1. Introduction
Bacillus and
Paenibacillus are well recognized as plant growth-promoting bacteria (PGPB), notably due to their production of bioactive compounds. These include nonribosomal peptides (NRPs), lipopeptides, and polyketides, which exhibit a broad range of activities such as antifungal and antimicrobial effects. Nonribosomal peptide synthetases (NRPS) constitute approximately 29.33% of all biosynthetic gene clusters (BGCs) found in
Bacillus genomes, representing one of the largest categories of BGCs in these bacteria. On average, about twelve biosynthetic clusters are identified per
Bacillus genome, with roughly one- third comprising NRPS clusters. Considering that
Bacillus genome sizes range from 4 to 8 megabase pairs (Mb), indicating that NRPS clusters occupy a significant portion of the genomic space [
1]. In
Paenibacillus, biosynthetic genes linked to antimicrobial activity encompass up to 13.9% of the genome, notably including NRPS clusters [
2]. The substantial representation of NRPS clusters in these bacteria underscores their functional importance in the synthesis of biologically active compounds, highlighting their central role in secondary metabolism. Therefore, NRPS clusters form a notable part of the
Paenibacillus genomic landscape, enabling bacterial adaptability and biosynthetic diversity.
Numerous studies have demonstrated that plant diseases caused by phytopathogenic fungi—such as
Fusarium and
Alternaria—can be effectively controlled by bioproducts based on
Bacillus and related genera. For instance,
B. paralicheniformis has shown considerable efficacy in managing vascular wilt of tomato caused by
Fusarium oxysporum and early blight induced by
Alternaria alternata, reducing disease severity by 50–66% [
3]. Similarly,
B. velezensis suppresses
A. alternata by modulating the microbial community on leaf surfaces, thereby limiting pathogen spread. Other studies confirm that
Bacillus and related genera employ diverse biocontrol mechanisms, including antibiotic production, fungal cell wall-degrading enzymes, and induction of systemic resistance in plants. This feature makes them promising biocontrol agents against phytopathogens such as
Fusarium and
Alternaria [
4,
5].
The pursuit of novel nonribosomal products remains critical, given their promise as new antibiotics, urgently needed in light of escalating antibiotic resistance [
6,
7,
8]. Hence, bioinformatic mining combined with in vitro methods such as mass spectrometry, presents a promising approach [
9,
10,
11]. These techniques facilitate not only the identification of biosynthetic genes for target compounds, but also confirm their effective expression in specific strains [
12,
13,
14].
Integrating biosynthetic gene cluster profiles with actual metabolite expression under varying physiological and environmental conditions enables the design of precisely targeted biocontrol agents. Genomic mining, paired with metabolomic profiling opens avenues for discovering previously unknown nonribosomal peptides and other bioactive molecules, while also enabling optimization of bacterial strains to enhance their efficacy as bioproducts. This approach expands the repertoire of environmentally sustainable alternatives to chemical fungicides. The complex regulation of BGCs, especially NRPS clusters, holds promise for the development of novel antibiotics and phytopathogen antagonists with unique modes of action. Realizing this potential in agriculture and medicine requires increasingly close collaboration among bioinformatics, microbiology, and analytical chemistry.
The objective of this study was to characterize the metabolite spectrum of a bioproduct based on ten bacterial strains—two Bacillus and eight Paenibacillus—registered in Russia as a biocontrol agent “Code of balance F1”, used against fusarium wilt in agricultural crops. This analysis aimed to elucidate the diverse array of bioactive compounds produced by these bacterial antagonists and their potential mechanisms of action against fungal pathogens affecting crop production systems.
2. Materials and Methods
2.1. Bacterial Strains
Eight
Paenibacillus strains, along with two
Bacillus strains, were used in this study. These strains were originally isolated from soil samples collected at agricultural sites, specifically from rhizosphere soil (0–10 cm depth near plant roots) sampled in a wheat field in the Krasnodar region, Russia. Previous investigations have confirmed the pronounced antifungal efficacy of these strains against fungi of the genus
Fusarium, demonstrated consistently under both controlled laboratory conditions and in situ field evaluations [
15,
16]. This bacterial formulation is registered under Russian patent law as “Code of balance F1”, and the genomic sequences of these bacterial strains are catalogued within the NCBI database, referenced under BioProject ID PRJNA806727 [
17] (
Table 1).
2.2. Sequencing
DNA extraction from bacterial cell suspension samples was performed using a modified protocol with the MagAttract HMW DNA Kit (Qiagen, Hilden, Germany). The quality of the extracted DNA was assessed by agarose gel electrophoresis, and DNA concentrations were measured using a Qubit 3.0 fluorometer ((Thermo Fisher Scientific, Waltham, MA, USA). Prior to library preparation, short fragments (<3000 bp) and inhibitors were removed via magnetic bead-based purification using Agencourt Ampure XP beads (Beckman Coulter, Brea, CA, USA).
Libraries were prepared from the extracted DNA according to the manufacturer’s Native Barcoding Genomic DNA protocol using kits EXP-NBD104 and SQK-LSK109 (Oxford Nanopore Technologies, Oxford, UK). Sequencing was performed on two flow cells using the MinION device (Oxford Nanopore Technologies, Oxford, UK). The number and quality of reads obtained from the Oxford Nanopore platform are shown in
Table S1.
For sequencing on the Illumina HiSeq 2500 platform, libraries were prepared using the QIAseq FX DNA Library kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The number and quality of reads obtained from the Illumina platform are presented in
Table S2. Quality control of the DNA fragment libraries was carried out using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) (
Figure S1). Sample preparation and sequencing were performed according to Illumina’s recommended protocols.
