Adopting Biochar as Immobilization Support for Hyper Ammonia-Producing Bacteria Proliferation
Abstract
1. Introduction
2. Materials and Methods
2.1. Media and Fermentation Conditions
2.2. Experimental Setup
2.3. Biochar Concentration Experiments
2.4. Temperature Experiments
2.5. Biochar Particle Size Experiments
2.6. Statistical Analysis
2.7. Scanning Electron Microscope Analysis (SEM)
3. Results
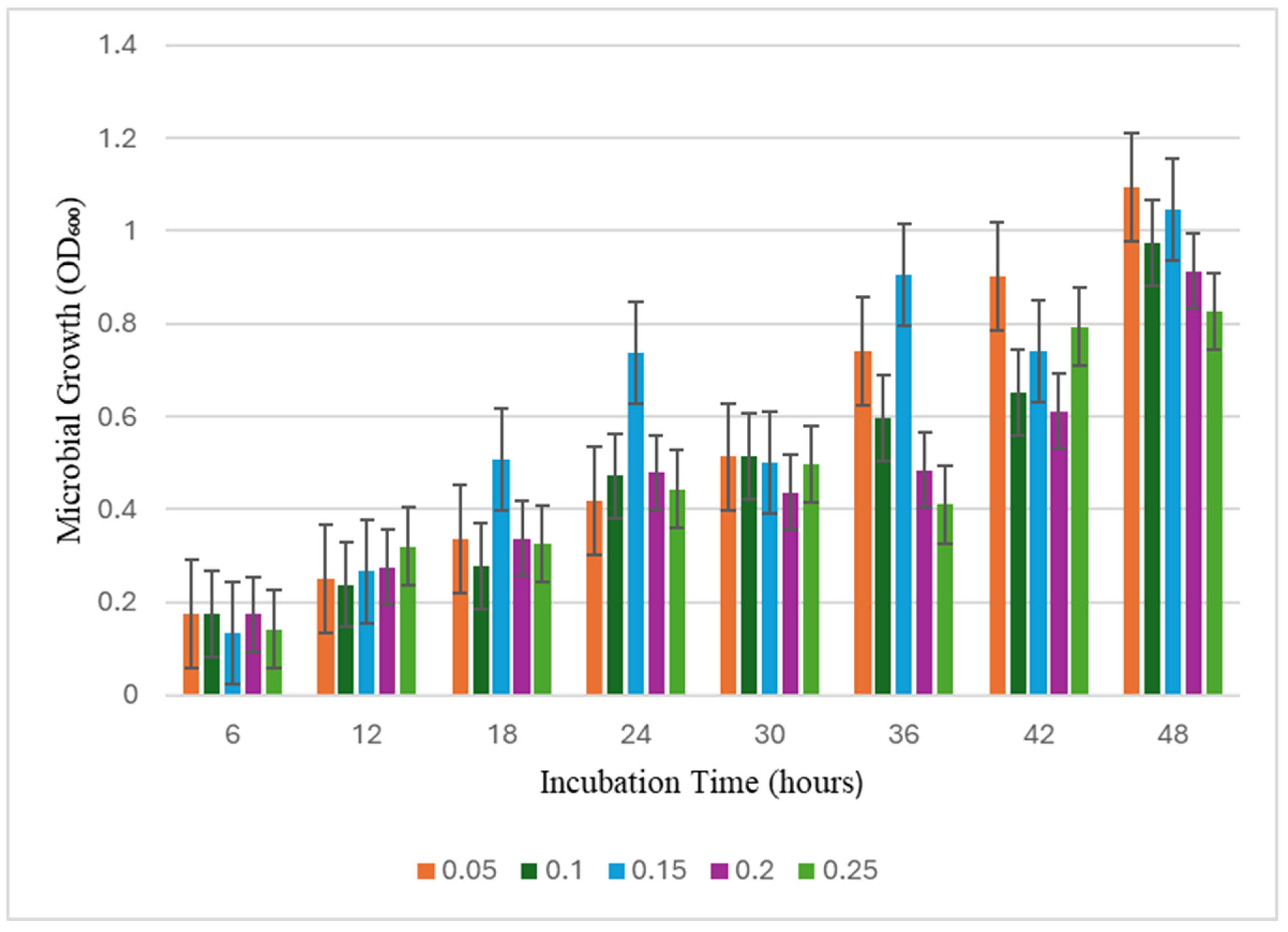
3.1. Biochar Concentration Effects
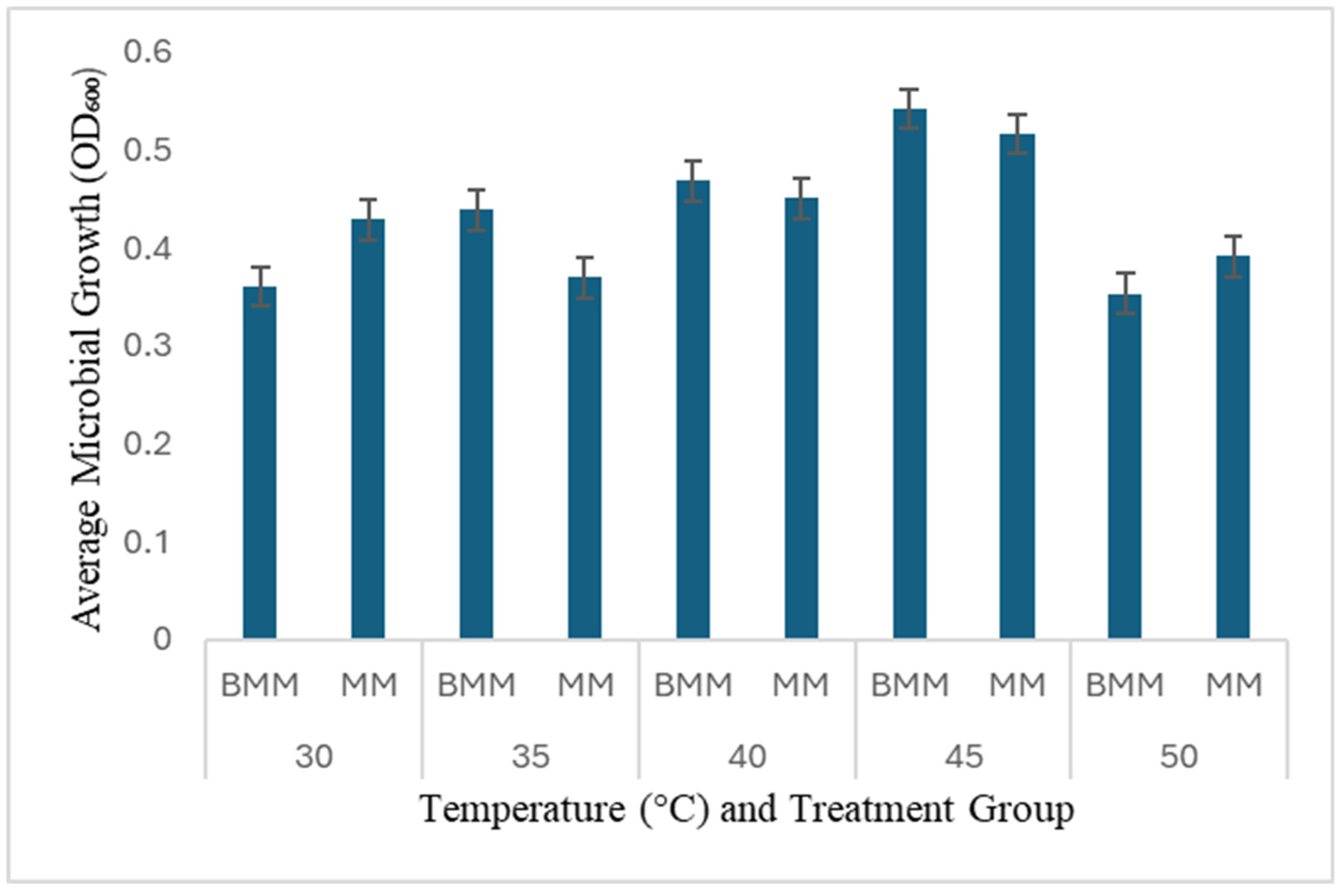
3.2. Temperature Effects
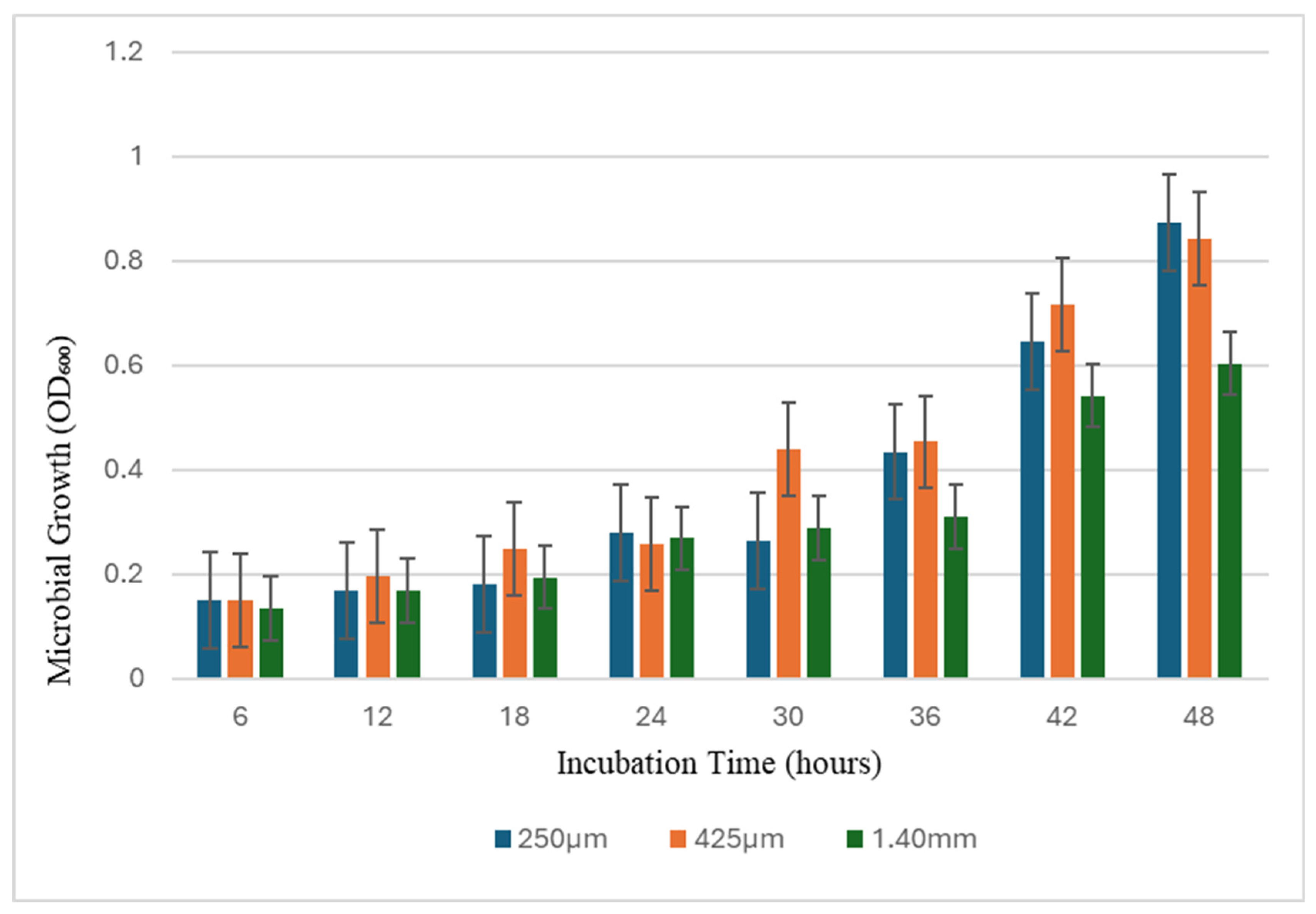
3.3. Particle Size Effects
3.4. Statistical Analysis Results
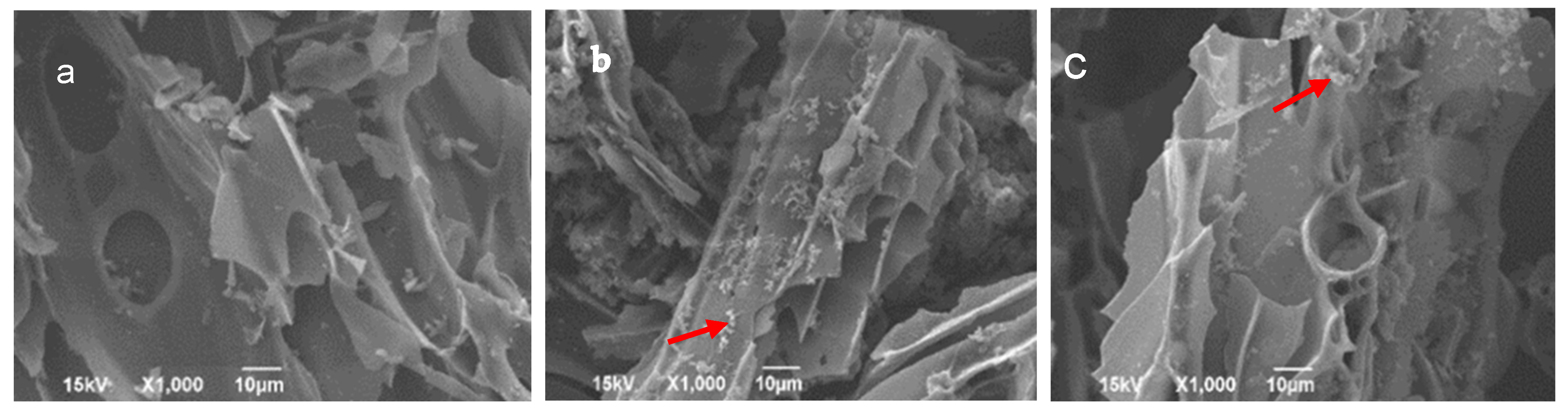
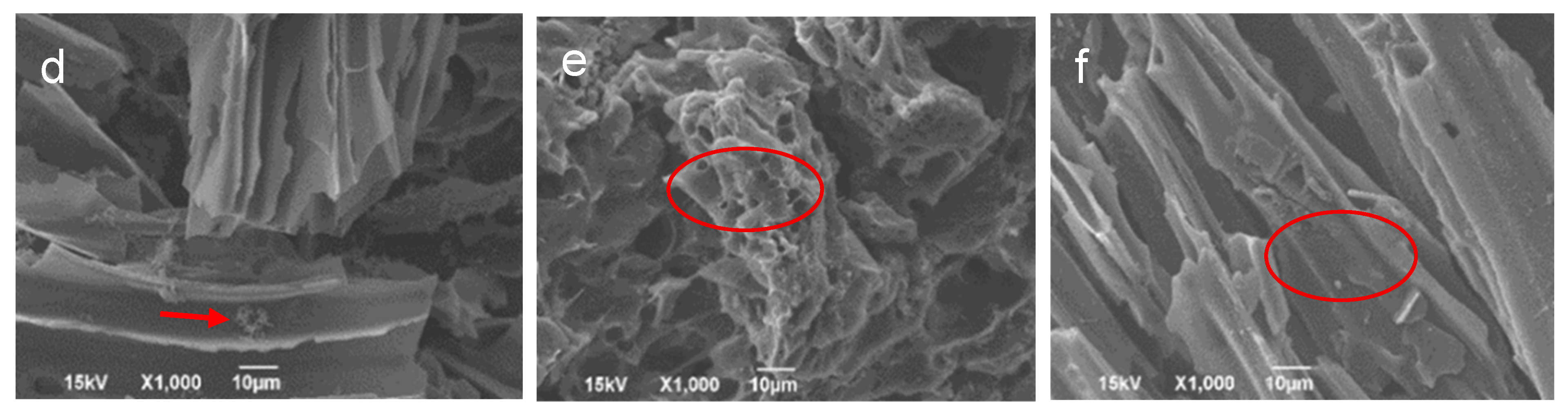
3.5. Biochar, Microbe, and Composite Morphology
4. Discussion
4.1. Scientific Novelty and Mechanistic Basis
4.2. Practical Implications and Environmental Considerations
4.3. Limitations and Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, S.A.; Erol, Z.; Rugji, J.; Taşçı, F.; Kahraman, H.A.; Toppi, V.; Musa, L.; Di Giacinto, G.; Bahmid, N.A.; Mehdizadeh, M.; et al. An Overview of Fermentation in the Food Industry—Looking Back from a New Perspective. Bioresour. Bioprocess. 2023, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Voidarou, C.; Antoniadou, M.; Rozos, G.; Tzora, A.; Skoufos, I.; Varzakas, T.; Lagiou, A.; Bezirtzoglou, E. Fermentative Foods: Microbiology, Biochemistry, Potential Human Health Benefits and Public Health Issues. Foods 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Piątek-Gołda, W.; Osińska-Jaroszuk, M.; Pawlik, A.; Komoń-Janczara, E.; Sulej, J. Chemical Versus Biological Approaches to the Synthesis of Lactobionic Acid: A Review. Molecules 2025, 30, 3330. [Google Scholar] [CrossRef] [PubMed]
