Influence of Native Leptospirillum ferriphilum Strains on Ferric Iron and Leached Copper Recovery from Chalcopyrite to Mesophilic Temperature Under Laboratory Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Bioleaching Bacterial Strains
2.2. Morphology of Leptospirillum ferriphilum by SEM
2.3. Phylogenetic Analysis of Leptospirillum ferriphillum Strains
2.4. Reactivation of the Strains
2.5. Recovery of Copper from Chalcopyrite ore Using Bioleaching with Leptospirillum ferriphilum Strains
2.6. Bioleaching Kinetics Applying M1D and M3E Strains
2.7. Statistical Analysis and Data Processing
3. Results
3.1. Characterization of Leptospirillum ferriphilum Isolated from Chalcopyrite Mineral
3.2. Comparative Analysis of Eight Leptospirillum ferriphilum Strains Isolated from Chalcopyrite
3.3. Growth Kinetics of Leptospirillum ferriphilum Strains M1D and M3E
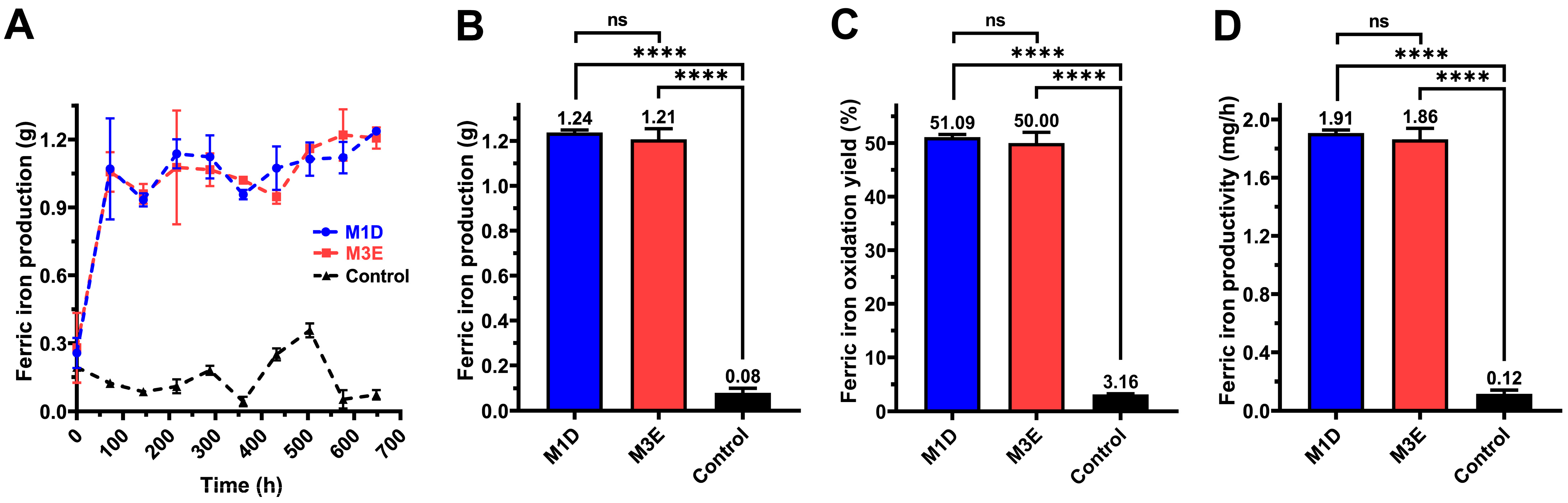
3.4. Ferric Iron Production
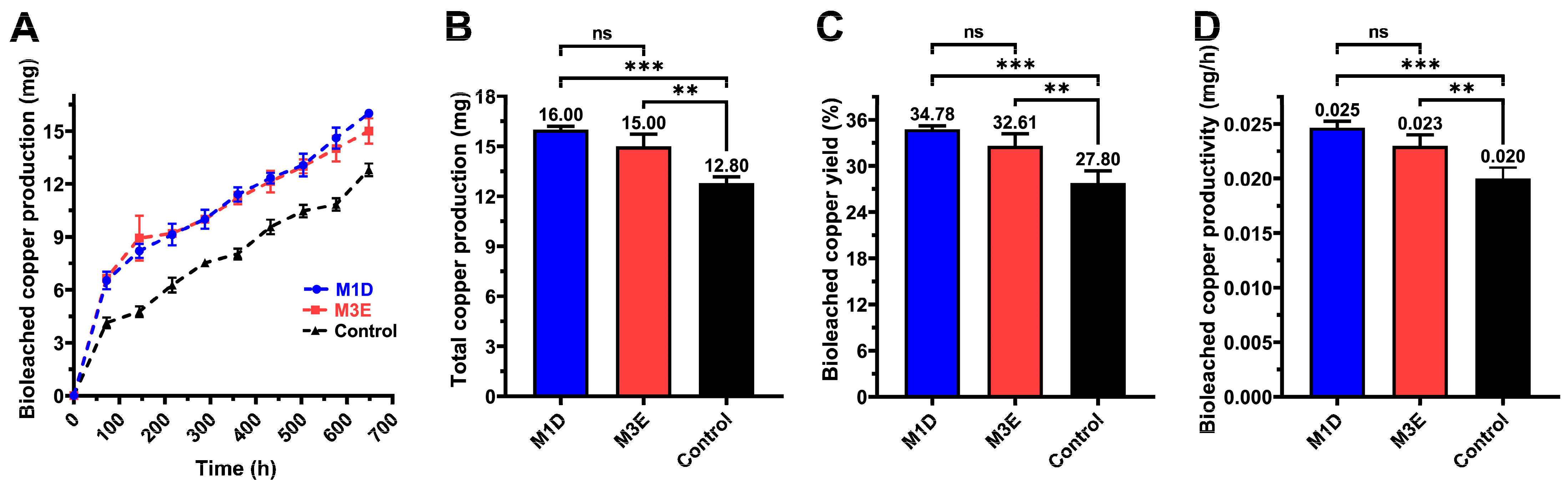
3.5. Copper Bioleaching Kinetics and Production
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Rawlings, D.E. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Fact. 2005, 4, 13. [Google Scholar] [CrossRef]
- Hua, Z.-S.; Han, Y.-J.; Chen, L.-X.; Liu, J.; Hu, M.; Li, S.-J.; Kuang, J.-L.; Chain, P.S.G.; Huang, L.-N.; Shu, W.-S. Ecological functions of dominant and rare prokaryotes in mine drainage revealed by metagenomics and metatranscriptomics. ISME J. 2015, 9, 1280–1294. [Google Scholar] [CrossRef] [PubMed]
- Vardanyan, A.; Khachatryan, A.; Castro, L.; Willscher, S.; Gaydardzhiev, S.; Zhang, R.; Vardanyan, N. Biolixiviación de minerales sulfurados por Leptospirillum ferriphilum CC de una mina polimetálica (Armenia). Minerals 2023, 13, 243. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.; Chen, X.; Zhou, H. Responses of microbial community to pH stress in bioleaching of low grade copper sulfide. Bioresour. Technol. 2018, 249, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Özkaya, B.; Sahinkaya, E.; Nurmi, P.; Kaksonen, A.H.; Puhakka, J.A. Kinetics of iron oxidation by Leptospirillum ferriphilum dominated culture at pH below one. Biotechnol. Bioeng. 2007, 97, 1121–1127. [Google Scholar] [CrossRef]