2.3. Genome Assembly
Raw Oxford Nanopore Technologies (ONT) data were initially obtained in FAST5 format, from which basecalled reads in FASTQ format were generated using the Guppy basecaller in high-accuracy mode (model dna_r9.4.1_450bps_hac.cfg). Paired-end reads of 100 base pairs acquired from the Illumina platform were converted to standard FASTQ format using the bcl-convert software V 3.9.3. The quality of the resulting reads was assessed with FastQC, seqkit stats, pycoQC, and MultiQC tools. Adapter sequences from Oxford Nanopore reads were trimmed using Porechop v0.2.4 [
18], while nucleotide bases with a quality score below 20 (Phred < 20) were trimmed from read ends employing Cutadapt v2.10 [
19]. Error correction of ONT reads was performed using Canu v2.1.1 [
20].
Similarly, adapter sequences and low-quality bases (Phred < 20) were removed from Illumina reads using Cutadapt v2.10. The processed Illumina reads were mapped onto the corresponding Oxford Nanopore-derived genome assemblies using bwa mem v0.7.17 [
21]. Genome assembly polishing was conducted with Pilon v1.23 [
22] to enhance sequence accuracy. De novo genome assemblies from the quality-filtered Oxford Nanopore reads were generated employing Flye v2.8.1 [
23].
2.4. Functional Genome Annotation
Species identification of microorganisms from extracted DNA samples was performed using the longest high-quality reads in conjunction with Kraken v.2.1.04 [
24], employing the standard database comprising all archaeal, bacterial, and viral genome assemblies available in RefSeq release 2020-10-13, as well as the human reference genome assembly GRCh38.p13.
Annotation of the genomic assemblies from the bacterial antagonist strains was conducted on a UNIX-based computational system utilizing the National Center for Biotechnology Information’s (NCBI) Prokaryotic Genome Annotation Pipeline (PGAP) (NCBI PGAP, version 2022-10-03.build6384).
For each bacterial strain, nucleotide sequence files in FASTA format were prepared following the guidelines provided with the PGAP software version 2022-10-03.build6384 [
25]. This preparation included preliminary marking of loci corresponding to genomic fragments, assessing assembly completeness of these fragments, and providing other requisite metadata to enhance annotation accuracy of the assembled genomes.
2.5. Search for Clusters of Secondary Metabolite Synthesis Genes in Annotated Genomes
The identification of secondary metabolite biosynthetic gene clusters, with particular emphasis on nonribosomal peptide synthetase (NRPS) genes present in the genomes of the studied bacterial strains, was conducted using the stand-alone version of antiSMASH v. 6.0.0 [
26].
Gene cluster searching and annotation were carried out on pre-annotated genome assemblies in .gbk format using the searching of all clusters around all databases. The script used for this is provided in the
Supplementary Materials.
2.6. Cultivation of Strains
To evaluate the spectrum of peptides synthesized via nonribosomal peptide synthetases by the bacterial strains, a co-cultivation experiment was performed with the target Fusarium strain (Fus1). Bacterial antagonist strains were re-plated six hours prior to the experiment to obtain actively growing, spore-free cultures. Subsequently, each bacterial strain was co-cultivated on Petri dishes with the phytopathogenic fungus Fusarium graminearum strain Fus1. Co-cultivation was carried out via an accelerated protocol for 3–5 days at 25 °C until clear zones of fungal growth inhibition appeared. This stage aimed to activate the expression of NRPS genes.
Following this, the bacterial biomass was transferred to a liquid medium formulated to produce inocula, consisting of 10 g/L peptone, 10 g/L sodium chloride, 5 g/L yeast extract, 5 g/L glucose, and 8.5 g/L Malt Extract Broth (Merck, Darmstadt, Germany). Cultivation was conducted for 18–24 h in a shaking incubator at 180 rpm and 30 °C. The resulting inoculum was introduced at 5% (
v/
v) into a modified Landy medium with the following composition: 20 g/L glucose, 5 g/L glutamic acid, 1 g/L yeast extract, 0.5 g/L MgSO
4·7H
2O, 0.5 g/L KCl, 0.005 g/L MnSO
4, 0.00016 g/L CuSO
4·5H
2O, 0.00015 g/L Fe
2(SO
4)
3·7H
2O, 1 g/L KH
2PO
4, 0.002 g/L phenylalanine, and 0.016 g/L tryptophan [
27]. Bacterial cultivation in Landy medium for NRPS peptide production was sustained for three days under shaking conditions at 180 rpm and 30 °C.
After 72 h, cell-free culture supernatants were obtained by centrifugation at 6000× g for 30 min. These were subsequently filtered using Stericup ultrafiltration systems (Millipore, St. Louis, MO, USA). A small aliquot of sterile supernatant was reserved for subsequent well diffusion assays to assess antifungal activity. The pH of the remaining supernatant was adjusted to 2.0 to precipitate peptides, followed by overnight precipitation at 4 °C. Peptide precipitates were collected by centrifugation at 12,000× g for 10 min and washed twice with sterile deionized water adjusted to pH 2.0. After removal of the supernatant, the pellet was subjected to methanol extraction overnight. The following morning, the supernatant was transferred to a clean tube, and the pellet was again extracted with methanol overnight to recover residual compounds. All methanolic extracts were combined and concentrated to dryness using a rotary vacuum evaporator at 45–50 °C. The resulting residue was weighed with microgram accuracy and subsequently dissolved in 96% ethanol.
2.7. Mass Spectrometric Analysis
The peptide mixture obtained was subjected to mass spectrometric analysis using a MALDI-TOF instrument (Bruker Daltonics, Billerica, MA, USA), employing α-cyano-4-hydroxycinnamic acid (HCCA) as the matrix. Standard reference compounds included fengycin, iturin, surfactin, polymyxin B (Sigma Aldrich, St. Louis, MO, USA), and polymyxin M sulfate (Kievmedpreparat, Kiev, Ukraine). Their ionization spectra are shown in
Figures S2–S6 (Supplementary Materials).