- Kotarska, K.; Dziemianowicz, W.; Świerczyńska, A. The Effect of Detoxification of Lignocellulosic Biomass for Enhanced Methane Production. Energies 2021, 14, 5650. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Shang, N.; Li, P. Microbial Fermentation Processes of Lactic Acid: Challenges, Solutions, and Future Prospects. Foods 2023, 12, 2311. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Berillo, D.; Malika, T.; Baimakhanova, B.B.; Sadanov, A.K.; Berezin, V.E.; Trenozhnikova, L.P.; Baimakhanova, G.B.; Amangeldi, A.A.; Kerimzhanova, B. An Overview of Microorganisms Immobilized in a Gel Structure for the Production of Precursors, Antibiotics, and Valuable Products. Gels 2024, 10, 646. [Google Scholar] [CrossRef]
- Najim, A.A.; Radeef, A.Y.; al-Doori, I.; Jabbar, Z.H. Immobilization: The Promising Technique to Protect and Increase the Efficiency of Microorganisms to Remove Contaminants. J. Chem. Technol. Biotechnol. 2024, 99, 1707–1733. [Google Scholar] [CrossRef]
- Verbelen, P.J.; De Schutter, D.P.; Delvaux, F.; Verstrepen, K.J.; Delvaux, F.R. Immobilized Yeast Cell Systems for Continuous Fermentation Applications. Biotechnol. Lett. 2006, 28, 1515–1525. [Google Scholar] [CrossRef]
- Willaert, R.G.; Baron, G.V. Gel Entrapment and Micro-Encapsulation: Methods, Applications and Engineering Principles. Rev. Chem. Eng. 1996, 12, 1–205. [Google Scholar] [CrossRef]
- Suzana, C.S.M.; Claudia, M.M.; Larissa, M.C.G.F.; Sandra, T.S. Immobilization of Microbial Cells: A Promising Tool for Treatment of Toxic Pollutants in Industrial Wastewater. Afr. J. Biotechnol. 2013, 12, 4412–4418. [Google Scholar] [CrossRef]