- Coram, N.J.; Rawlings, D.E. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates South African commercial biooxidation tanks that operate at 40 °C. Appl. Environ. Microbiol. 2002, 68, 838–845. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron (III) ions and acidophilic bacteria. Res. Microbiol. 2006, 157, 49–56. [Google Scholar] [CrossRef]
- Christel, S.; Herold, M.; Bellenberg, S.; El Hajjami, M.; Buetti-Dinh, A.; Pivkin, I.V.; Sand, W.; Wilmes, P.; Poetsch, A.; Dopson, M. Multi-omics reveals the lifestyle of the acidophilic, mineral-oxidizing model species Leptospirillum ferriphilum T. Appl. Environ. Microbiol. 2018, 84, e02091-17. [Google Scholar] [CrossRef]
- Huo, X.; Liu, J.; Hong, X.; Bai, H.; Chen, Z.; Che, J.; Yang, H.; Tong, Y.; Feng, S. Enhancing Column Bioleaching of Chalcocite by Isolated Iron Metabolism Partners Leptospirillum ferriphilum/Acidiphilium sp. Coupling with Systematically Utilizing Cellulosic Waste. Bioresour. Technol. 2024, 394, 130193. [Google Scholar] [CrossRef]
- Merino, M.P.; Andrews, B.A.; Parada, P.; Asenjo, J.A. Characterization of Ferroplasma acidiphilum Growing in Pure and Mixed Culture with Leptospirillum ferriphilum. Biotechnol. Prog. 2016, 32, 1390–1396. [Google Scholar] [CrossRef]
- Zhou, Z.; Dopson, M.; Bellenberg, S.; Salas, B.; Jorquera, C.; Valenzuela, M.L.; Buetti-Dinh, A.; Unelius, C.R.; Vera, M. Interspecies Quorum Sensing Signals Control Bioleaching Activity and Niche Protection in Acidophilic, Mineral-Oxidizing Leptospirillum spp. Sci. Rep. 2021, 11, 16275. [Google Scholar] [CrossRef]
- Arias, D.; Zepeda, V.; Nancucheo, I.; Saldaña, M.; Galleguillos, P.A. Osmotic response in Leptospirillum ferriphilum isolated from an industrial copper bioleaching environment to sulfate. Front. Microbiol. 2024, 15, 1369244. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.; Medina, J.; Gamarra, F.; Delgado, S.; Chipana, V.; Clavijo, C.; Ccorahua-Santo, R.; Peceros-Melchor, M. Metagenomic Influential Insights in the Formation of Biogenic Iron Hydroxysulfate Precipitates by Ferrous Oxidative Microbial Consortia. Geomicrobiol. J. 2023, 39, 791–804. [Google Scholar] [CrossRef]
