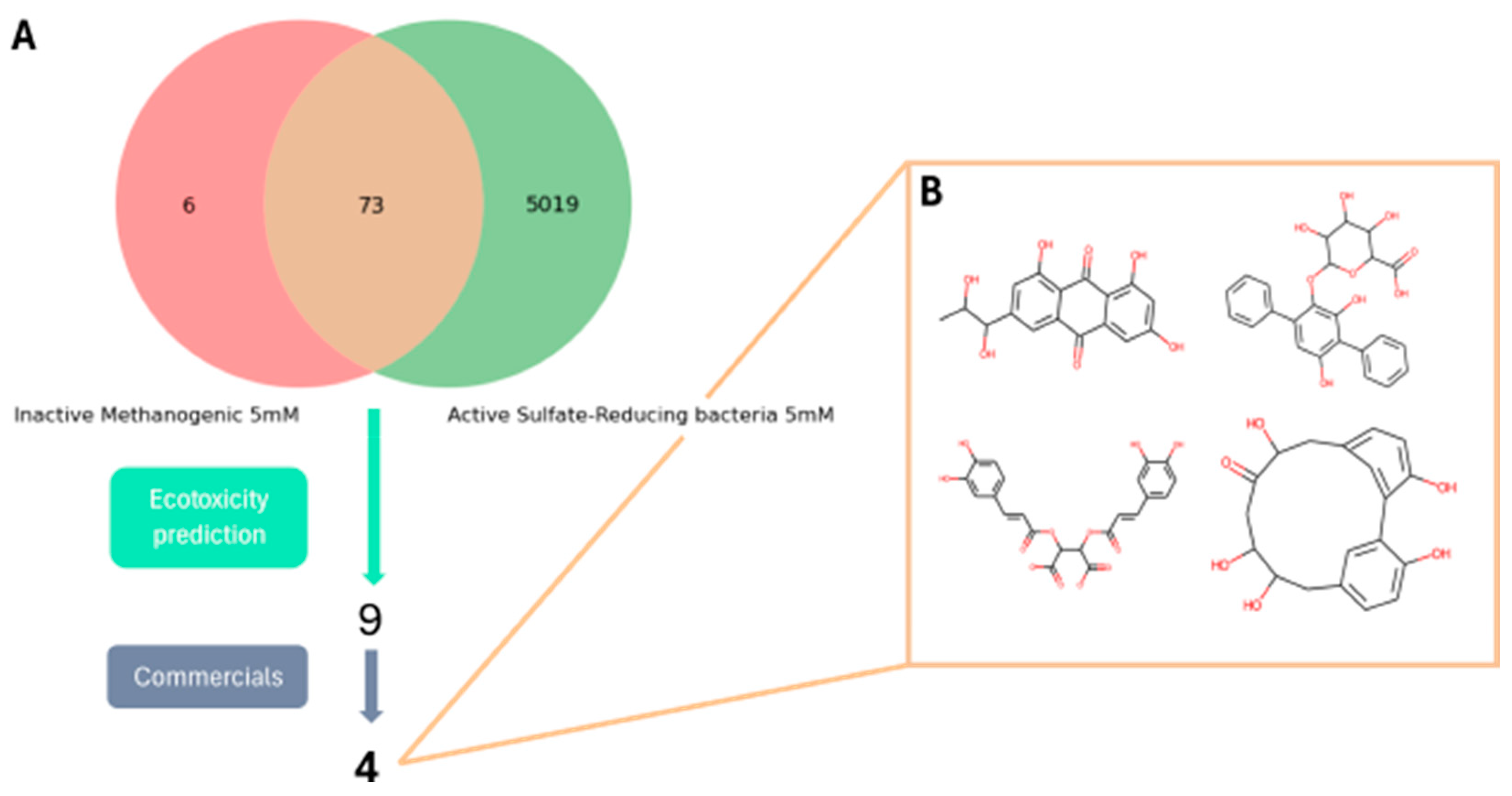
In the present work, through the combination of a database search-driven approach and QSAR-based virtual screening, nine candidates for sulphate-reduction inhibition have been identified—four and five molecules, respectively—that might inhibit SRB without interfering with methanogenic metabolism.