Potentially similar peptides were further investigated via database comparisons using npAtlas [
28], Norine [
29], PubChem [
30], alongside predictive chemical formula analyses conducted with the Kendrick Formula Predictor [
31]. The search was focused on compounds exhibiting mass-to-charge ratios (
m/
z) greater than 124.
2.8. In Vitro Evaluation of Antifungal Activity of the Studied Strains
Preliminary antifungal activity was assessed via agar well diffusion assays to evaluate fungal growth inhibition by bacterial culture supernatants. For this assay, a mycological loop was used to inoculate a Fusarium culture centrally on Petri dishes containing meat peptone agar supplemented with wort (MPA plus malt wort). Subsequently, four wells were aseptically bored into the agar at the periphery using a sterile cork borer. Each well received 100 μL of culture supernatant from individual bacterial strains. Plates were incubated at 37 °C for 5 to 8 days, with assessments made at day 5. The radius (mm) of the fungal growth inhibition zone around the well containing the supernatant was then measured. The result was expressed as the mean ± SE (Standard Error). The experiments were conducted in triplicate.
Culture supernatants from bacterial strains were produced in flasks containing liquid modified Landy medium at 30 °C, under both static and shaking conditions for 48 h. The activity of the culture fluids was analyzed after various treatments, including centrifugation, protein precipitation, filtration through 3 kDa and 10 kDa membranes, and storage under different temperatures and lyophilization. Ion-exchange syringe columns were also employed to probe the properties of the culture fluids.
3. Results
3.1. Genome Analysis
The genomic identification of bacterial strains revealed that the genomes of strains V3.14 and R4.6 displayed the highest similarity to B. velezensis (genome size approximately 4 million base pairs), whereas the remaining samples corresponded to P. polymyxa (genome size approximately 5.8 million base pairs).
The results of the genome assembly and annotation of the bacterial strains are summarised in
Table 2.
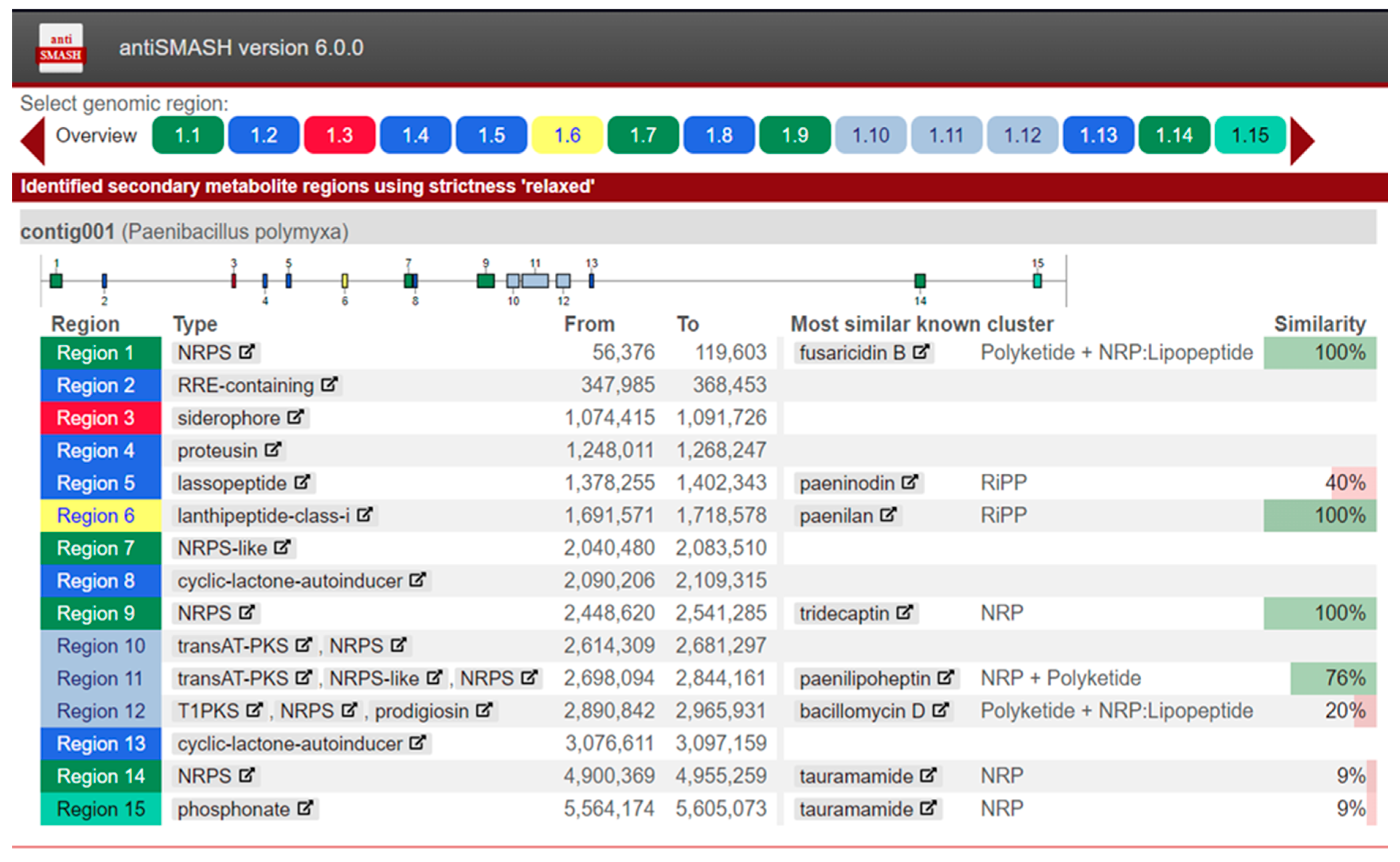
In the annotated genomic assemblies, secondary metabolite biosynthetic gene clusters were identified using antiSMASH v6.0.0. For example, analysis of the genome of
P. polymyxa strain K1.14 revealed 15 gene clusters responsible for the biosynthesis of various secondary metabolites (
Figure 1).