- Amaro, A.M.; Chamorro, D.; Seeger, M.; Arredondo, R.; Peirano, I.; Jerez, C.A. Effect of external pH perturbations on in vivo protein synthesis by the acidophilic bacterium Thiobacillus ferrooxidans. J. Bacteriol. 1991, 173, 910–915. [Google Scholar] [CrossRef]
- Johnson, D.B.; Macvicar, J.H.M.; Rolfe, S. A new solid medium for the isolation and enumeration of Thiobacillus ferrooxidans and acidophilic heterotrophic bacteria. J. Microbiol. Methods 1987, 7, 9–18. [Google Scholar] [CrossRef]
- Liu, J.-S.; Xie, X.-H.; Xiao, S.-M.; Wang, X.-M.; Zhao, W.-J.; Tian, Z.-L. Isolation of Leptospirillum ferriphilum by single-layered solid medium. J. Cent. South Univ. 2007, 14, 467–473. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- NCBI Resource Coordinators. BLAST: Basic Local Alignment Search Tool. National Center for Biotechnology Information. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 8 April 2025).
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Ostell, J.; Pruitt, K.D.; Sayers, E.W. GenBank. Nucleic Acids Res. 2018, 46, D41–D47. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information (NCBI). GenBank. Available online: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 8 April 2025).
- Edgar, R.C. Muscle5: High-accuracy alignment ensembles enable unbiased assessments of sequence homology and phylogeny. Nat. Commun. 2022, 13, 6968. [Google Scholar] [CrossRef]
- Criscuolo, A.; Gribaldo, S. BMGE (Block Mapping and Gathering with Entropy): A new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol. Biol. 2010, 10, 210. [Google Scholar] [CrossRef]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis, Version 3.81; Mesquite Project: Tucson, AZ, USA, 2023. [Google Scholar]
- Felsenstein, J. PHYLIP (Phylogeny Inference Package), Version 3.6; Department of Genome Sciences, University of Washington: Seattle, WA, USA, 2005. Available online: http://evolution.genetics.washington.edu/phylip.html (accessed on 8 April 2025).
- Maddison, W.P.; Swofford, D.L.; Maddison, D.R. Nexus: An extensible file format for systematic information. Syst. Biol. 1997, 46, 590–621. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2018. [Google Scholar]
- Inkscape Project. Inkscape, Version 1.2.2; Inkscape Project: Boston, MA, USA, 2022.