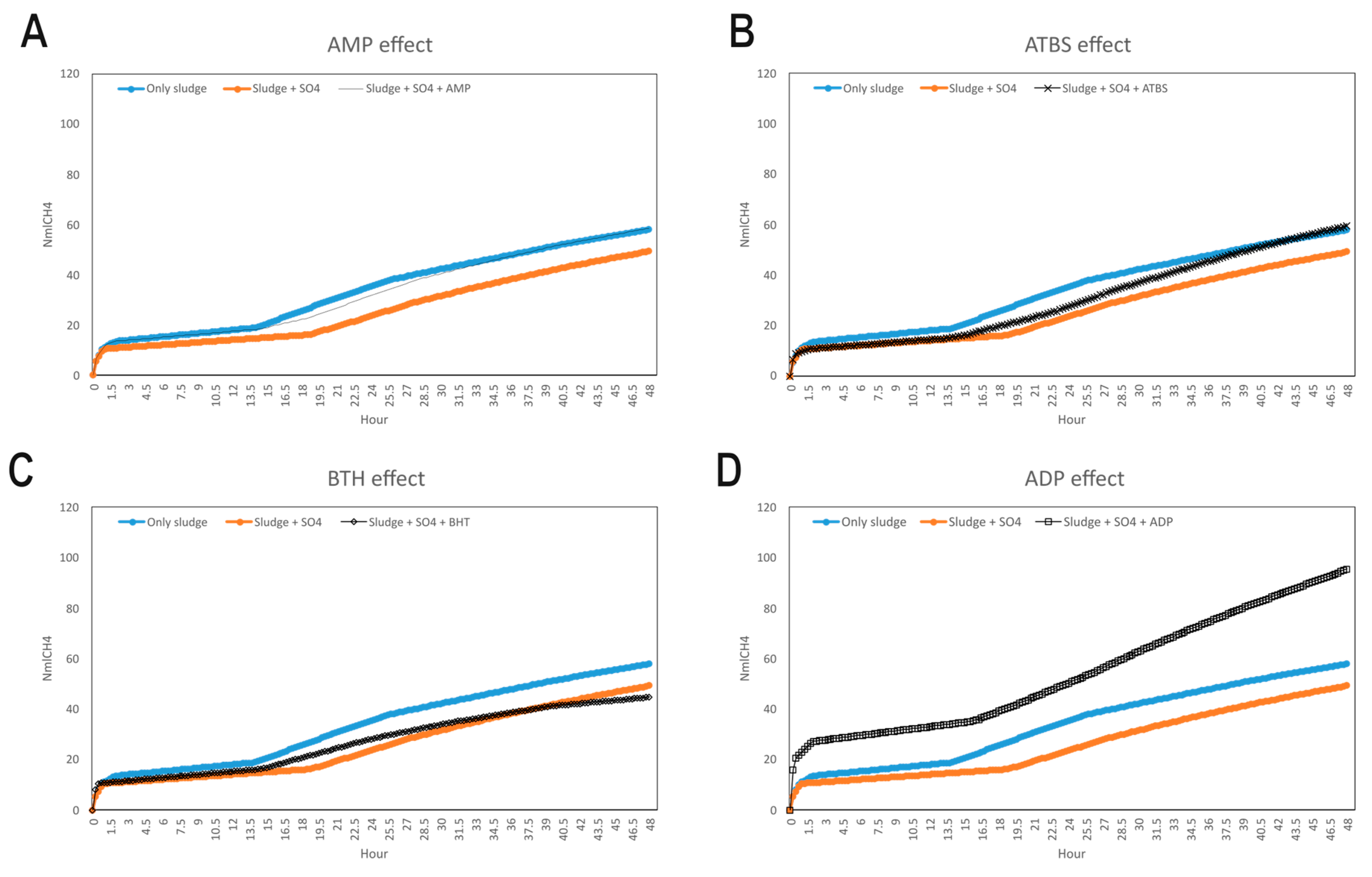
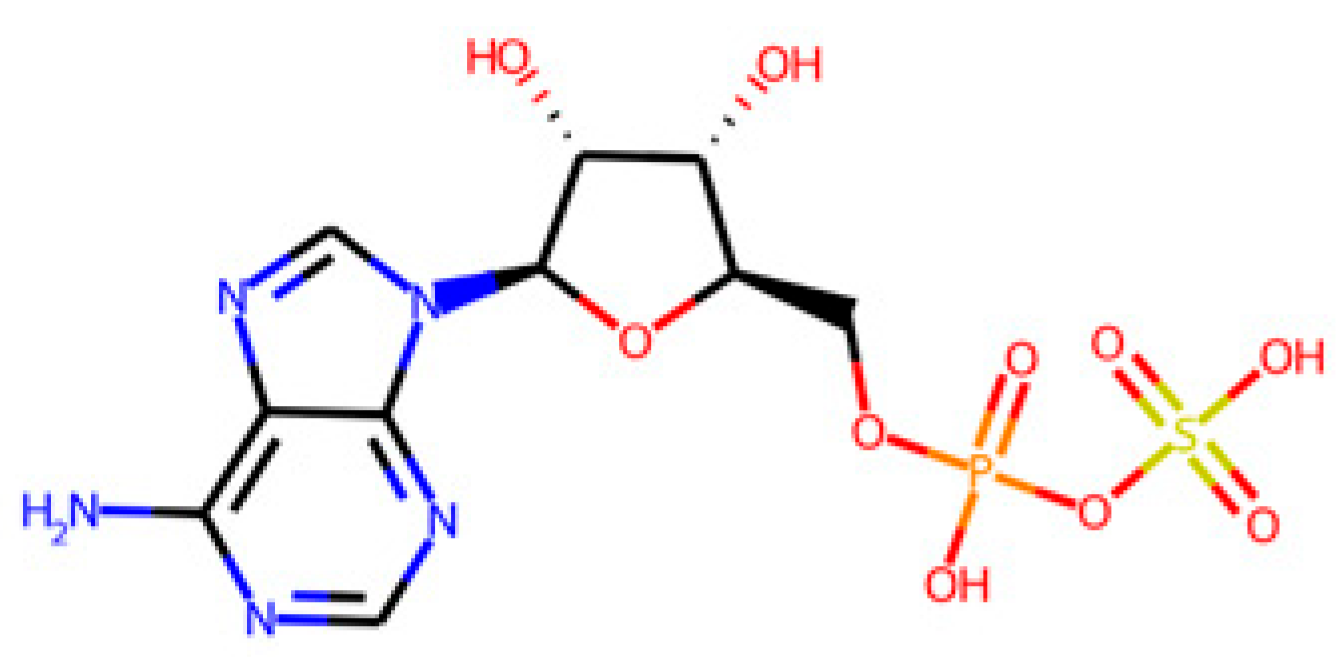
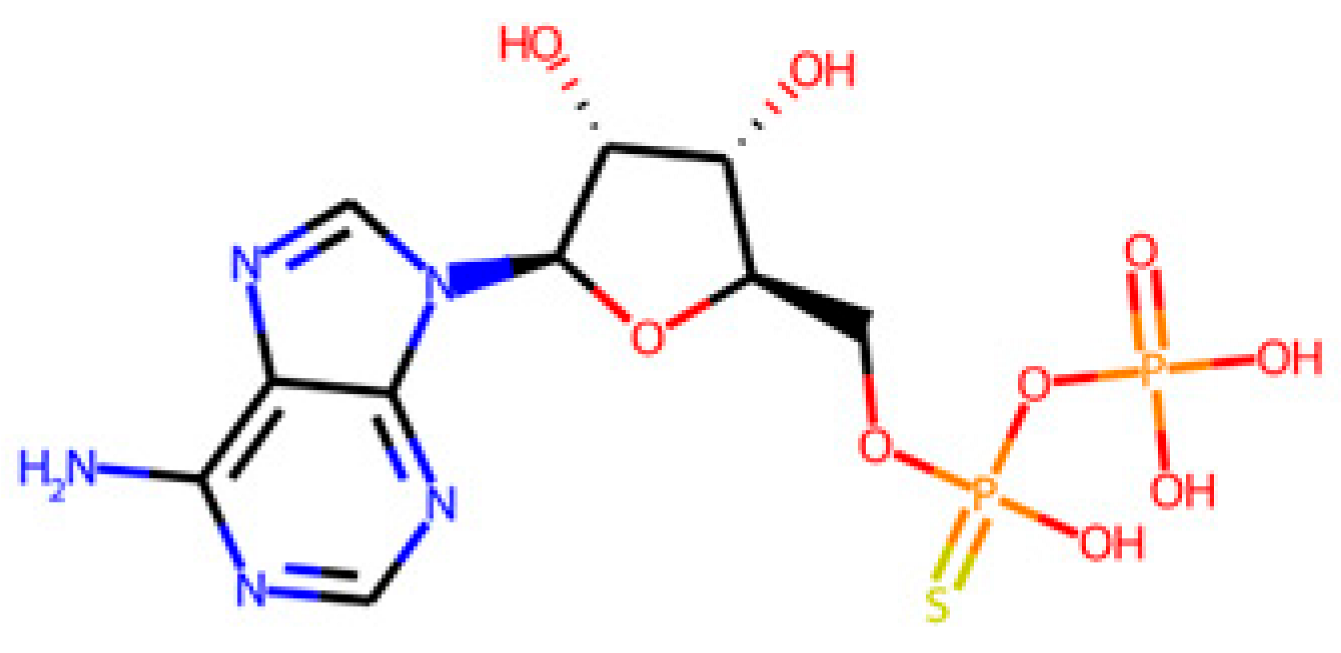
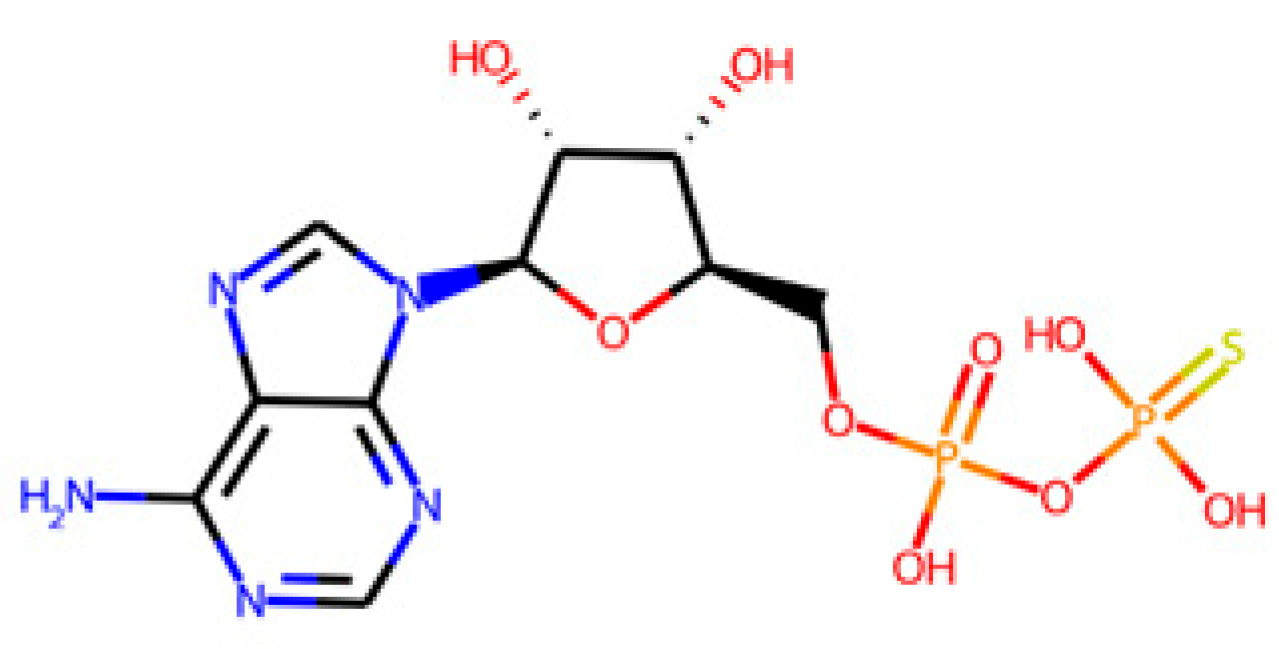
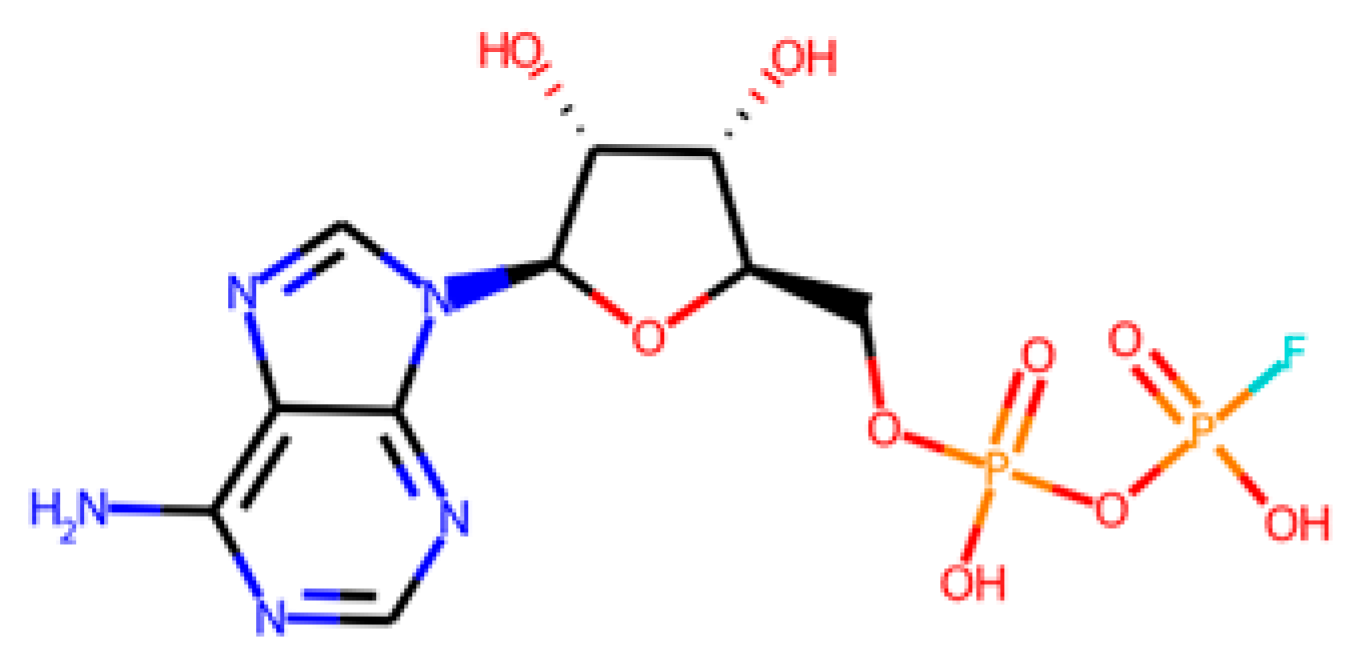
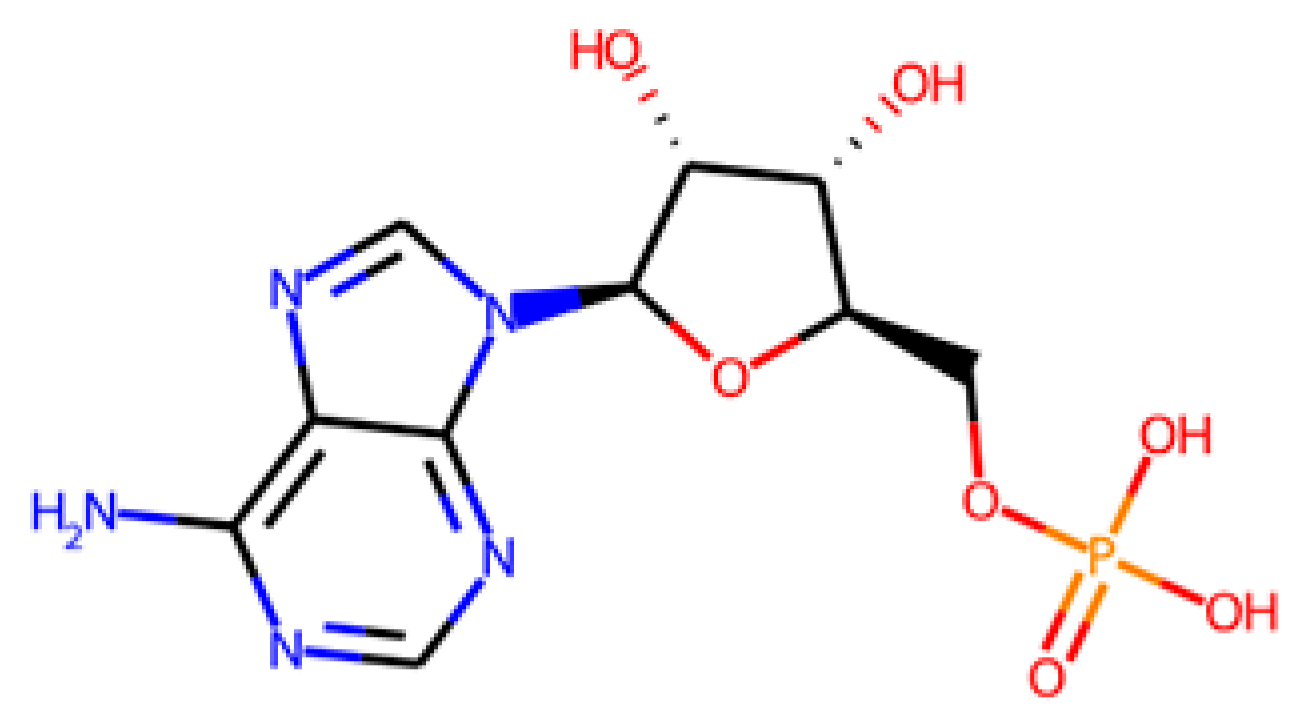
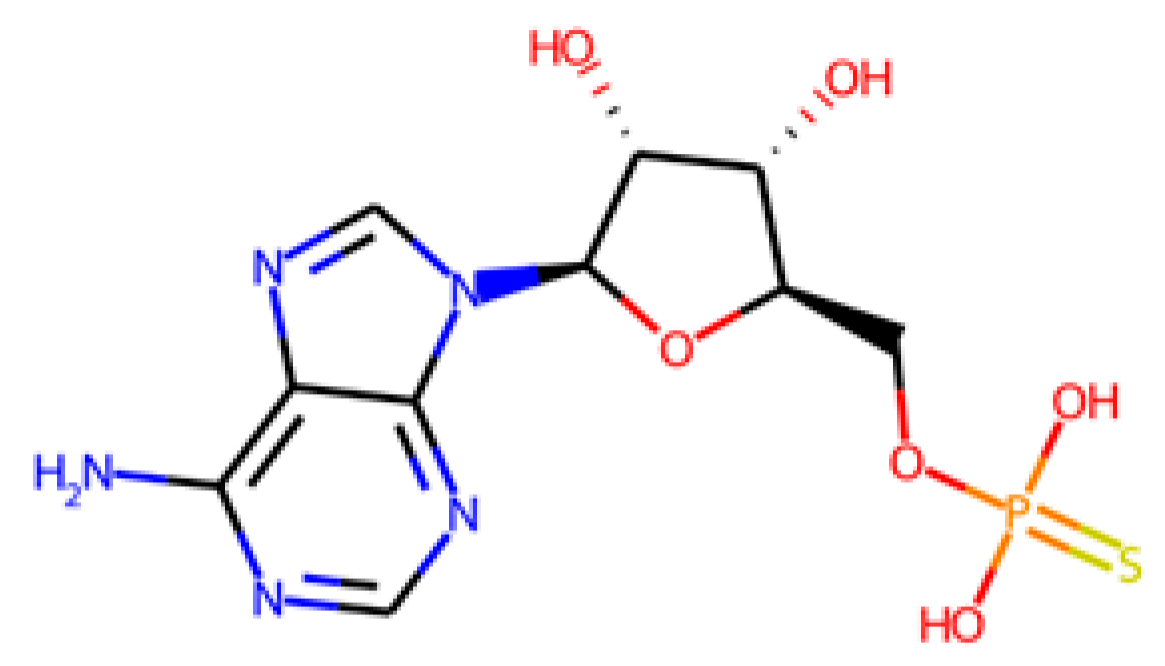
The database search-driven strategy yielded 12 diverse molecules whose specific anti-SRB activities were reported. From the selected molecules that were subjected to experimental testing (ADP, AMP, ABTS, and BHT), only ADP efficiently increased methane production, successfully optimising the levels of this gas in the reactors. However, BHT and ABTS showed an eventual decrease in methane production. It is important to note that the BMP values obtained are lower than the theoretical values, mainly for the following reasons First, the theoretical value assumes the complete conversion of all the substrate carbon into CH