- Duarte, J.C.; Rodrigues, J.A.R.; Moran, P.J.S.; Valença, G.P.; Nunhez, J.R. Effect of Immobilized Cells in Calcium Alginate Beads in Alcoholic Fermentation. AMB Express 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, S.K.; Pallavi, P.; Shetty, K.; Bhattacharjee, D.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Nair, V. Effective Usage of Biochar and Microorganisms for the Removal of Heavy Metal Ions and Pesticides. Molecules 2023, 28, 719. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; Hassanat, F.; Côrtes, C. Assessment of the Effects of Commercial or Locally Engineered Biochars Produced from Different Biomass Sources and Differing in Their Physical and Chemical Properties on Rumen Fermentation and Methane Production In Vitro. Animals 2023, 13, 3280. [Google Scholar] [CrossRef]
- Huff, M.D.; Marshall, S.; Saeed, H.A.; Lee, J.W. Surface Oxygenation of Biochar through Ozonization for Dramatically Enhancing Cation Exchange Capacity. Bioresour. Bioprocess. 2018, 5, 18. [Google Scholar] [CrossRef]
- Wijitkosum, S.; Jiwnok, P. Elemental Composition of Biochar Obtained from Agricultural Waste for Soil Amendment and Carbon Sequestration. Appl. Sci. 2019, 9, 3980. [Google Scholar] [CrossRef]
- Kayoumu, M.; Wang, H.; Duan, G. Interactions between Microbial Extracellular Polymeric Substances and Biochar, and Their Potential Applications: A Review. Biochar 2025, 7, 62. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Q.; Peng, F.; Yu, J. Biochar Loaded with a Bacterial Strain N33 Facilitates Pecan Seedling Growth and Shapes Rhizosphere Microbial Community. Plants 2024, 13, 1226. [Google Scholar] [CrossRef]