Inspection of the data indicates that approximately half of the identified and annotated secondary metabolite clusters exhibit low homology to known clusters in the antiSMASH database. For instance, the cluster designated as bacillomycin D (region 12) shows only 20% similarity to the reference sequence of that cluster. Clusters with low homology are of particular interest for further study, as they may represent novel secondary metabolites and associated genes with diverse bioactivities, including antifungal properties.
This analysis confirmed the presence of a fusaricidin synthetase biosynthetic gene cluster (region 1). In addition to fusaricidin synthetase, clusters corresponding to synthetases for tridecaptin (region 9) and paenilipoheptin (region 11) were identified. Moreover, a siderophore biosynthetic gene cluster was detected (region 3).
Subsequent analyses were conducted for other bacterial strains. The results of secondary metabolite biosynthetic gene cluster searches for bacterial antagonist strains are presented in
Table 3 and
Table 4, organized according to their genus.
Among the clusters identified in the genomes of strains V3.14 and R4.6 were those encoding surfactin and fengycin synthetases. In addition to fengycin and surfactin synthetases, gene clusters corresponding to bacillibactin, difficidin, bacilysin, and macrolactin synthetases were also discovered.
The consolidated results of secondary metabolite biosynthetic gene cluster searches in strains identified as
P. polymyxa are presented across the tables below (
Table 4 and
Table 5).
The numbers in each column denote the count of detected clusters; the percentages in the table indicate the homology to gene clusters present within the AntiSMASH database.
From the data, it can be inferred that all bacterial strains of the species
P. polymyxa possessed fusaricidin synthetase gene clusters (
Table 4); all strains except K1.14 and R4.24 harboured polymyxin synthetase gene clusters (
Table 5).
Additionally, all strains contained gene clusters encoding tridecaptin synthetase and a close analogue of paeninodin synthetase (