4; however, some carbon is transformed into CO
2, since biogas production involves several metabolic pathways and not just methanogenesis (see
Figure 1). Second, the biogas generated in the tests was passed through a CO
2 trap before measurement, so only the methane fraction generated was quantified. Third, the equilibrium between CH
4 and CO
2 depends on the predominant microbial pathways at any given time, since bacteria and archaea compete for electron acceptors and substrates. This microbial competition can modify the percentage of CH
4 and CO
2 in the generated biogas (for example, it depends on the proportion of acetoclastic and hydrogenotrophic methanogens participating in the process). Finally, to verify the effect of the compounds on sulphate reduction, the CH
4 values used for comparison between replicates were those obtained at 48 h, an insufficient time to achieve complete methane conversion. This short duration was chosen to capture the initial effect of sulphate reduction, whose kinetics are faster than those of methanogenesis, thus allowing for comparison of the effect on methanogenesis among the different replicates analysed.
As mentioned previously, the higher methane production might be explained by potential pleiotropic effects, affecting several enzymes from the sulphur metabolism. This could lead to the alteration of the levels of metabolic intermediates and promote higher levels of methane. Additionally, ADP is structurally closer to APS, PAPS, and other metabolites involved in the sulphur metabolism than AMP, BHT, or ABTS, performing better in the competition for the enzymes against their endogenous ligands. However, nucleotides such as AMP have been reported to deregulate energetic metabolic pathways (i.e., tricarboxylic acid cycle) and have been proposed as a therapeutic complement to antibiotics in tackling infections caused by Gram-negative resistant bacteria [
92]. Therefore, the observed effect of AMP could be optimised with another inhibitor. The exact mechanisms through which these compounds affect methanogenesis remain unclear. However, it has already been reported that some additives can affect the sludge structure and interfere with different phases of the anaerobic consortium, ultimately leading to reduced methane yields. For instance, Pasciucco et al. reported that aluminium-based coagulants modified sludge characteristics and inhibited methane production during anaerobic digestion, probably by disrupting hydrolysis and acidogenesis phases [
30]. These findings highlight the need for more global approaches to better understand microbial and physicochemical interactions in sludge systems.