- Lu, J.-H.; Chen, C.; Huang, C.; Lee, D.-J. Glucose Fermentation with Biochar-Amended Consortium: Microbial Consortium Shift. Bioengineered 2020, 11, 272–280. [Google Scholar] [CrossRef]
- Wang, W.; Dai, L.; Wu, B.; Qi, B.; Huang, T.; Hu, G.; He, M. Biochar-Mediated Enhanced Ethanol Fermentation (BMEEF) in Zymomonas mobilis under Furfural and Acetic Acid Stress. Biotechnol. Biofuels 2020, 13, 28. [Google Scholar] [CrossRef]
- Chanu, Y.M.; Paul, S.S.; Dey, A.; Andonissamy, J. Deciphering Hyperammonia-Producing Bacteria (HAB) in the Rumen of Water Buffaloes (Bubalus bubalis) and Their Inhibition through Plant Extracts and Essential Oils. Microorganisms 2024, 12, 2040. [Google Scholar] [CrossRef] [PubMed]
- Bello, I.; Adeniyi, A.; Mukaila, T.; Monono, E.; Hammed, A. Biological Ammonia Production via Anaerobic Fermentation of Soy Meal Protein. Front. Biosci. (Elite Ed.) 2023, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, A.; Bello, I.; Mukaila, T.; Monono, E.; Hammed, A. Developing Rumen Mimicry Process for Biological Ammonia Synthesis. Bioprocess. Biosyst. Eng. 2023, 46, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, R.; Neves, A.L.A.; Sapkota, R.; Khanal, P.; Hansen, H.H. Prokaryote Composition and Structure of Rumen Fluid before and after In Vitro Rumen Fermentation. Fermentation 2024, 10, 108. [Google Scholar] [CrossRef]
- O’Reilly, G.C.; Huo, Y.; Meale, S.J.; Chaves, A.V. Dose Response of Biochar and Wood Vinegar on in Vitro Batch Culture Ruminal Fermentation Using Contrasting Feed Substrates. Transl. Anim. Sci. 2021, 5, txab107. [Google Scholar] [CrossRef]