Table 5), suggesting that the peptides produced by these synthetases may contribute to the antifungal activity of these strains.
Interestingly, some strains within this genus exhibited homologues of gene clusters for aurantinin, bacillomycin, and brevicidine synthetases, which are characteristic of other spore-forming bacteria genera such as Bacillus and Brevibacillus. The low similarity of these identified gene clusters to reference clusters in the AntiSMASH database indicates the possibility of novel and as yet undescribed clusters of nonribosomal peptide synthetase genes.
It is also noteworthy that
P. polymyxa strains demonstrated a substantially greater number of secondary metabolite biosynthetic gene clusters compared to
B. velezensis strains (
Table 3).
3.2. Mass Spectrometry
During preliminary analysis, pure peptide standards were acquired against which peptides produced by the selected bacterial strains were analysed. Subsequently, mass spectrometric analysis was conducted on extracts obtained following bacterial cultivation in Landy medium.
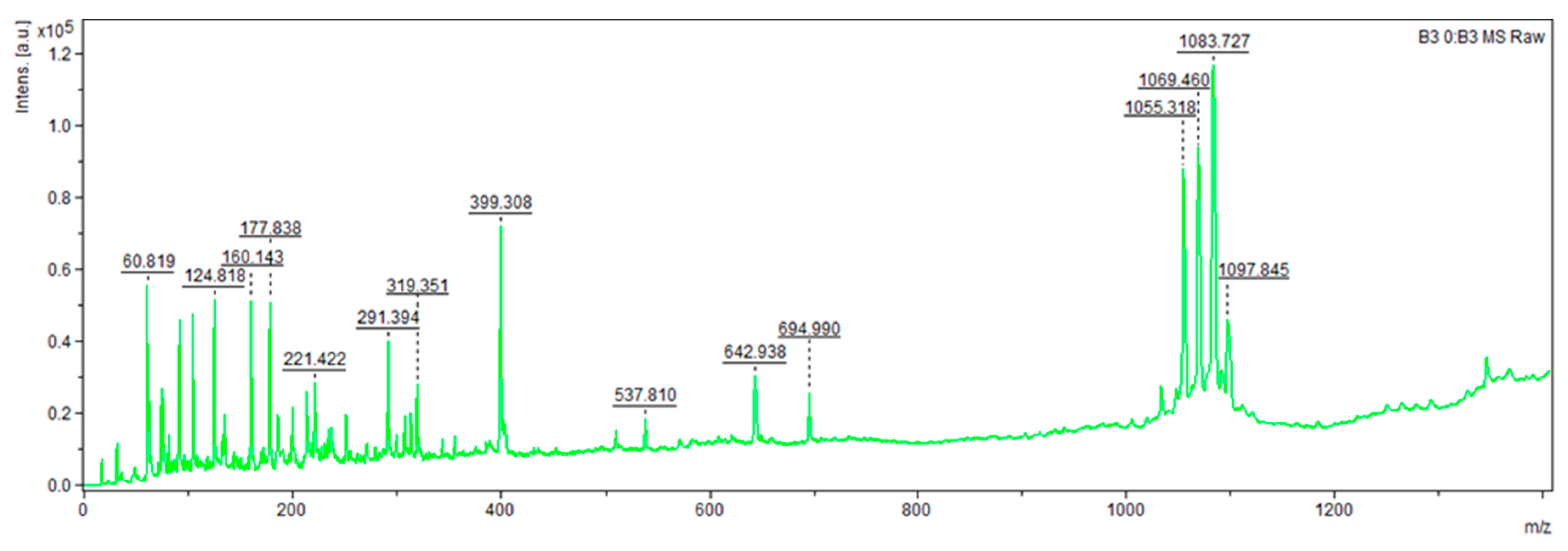
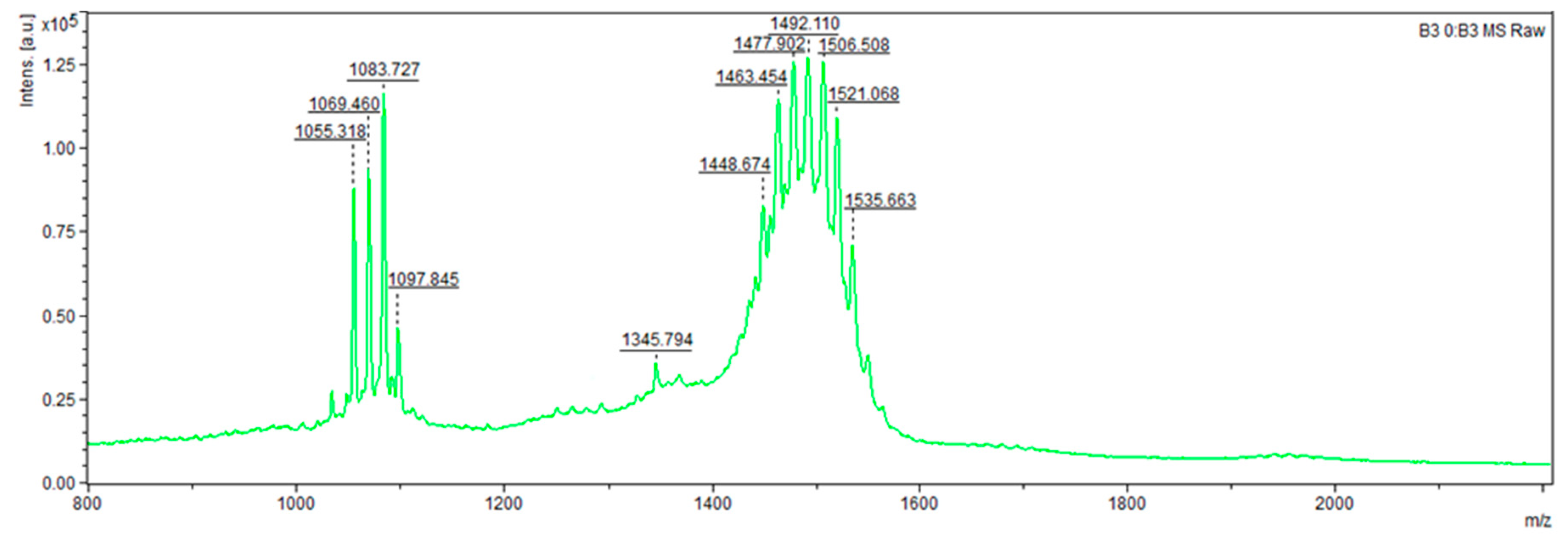
For instance, strain
B. velezensis V3.14 (
Figure 2 and
Figure 3) presented compounds with
m/
z values of 124.818, 160.143, 177.838, 221.422, 291.394, 319.351, 399.308 (potentially an analogue of macrolactin NPA018354 or a homologue of the recently described antifungal compound YM-47522 NPA014036), 537.810 (possible homologue of 3′,4′-Dideoxy-6′-C-methylbutirosin B NPA009107), 642.938 (putative homologue of Ieodoglycolipid NPA029890), 694.990 (potential homologue of Bacilotetrin A NPA022488), 1055.318 and 1069.460 (likely surfactin isoforms NOR00218), 1083.727 (potential mycosubtilin analogue NPA032741), 1097.845 (possible mojavensin analogue NPA029829), 1345.794 (putative brevistin analogue NPA021145), and a series of peptides ranging from 1448.674 to 1535.663, likely representing isoforms of fengycin given their strong similarity to the reference standard. The remaining peptides did not have identifiable homologues.