It is important to recognise that this study represents a first approximation, establishing the computational and experimental feasibility of the inhibition strategy. ADP demonstrated high efficacy with an 86% increase in methane production, which could mean high production on a real scale, resulting in direct energy savings. The effective use of the compounds studied at relatively low concentrations and in single doses (e.g., 50 µM tested in the assays) suggests that the operational cost is manageable. The price of the products is currently high, as they have been produced on demand, but it is foreseeable they could be substantially cheaper when manufactured on a larger industrial scale, which will offset the initial costs associated with smaller-scale synthesis. Likewise, it is necessary to analyse on a larger scale the economic savings resulting from increased methane production.
The successful and selective inhibition of SRB presents ecological, energetic, and economic advantages, promoting a shift to a circular economy and sustainable model in the WWTP sector. In addition to the increase in methane production, SRB inhibition directly addresses the significant issues derived from the H2S generation. This gas can not only affect the methanogens but also damage the structural integrity of the facilities and reduce the useful life of cogeneration engines, entailing additional costs for their removal. The reduction in the SRB enzymes activity (e.g., ATPs, APSK, and APSr) thus greatly diminishes the production and accumulation of H2S. Traditional control strategies, such as the addition of metal salts, can compromise the quality and the potential valorisation of the digested sludge as agricultural fertiliser. The identification and validation of selective and non-ecotoxic candidates is essential to have alternative control strategies.
The capacity of a QSAR model to distinguish between active and inactive compounds mainly depends on the molecular descriptors used to encode the chemical information [
93]. Understanding the role of the selected descriptors allows us to draw a clear relationship between certain structural and physicochemical characteristics and the biological activity of interest and comprehend the underlying mechanisms. Among the descriptors employed in the generation of the anti-SRB QSAR model (
Supplementary Materials Table S1) and in the generation of the anti-methanogenic QSAR model (