- He, P.; Zhang, H.; Duan, H.; Shao, L.; Lü, F. Continuity of Biochar-Associated Biofilm in Anaerobic Digestion. Chem. Eng. J. 2020, 390, 124605. [Google Scholar] [CrossRef]
- Kinyua, E.M.; Mwangi, I.W.; Wanjau, R.N.; Ngila, J.C. Clarification of Colloidal and Suspended Material in Water Using Triethanolamine Modified Maize Tassels. Env. Sci. Pollut. Res. Int. 2016, 23, 5214–5221. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M. Role of Coagulation/Flocculation as a Pretreatment Option to Reduce Colloidal/Bio-Colloidal Fouling in Tertiary Filtration of Textile Wastewater: A Review and Future Outlooks. Front. Environ. Sci. 2023, 11, 1142227. [Google Scholar] [CrossRef]
- Waqas, M.; Shahzad, R.; Hamayun, M.; Asaf, S.; Khan, A.L.; Kang, S.-M.; Yun, S.; Kim, K.-M.; Lee, I.-J. Biochar Amendment Changes Jasmonic Acid Levels in Two Rice Varieties and Alters Their Resistance to Herbivory. PLoS ONE 2018, 13, e0191296. [Google Scholar] [CrossRef]
- Frenkel, O.; Jaiswal, A.K.; Elad, Y.; Lew, B.; Kammann, C.; Graber, E.R. The Effect of Biochar on Plant Diseases: What Should We Learn While Designing Biochar Substrates? J. Environ. Eng. Landsc. Manag. 2017, 25, 105–113. [Google Scholar] [CrossRef]
- Li, K.; Yang, B.; Wang, H.; Xu, X.; Gao, Y.; Zhu, Y. Dual Effects of Biochar and Hyperaccumulator Solanum nigrum L. on the Remediation of Cd-Contaminated Soil. PeerJ 2019, 7, e6631. [Google Scholar] [CrossRef]
- Shan, S.; Coleman, M.D. Biochar Influences Nitrogen Availability in Andisols of North Idaho Forests. SN Appl. Sci. 2020, 2, 362. [Google Scholar] [CrossRef]