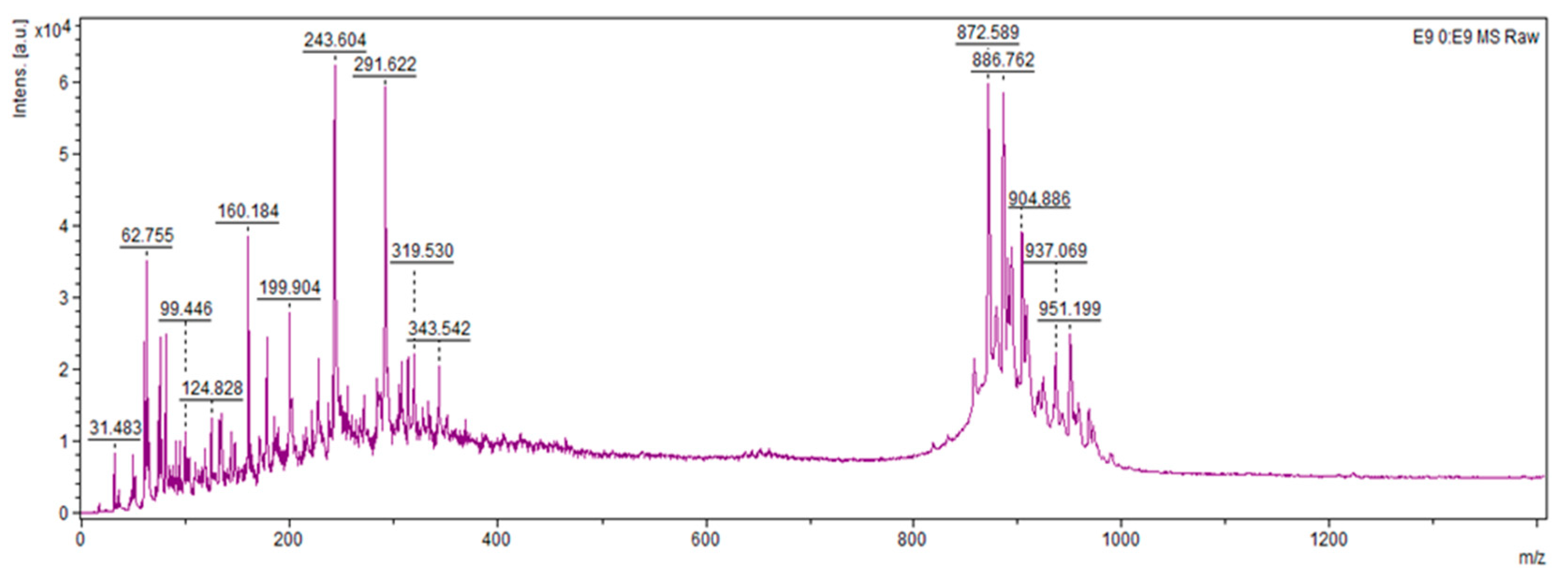
From the data, it can be concluded that all bacterial strains of the species
P. polymyxa possessed fusaricidin synthetase gene clusters (
Table 4); all strains except K1.14 and R4.24 harboured polymyxin synthetase gene clusters (
Table 5). Compounds up to 300
m/
z can be represented by Paleic acid (298.2508, NPA005448) and Paenibacillin A (194.1419, NPA005833)—compounds with pronounced antibacterial activity (
Figure 4). A close homologue to the compound with 951
m/
z can be Paenilamicin A2 (994.6499, NPA024854), which has antibacterial, antifungal and cytotoxic activities. The remaining compounds found in the 870–970
m/
z range could not be found in the NPA Atlas database, which opens up the possibility and scope for analysis to characterize promising metabolites.
In most cases, compounds with m/z up to 124 are protein fragments, organic acids, or amino acids. Analysis of these compounds requires higher-resolution analysis, including HPLC separation, which was not performed since these compounds were not the focal point of our research.
It is noteworthy that the antifungal activity of Paenibacillus strains is likely attributable to compounds with m/z values ranging from 872 to 969, as evidenced by the results of the well diffusion assay. Such compounds were detected exclusively in bacterial strains whose culture supernatants exhibited fungal growth inhibition. Notably, no polymyxin homologues were identified in strains of this genus, possibly reflecting limitations in their biosynthetic capacity due to the relatively nutrient-poor Landy medium used for cultivation.
Conversely, Bacillus strains were found to produce peptides belonging to the surfactin and fengycin families, further supporting the involvement of these peptides in the suppression of Fusarium spp.
3.3. In Vitro Evaluation of Antifungal Activity of the Studied Strains Results
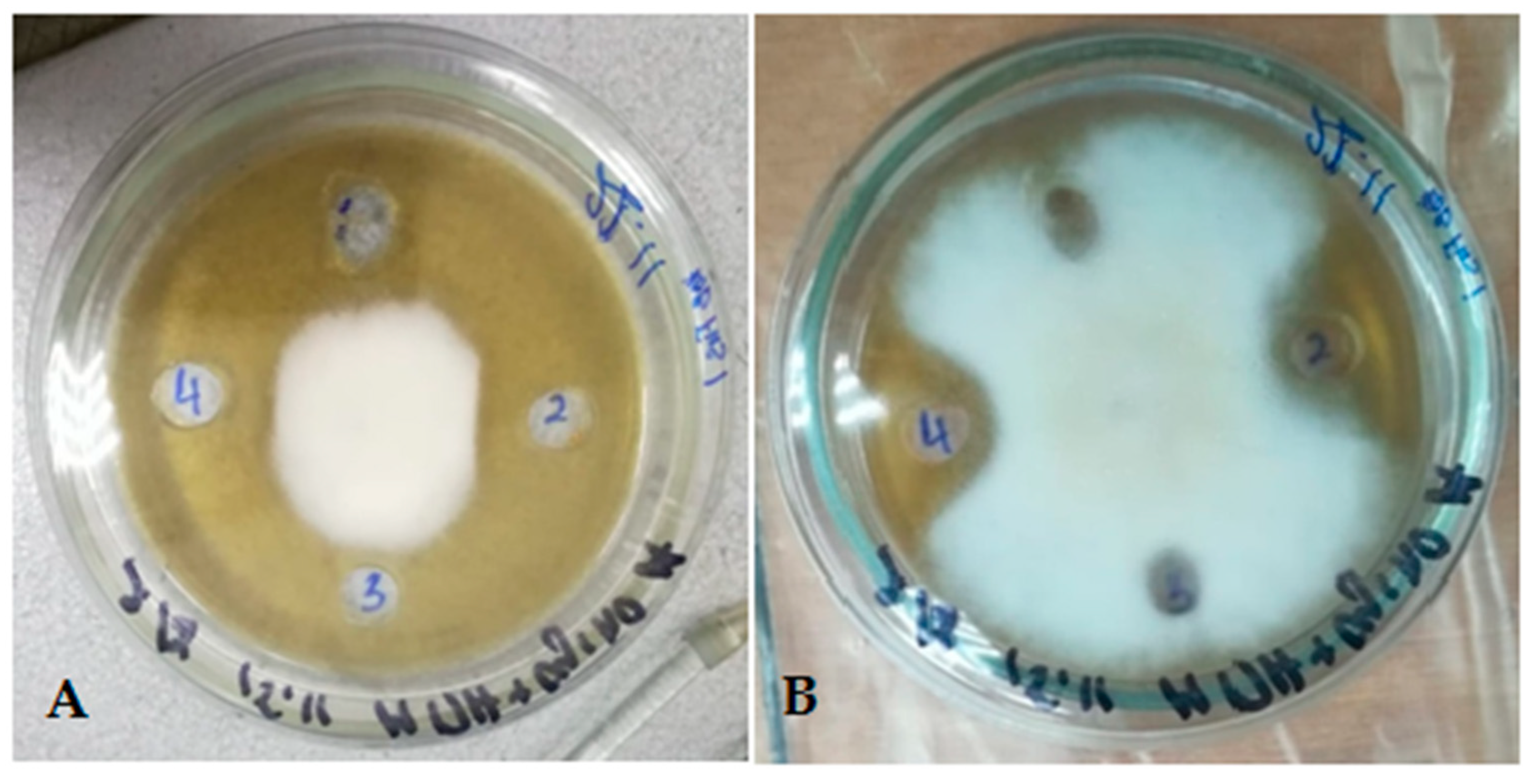
Testing revealed that culture supernatants demonstrated pronounced bioactivity, manifested as clear, well-defined, circular zones of fungal growth inhibition surrounding the applied wells, clearly indicating antagonistic effects. Further investigation established that culture supernatant from
B. subtilis strain R 4.6 cultivated in a modified Landy medium exhibited robust and stable antagonistic activity against
Fusarium species. In contrast,
Paenibacillus strain R 4.5 showed no activity under identical conditions (
Figure 5).
Therefore, culture fluids obtained under shaking conditions were used for subsequent tests (
Table 6).
During this phase of the work, nutrient media with artificial compositions based on simple carbohydrates, amino acids, and dense carriers were selected to replace agar-agar when grown on solid media. This minimized the presence of impurities that could interfere with qualitative analysis by mass spectrometry. Various approaches to purifying the target compounds were also tested.
It was revealed that the medium composition was a critical factor in the synthesis of active metabolites, as active culture supernatants demonstrating inhibition of fungal growth were not obtained using a simple synthetic medium or with the addition of bran, despite good bacterial growth.
Aeration conditions (static or shaking incubation) had no significant effect, as demonstrated by the photograph of the well test.
Passing the culture supernatants through syringes filled with ion-exchange resin resulted in the disappearance of the antagonistic activity of the supernatant, indicating potential adsorption of the compounds onto the resin particles.