- Zhao, X.; Wang, R.; Lu, X.; Lu, J.; Li, C.; Li, J. Bioleaching of chalcopyrite by Acidithiobacillus ferrooxidans. Miner. Eng. 2013, 53, 184–192. [Google Scholar] [CrossRef]
- Gompertz, B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Philos. Trans. R. Soc. Lond. 1825, 115, 513–585. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; van ’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Vardanyan, A.; Vardanyan, N.; Khachatryan, A.; Zhang, R.; Sand, W. Adhesion to Mineral Surfaces by Cells of Leptospirillum, Acidithiobacillus and Sulfobacillus from Armenian Sulfide Ores. Minerals 2019, 9, 69. [Google Scholar] [CrossRef]
- Vardanyan, A.K.; Vardanyan, N.S.; Markosyan, L.; Sand, W.; Vera, M.; Zhang, R.Y. Biofilm Formation and Extracellular Polymeric Substances (EPS) Analysis by New Isolates of Leptospirillum, Acidithiobacillus and Sulfobacillus from Armenia. Adv. Mater. Res. 2015, 1130, 153–156. Available online: https://doi.org/10.4028/www.scientific.net/AMR.1130.153 (accessed on 20 August 2025). [CrossRef]
- Henke, J.M.; Bassler, B.L. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J. Bacteriol. 2004, 186, 6902–6914. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, X.; Yang, W.; Ma, L.; Li, H.; Liu, R.; Qiu, J.; Li, Y. Insights into Adaptive Mechanisms of Extreme Acidophiles Based on Quorum Sensing/Quenching-Related Proteins. mSystems 2022, 7, e01491-21. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, Y.; Chen, S.; Liang, Y.; Liu, X. Roles and Regulation of Quorum Sensing of Acidophiles in Bioleaching: A Review. Microorganisms 2024, 12, 422. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Fu, C.-A.; Hao, L.; Gu, X.-F.; Wang, R.; Lin, J.-Q.; Liu, X.-M.; Pang, X.; Zhang, C.-J.; Lin, J.-Q.; et al. The substrate-dependent regulatory effects of the AfeI/R system in Acidithiobacillus ferrooxidans reveals the novel regulation strategy of quorum sensing in acidophiles. Environ. Microbiol. 2021, 23, 757–773. [Google Scholar] [CrossRef]
- Africa, C.-J.; van Hille, R.P.; Harrison, S.T.L. Attachment of Acidithiobacillus ferrooxidans and Leptospirillum ferriphilum cultured under varying conditions to pyrite, chalcopyrite, low-grade ore and quartz in a packed column reactor. Appl. Microbiol. Biotechnol. 2013, 97, 1317–1324. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, J.; Li, S.; Zhang, R.; Xiao, T.; Sand, W. Interactions between Cells of Sulfobacillus thermosulfidooxidans and Leptospirillum ferriphilum during Pyrite Bioleaching. Front. Microbiol. 2020, 11, 44. [Google Scholar] [CrossRef]
- Nguyen, K.A.; Borja, D.; You, J.; Hong, G.; Jung, H.; Kim, H. Chalcopyrite bioleaching using adapted mesophilic microorganisms: Effects of temperature, pulp density, and initial ferrous concentrations. Mater. Trans. 2018, 59, 1860–1866. [Google Scholar] [CrossRef]
- Khachatryan, A.; Vardanyan, N.; Vardanyan, A.; Zhang, R.; Castro, L. The Effect of Metal Ions on the Growth and Ferrous Iron Oxidation by Leptospirillum ferriphilum CC Isolated from Armenia Mine Sites. Metals 2021, 11, 425. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Feng, X.; Liang, Y.; Xiao, Y.; Hao, X.; Yin, H.; Liu, H.; Liu, X. Co-Culture Microorganisms with Different Initial Proportions Reveal the Mechanism of Chalcopyrite Bioleaching Coupling with Microbial Community Succession. Bioresour. Technol. 2017, 223, 121–130. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Liu, H.; Yin, H.; Liu, X.; Qiu, G.; Liu, Y. Effect of the Introduction of Exogenous Strain Leptospirillum ferriphilum YSK on Functional Gene Expression, Structure, and Function of Indigenous Consortium during Pyrite Bioleaching. J. Cent. South Univ. 2020, 27, 1453–1465. [Google Scholar] [CrossRef]
- Kinnunen, P.H.-M.; Puhakka, J.A. High-Rate Iron Oxidation at below pH 1 and at Elevated Iron and Copper Concentrations by a Leptospirillum ferriphilum Dominated Biofilm. Process Biochem. 2005, 40, 3536–3541. [Google Scholar] [CrossRef]