- Schommer, V.A.; Nazari, M.T.; Melara, F.; Braun, J.C.A.; Rempel, A.; dos Santos, L.F.; Ferrari, V.; Colla, L.M.; Dettmer, A.; Piccin, J.S. Techniques and Mechanisms of Bacteria Immobilization on Biochar for Further Environmental and Agricultural Applications. Microbiol. Res. 2024, 278, 127534. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zeng, H.; Yang, F.; Yang, Y.; Qiao, Z.; Zhao, X.; Wang, L.; Wu, F. Functional Biochar as Sustainable Precursors to Boost the Anaerobic Digestion of Waste Activated Sludge from a Circular Economy Perspective: A Review. Biochar 2024, 6, 60. [Google Scholar] [CrossRef]
- Hill, R.A.; Hunt, J.; Sanders, E.; Tran, M.; Burk, G.A.; Mlsna, T.E.; Fitzkee, N.C. Effect of Biochar on Microbial Growth: A Metabolomics and Bacteriological Investigation in E. coli. Environ. Sci. Technol. 2019, 53, 2635–2646. [Google Scholar] [CrossRef]
- Ding, J.; Zhen, F.; Kong, X.; Hu, Y.; Zhang, Y.; Gong, L. Effect of Biochar in Modulating Anaerobic Digestion Performance and Microbial Structure Community of Different Inoculum Sources. Fermentation 2024, 10, 151. [Google Scholar] [CrossRef]
- Ahmad, W.; Nepal, J.; Zou, Z.; Munsif, F.; Khan, A.; Ahmad, I.; Zaheer, S.; Khan, M.S.; Jadoon, S.A.; Tang, D. Biochar Particle Size Coupled with Biofertilizer Enhances Soil Carbon-Nitrogen Microbial Pools and CO2 Sequestration in Lentil. Front. Environ. Sci. 2023, 11, 1114728. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, H.; Liang, X.; Fu, R.; Li, M.; Chen, C. Effect of Different Biochar Particle Sizes Together with Bio-Organic Fertilizer on Rhizosphere Soil Microecological Environment on Saline–Alkali Land. Front. Environ. Sci. 2022, 10, 949190. [Google Scholar] [CrossRef]
- Tang, E.; Liao, W.; Thomas, S.C. Optimizing Biochar Particle Size for Plant Growth and Mitigation of Soil Salinization. Agronomy 2023, 13, 1394. [Google Scholar] [CrossRef]