Subsequent analysis showed that the antagonistic properties were retained after filtration and protein precipitation, but were lost upon lyophilization, indicating that the active compounds are low-molecular-weight substances (presumably less than 3 kDa), stable during storage, but sensitive to certain treatments.
4. Discussion
4.1. Biosynthetic Potential of Bacillus and Paenibacillus
Genome mining revealed that the genomes of these strains contain numerous secondary metabolite biosynthetic gene clusters, including those encoding nonribosomal peptide synthetases (NRPS), polyketide synthases (PKS), lanthipeptides, bacteriocins, and siderophores.
These findings provide new insights into the biosynthetic versatility of Bacillus and Paenibacillus species, emphasizing their significance as sources of secondary metabolites with strong antifungal potential.
Notably, all strains contained gene clusters for fusaricidin synthetase, tridecaptin synthetase, and analogues of paeninodin, implying a role in antifungal activity. Clusters typical of other spore-forming bacterial genera, such as bacillomycin and brevicidine, were also detected, possibly reflecting horizontal gene transfer events or extensive biochemical diversity.
B. velezensis and related
Bacillus strains characteristically produce lipopeptides such as surfactin, fengycin, and bacillomycin D, alongside polyketides like macrolactin, bacillaene, and difficidin. These compounds are well-characterized antimicrobials with documented roles in plant pathogen suppression and biocontrol. Multiple studies corroborate the presence and activity of these synthetases within
B. velezensis, consistent with the gene clusters identified [
32,
33,
34].
These metabolites plausibly contribute to antifungal effects, according to other studies. Fusaricidin exhibits strong inhibitory activity against various phytopathogenic fungi including
F. oxysporum, the causal agent of vascular wilt, acting through membrane permeability disruption and inhibition of spore and hyphal growth leading to fungal cell death [
35]. Fengycin, a cyclic lipopeptide, demonstrates antifungal activity by damaging fungal cell membranes, causing permeability loss, spore germination inhibition, and apoptosis; these effects have been confirmed against pathogens such as
Botrytis cinerea and
Fusarium spp. [
36]. Polymyxins also possess documented antifungal activity, effectively inhibiting
Fusarium,
Candida albicans, and
Cryptococcus neoformans through membrane disruption [
37]. Strain
P. polymyxa K1.14 harbored 15 such clusters, more than half of which displayed low homology to known databases, suggesting the presence of potentially novel, previously uncharacterized metabolites.
The identified biosynthetic gene clusters (BGCs) collectively highlight the evolutionary adaptation of these bacteria to competitive soil niches, where metabolic diversity ensures ecological success and pathogen suppression. Instead of focusing on individual compounds, the data as a whole suggest that the functional redundancy among distinct BGCs may enhance resistance to environmental stress and sustain persistent antifungal activity under variable conditions. Such redundancy might represent an evolutionary strategy ensuring that critical ecological functions, such as competition with filamentous fungi, remain active even when specific pathways are downregulated.