Supplementary Materials Table S2), we found that most descriptors are low-explainable and complex molecular descriptors, such as the 3D-MoRSe series. This may be due to the heterogeneity of the employed dataset used to develop the model, as it contains compounds that affect different targets [
54,
55]. Such a limitation could be overcome through more specific investigations on both microorganism targets in future studies.
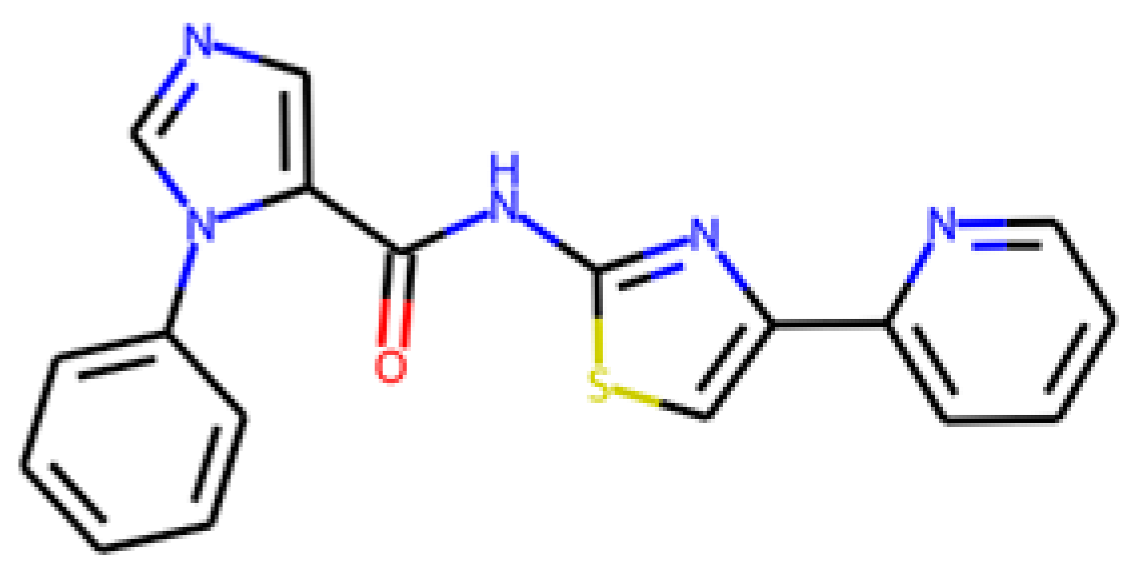
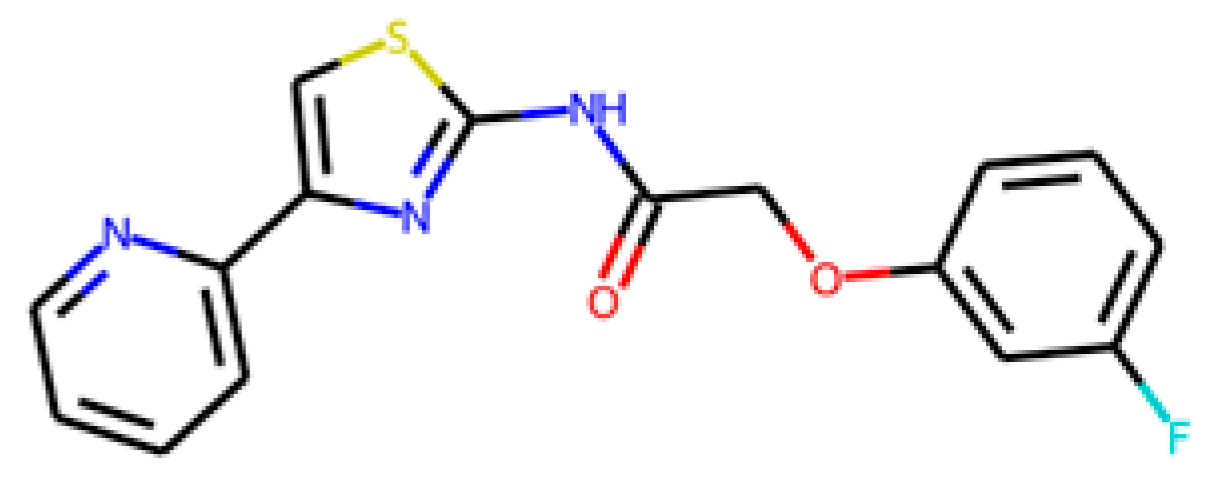
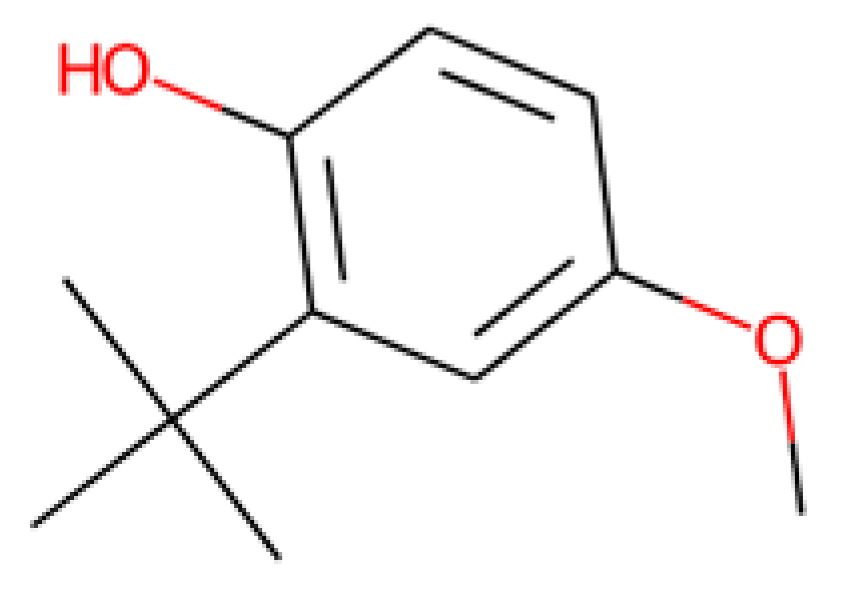
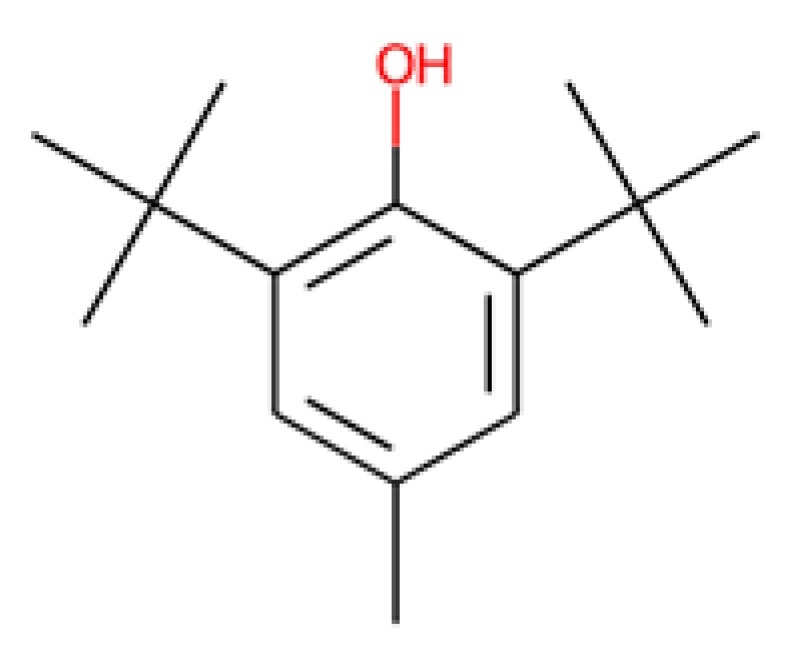
Nevertheless, some of the captured descriptors are directly related to structural features or specific functional groups. Our anti-SRB QSAR model identified the ‘N-079’ descriptor (i.e., counts the presence of nitrogen atoms charged positively in the molecules). It has been reported that quaternary ammonium salts are antimicrobial molecules with great potential, known for adsorbing to the bacterial membranes due to the cationic charge [
94]. Moreover, molecules with charged nitrogen could be competing for the active site of the enzymes involved in sulphur metabolism. The model also identified the descriptor SLogP_VSA3 (which captures the contributions of specific atom type to the surface area and therefore the local lipophilicity). This descriptor reflects the relevance of the passive intake of compounds through lipidic membranes in the case of SRB, which are commonly Gram-negative bacteria. In the case of the anti-methanogenic QSAR model, the following seven molecular descriptors presented a high explainability: ‘H-052’, ‘O-059’, ‘B03[N-O]’, ‘B03[N-Cl]’, ‘F04[O-Cl]’, ‘nRCOO’, and ‘nAl_OH’. Together, these descriptors indicate the presence of hydroxyl, carboxylic, and chloro-substituted groups, suggesting that these species may have a potential relationship with the inhibitory activity against methanogenic archaea. Indeed, some studies have reported inhibition of methanogens by halogenated compounds such as 2-bromoethanesulfonate or bromochloromethane, and diverse carboxylic acids like pterin-6-carboxylic acid [
95,
96]. The combination of these atom-centred descriptors and functional groups with some complex 3D descriptors likely captures the conformational and electronic diversity required to interfere with the metabolic pathways or membrane integrity of these microorganisms.