- Tahery, S.; Parra, M.C.; Munroe, P.; Mitchell, D.R.G.; Meale, S.J.; Joseph, S. Developing an Activated Biochar-Mineral Supplement for Reducing Methane Formation in Anaerobic Fermentation. Biochar 2025, 7, 26. [Google Scholar] [CrossRef]
- Zhang, L.; Lim, E.Y.; Loh, K.-C.; Ok, Y.S.; Lee, J.T.E.; Shen, Y.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Biochar Enhanced Thermophilic Anaerobic Digestion of Food Waste: Focusing on Biochar Particle Size, Microbial Community Analysis and Pilot-Scale Application. Energy Convers. Manag. 2020, 209, 112654. [Google Scholar] [CrossRef]
- Wu, S.-L.; Wei, W.; Xu, Q.; Huang, X.; Sun, J.; Dai, X.; Ni, B.-J. Revealing the Mechanism of Biochar Enhancing the Production of Medium Chain Fatty Acids from Waste Activated Sludge Alkaline Fermentation Liquor. ACS EST Water 2021, 1, 1014–1024. [Google Scholar] [CrossRef]
- Abd-Elhamied, A.S.; El-Shiekha, A.M. Effect of Biochar Source, Particle Size and Application Rates on Soil Properties and Maize Yield (Zea mays L.) under Sandy Soil Conditions. J. Soil Sci. Agric. Eng. 2021, 12, 71–80. [Google Scholar] [CrossRef]
- Chang, X.; Wang, S.; Luo, C.; Zhang, Z.; Duan, J.; Zhu, X.; Lin, Q.; Xu, B. Responses of Soil Microbial Respiration to Thermal Stress in Alpine Steppe on the Tibetan Plateau. Eur. J. Soil Sci. 2012, 63, 325–331. [Google Scholar] [CrossRef]
- Ye, J.-S.; Bradford, M.A.; Maestre, F.T.; Li, F.-M.; García-Palacios, P. Compensatory Thermal Adaptation of Soil Microbial Respiration Rates in Global Croplands. Glob. Biogeochem. Cycles 2020, 34, e2019GB006507. [Google Scholar] [CrossRef]