4.2. Metabolic Diversity In Vitro
Mass spectrometric analysis of bacterial extracts revealed that Bacillus strains produced well-characterized antimicrobial lipopeptides with m/z ratios matching known antimicrobial compounds including surfactin, fengycin, and macrolactin. Strains of B. velezensis V3.14 and R4.6, as well as P. peoriae O1.27, displayed the broadest peptide spectra on minimal media, with surfactin and fengycin production confirmed in the Bacillus strains.
In contrast, Paenibacillus strains, particularly P. peoriae O1.27 and several P. polymyxa and P. jamilae isolates, synthesized distinct peptide profiles with notable compounds in the m/z 850–970 range that correlated with antifungal activity, suggesting production of novel or less-studied bioactive metabolites.
The absence of polymyxin detection in Paenibacillus cultures, while corresponding genes were present, likely reflects the specific nutrient limitations of the Landy medium used, which may not support expression of this specific biosynthetic pathway.
These findings support the hypothesis that the observed antimicrobial activities stem from strain-specific peptide production. It seems that Bacillus strains rely on established lipopeptide families while Paenibacillus isolates contribute through alternative peptide-based mechanisms.
Comparison of genomic and mass spectrometric data confirmed that gene clusters encoding biosynthesis of secondary metabolites in Bacillus and Paenibacillus correlate with detected peptides having m/z values corresponding to known antimicrobial compounds—surfactin, fengycin, macrolactin, and fusaricidin. The peptide masses and structures affirm the biosynthetic potential of the strains to produce these bioactive and antifungal compounds secondary metabolites with antifungal activity. Peptides with m/z 872–969 were identified in Paenibacillus, likely associated with fusaricidin and other nonribosomal peptides, consistent with genetic evidence and their antifungal role. Polymyxins, despite gene presence in some strains, were not detected via mass spectrometry, possibly due to cultivation constraints. Thus, integration of genomics and mass spectrometry provides a comprehensive evaluation of the biosynthetic capacity and antifungal metabolite production in these strains.
In vitro data demonstrated that culture supernatants of the studied microorganisms effectively inhibited phytopathogenic fungal growth, exhibiting stable antagonistic properties influenced by cultivation conditions and medium composition. The decrease in activity following lyophilisation and ion-exchange resin treatments indicates the labile nature of active metabolites, characterized as low-molecular-weight compounds.
4.3. Limitations and Future Perspectives
As a strategy for subsequent functional analysis, additional rounds of functional genome annotation are planned using both other gene annotation programs and functional annotation based on databases, including KEGG, GO, etc., followed by analysis of the identified loci, taking into account the search for homologous amino acid sequences.
It is also necessary to take into account the possible ecological consequences or off-target effects of introducing new bacterial strains or their metabolites into agricultural systems. Since these strains were isolated from soils of agricultural lands, there are potential risks of displacing the native soil microbiota in adjacent field areas, as well as in nearby water bodies. This will require a thorough analysis of long-term consequences, given that a high degree of antagonism may also affect other bacteria.
The identified antifungal compounds have potential applications in agriculture mainly through the use of live bacterial inoculants that produce these metabolites in situ, providing sustainable biocontrol of fungal pathogens and reducing reliance on chemical fungicides, as direct application of purified compounds is economically unfeasible. In pharmaceuticals, these compounds could be developed as antimicrobial agents primarily for veterinary use or as last-resort antibiotics similar to polymyxins, subject to rigorous toxicological and resistance assessments. Practical implementation requires scaling up production, conducting thorough safety and ecological risk assessments, developing stable formulations, obtaining regulatory approvals, and performing field or clinical trials to confirm efficacy and monitor long-term impacts. This combination of steps is essential to transform promising antifungal metabolites from laboratory findings into viable tools for agricultural and medical use.
Although potentially new NRPS were found, there are limitations to the current research, namely the absence of deep analysis of the full composition of all produced compounds by proteomic profiling methods, including amino acid composition analysis using NMR, as well as RNAseq for identifying activated loci and correlating them with metabolites detected in the cell-free culture fluid. This comprehensive approach is necessary for a complete understanding of the biosynthetic output beyond preliminary identification.
Future work should aim to connect these genomic predictions with expression profiles under plant-associated or stress-induced conditions, using transcriptomic or proteomic approaches. RNAseq analysis could identify which biosynthetic loci are selectively upregulated during fungal challenge, guiding the discovery of novel peptides and assessing their regulatory networks. Likewise, metabolomic profiling under different ecological stimuli, including root exudates or soil microcosm conditions, would clarify how bacterial communication and co-cultivation trigger antifungal compound production. This would provide the mechanistic understanding necessary to optimize field applications.
From an applied perspective, the study underscores the potential utility of these strains not through direct metabolite extraction but as living biocontrol agents capable of continuous metabolite production in situ. Such bioformulations could serve as ecologically safe alternatives to chemical fungicides, promoting sustainable crop protection. Implementation in agriculture, however, must consider long-term ecological effects, including potential disturbances of indigenous microbial communities. Evaluating the persistence, competitiveness, and horizontal gene transfer risks of these strains will be crucial before large-scale deployment. In pharmaceutical contexts, some identified metabolite classes—particularly cyclic lipopeptides—may warrant exploration as candidates for veterinary or last-resort antimicrobial therapies, pending thorough toxicological evaluation.
Overall, this study contributes to redefining Bacillus and Paenibacillus as dynamic producers of bioactive molecules at the interface of agriculture, microbiology, and biotechnology. It demonstrates that integrating genomic mining, microbial ecology, and metabolomic validation can bridge the gap between sequence-level discovery and functional antifungal application. Future experimental strategies should therefore combine systems-level omics approaches with ecological modeling to translate these findings into effective and safe biocontrol technologies.