- Xia, L.; Yin, C.; Dai, S.; Qiu, G.; Chen, X.; Liu, J. Bioleaching of Chalcopyrite Concentrate Using Leptospirillum ferriphilum, Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans in a Continuous Bubble Column Reactor. J. Ind. Microbiol. Biotechnol. 2010, 37, 289–295. [Google Scholar] [CrossRef]
- Ngom, B.; Liang, Y.; Liu, X. Cross-Comparison of Leaching Strains Isolated from Two Different Regions: Chambishi and Dexing Copper Mines. Biomed Res. Int. 2014, 2014, 787034. [Google Scholar] [CrossRef]
- Patel, B.C.; Tipre, D.R.; Dave, S.R. Development of Leptospirillum ferriphilum Dominated Consortium for Ferric Iron Regeneration and Metal Bioleaching under Extreme Stresses. Bioresour. Technol. 2012, 118, 483–489. [Google Scholar] [CrossRef]
- Remonsellez, F.; Galleguillos, F.; Moreno-Paz, M.; Parro, V.; Acosta, M.; Demergasso, C. Dynamic of Active Microorganisms Inhabiting a Bioleaching Industrial Heap of Low-Grade Copper Sulfide Ore Monitored by Real-Time PCR and Oligonucleotide Prokaryotic Acidophile Microarray. Microb. Biotechnol. 2009, 2, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Yévenes, L.; Malverde, S.; Quezada, V. A Sustainable Bioleaching of a Low-Grade Chalcopyrite Ore. Minerals 2022, 12, 487. [Google Scholar] [CrossRef]
- Bakhti, A.; Moghimi, H.; Bozorg, A.; Stanković, S.; Manafi, Z.; Schippers, A. Comparison of Bioleaching of a Sulfidic Copper Ore (Chalcopyrite) in Column Percolators and in Stirred-Tank Bioreactors Including Microbial Community Analysis. Chemosphere 2023, 341, 140945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Schippers, A. Stirred-Tank Bioleaching of Copper and Cobalt from Mine Tailings in Chile. Miner. Eng. 2022, 180, 107514. [Google Scholar] [CrossRef]
- Kiskira, K.; Lymperopoulou, T.; Lourentzatos, I.; Tsakanika, L.-A.; Pavlopoulos, C.; Papadopoulou, K.; Ochsenkühn, K.-M.; Tsopelas, F.; Chatzitheodoridis, E.; Lyberatos, G.; et al. Bioleaching of Scandium from Bauxite Residue Using Fungus Aspergillus niger. Waste Biomass Valorization 2023, 14, 3377–3390. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zea-Gamboa, F.; Clavijo-Koc, C.; Sandoval-Niebles, J.F.; Chipana-Laura, V.L.; Paredes-Escobar, J.; Condori-Pacoricona, D.A.; Castillo-Cotrina, D. Influence of Native Leptospirillum ferriphilum Strains on Ferric Iron and Leached Copper Recovery from Chalcopyrite to Mesophilic Temperature Under Laboratory Conditions. Appl. Microbiol. 2025, 5, 127. https://doi.org/10.3390/applmicrobiol5040127
Zea-Gamboa F, Clavijo-Koc C, Sandoval-Niebles JF, Chipana-Laura VL, Paredes-Escobar J, Condori-Pacoricona DA, Castillo-Cotrina D. Influence of Native Leptospirillum ferriphilum Strains on Ferric Iron and Leached Copper Recovery from Chalcopyrite to Mesophilic Temperature Under Laboratory Conditions. Applied Microbiology. 2025; 5(4):127. https://doi.org/10.3390/applmicrobiol5040127
Chicago/Turabian StyleZea-Gamboa, Francisco, Claudia Clavijo-Koc, Jose Fernando Sandoval-Niebles, Virginia Liliana Chipana-Laura, Jhonny Paredes-Escobar, Dayana Araceli Condori-Pacoricona, and Daladier Castillo-Cotrina. 2025. "Influence of Native Leptospirillum ferriphilum Strains on Ferric Iron and Leached Copper Recovery from Chalcopyrite to Mesophilic Temperature Under Laboratory Conditions" Applied Microbiology 5, no. 4: 127. https://doi.org/10.3390/applmicrobiol5040127
APA StyleZea-Gamboa, F., Clavijo-Koc, C., Sandoval-Niebles, J. F., Chipana-Laura, V. L., Paredes-Escobar, J., Condori-Pacoricona, D. A., & Castillo-Cotrina, D. (2025). Influence of Native Leptospirillum ferriphilum Strains on Ferric Iron and Leached Copper Recovery from Chalcopyrite to Mesophilic Temperature Under Laboratory Conditions. Applied Microbiology, 5(4), 127. https://doi.org/10.3390/applmicrobiol5040127