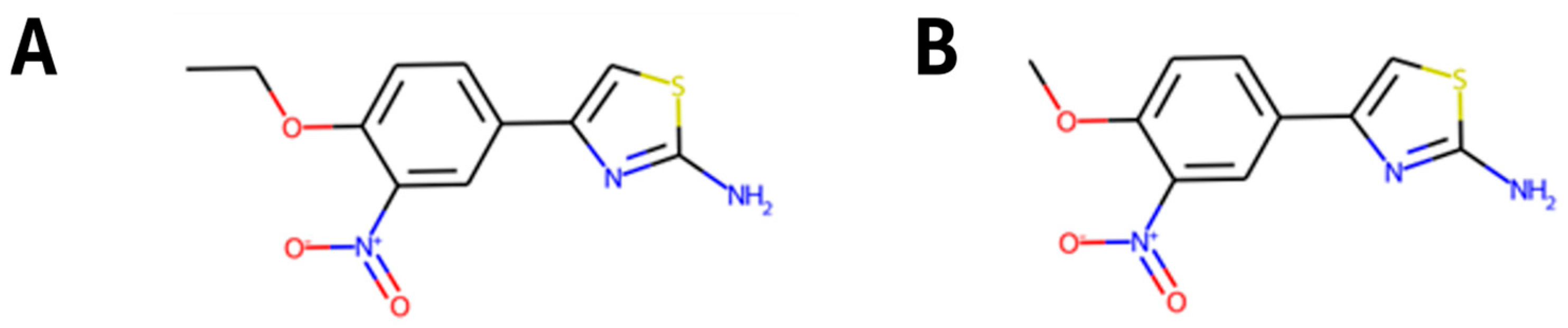
In summary, despite the fact that previous QSAR models have been used to describe the inhibition of SRB-related enzymes or methanogenic microorganisms [
97,
98], it is remarkable that this is the first time, to the best of our knowledge, that SRB and methanogenic microorganisms have both been taken into account in a single tool to identify safe and specific candidates against this type of bacteria and archaea. Moreover, robust and competent performances were accomplished. They were able not only to successfully screen a subset from the COCONUT database and confirm some of the results of the experimental studies, but also to identify significant functional groups such as aminothiazole, hydroxide, or aromatic groups, molecular elements widely present among common and novel antimicrobials [
35,
89,
90,
99]. Despite the limited amount of data, the QSAR models showed acceptable performance and successfully captured functional groups or substructures present in known antimicrobial agents. However, more diverse data is needed, and this could mean an improvement of the QSAR models in the future, trying to enhance not only the predictive ability of the model but also enlarge the chemical space in which the model is reliable. In addition, ecotoxicological predictions were also incorporated, because industrial optimisation should go hand in hand with environmental safety in circular economies.
Little has been studied concerning the inhibition of SRB through modelling techniques, especially QSAR models. Of particular note are the molecular docking studies on SRB proteins that have helped in the identification of novel SRB inhibitors [
81,
82,
100]. In them, dos Santos et al. selected APSr and ATPs as inhibition targets of SRB and identified some compounds with inhibitory activities that were of interest in our work. From that point, the approaches presented in this work contribute to refinement and better selection of potential candidates by taking into account the likely inhibition of methanogenesis, as has been studied [
28,
55,
101], by some of the previously studied molecules.
Other studies have also applied computational tools in the identification of antimicrobials with different applications, such as industrial biocidal control [
100,
102,
103] or clinical disinfection [
104]. Some combine QSAR modelling with docking and molecular dynamics, like the work by Ye et al. [
105] on Streptococci or the one by Tejera et al. [
103]. Other works, like this study, focus on QSAR modelling, like Abdullahi et al. [
102], to identify potential antimicrobials. Both strategies are equally valid, and in fact the results indicate that they serve as successful approaches to discover novel molecules with antimicrobial potential after experimental validation [
100,
106,
107,
108]. The experimental validation has an essential role in improving the in silico methodology and evaluating the efficiency of the predicted candidates in a real context.
Based on the results obtained, other types of inhibitors, such as synthetic molecules, could be further explored, and the already selected inhibitors could be further studied using different techniques, such as molecular modelling approaches. All of this would add to the knowledge of wastewater microbiology, and we hope to contribute to a shift towards a more sustainable paradigm. Briefly, the combination of available published data and computational tools has shown the potential of QSAR models in the identification of novel molecules of interest in the field of environmental chemistry. With the opportunity to test some of the compounds, the computational evidence of the generated models was supported.