- Bednik, M.; Medyńska-Juraszek, A.; Ćwieląg-Piasecka, I.; Dudek, M. Enzyme Activity and Dissolved Organic Carbon Content in Soils Amended with Different Types of Biochar and Exogenous Organic Matter. Sustainability 2023, 15, 15396. [Google Scholar] [CrossRef]
- Bergman, R.; Sahoo, K.; Englund, K.; Mousavi-Avval, S.H. Lifecycle Assessment and Techno-Economic Analysis of Biochar Pellet Production from Forest Residues and Field Application. Energies 2022, 15, 1559. [Google Scholar] [CrossRef]
- Crowder, D.W.; Reganold, J.P. Financial Competitiveness of Organic Agriculture on a Global Scale. Proc. Natl. Acad. Sci. USA 2015, 112, 7611–7616. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic Agriculture in the Twenty-First Century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, Soil and Land-Use Interactions That Reduce Nitrate Leaching and N2O Emissions: A Meta-Analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil Microbial Inoculants for Sustainable Agriculture: Limitations and Opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitrus, C.; Hammed, A.; Ayodele, T.; Alarape, K.; Chandra Sarker, N.; Clementson, C.; Monono, E. Adopting Biochar as Immobilization Support for Hyper Ammonia-Producing Bacteria Proliferation. Appl. Microbiol. 2025, 5, 111. https://doi.org/10.3390/applmicrobiol5040111
Bitrus C, Hammed A, Ayodele T, Alarape K, Chandra Sarker N, Clementson C, Monono E. Adopting Biochar as Immobilization Support for Hyper Ammonia-Producing Bacteria Proliferation. Applied Microbiology. 2025; 5(4):111. https://doi.org/10.3390/applmicrobiol5040111
Chicago/Turabian StyleBitrus, Christiana, Ademola Hammed, Tawakalt Ayodele, Kudirat Alarape, Niloy Chandra Sarker, Clairmont Clementson, and Ewumbua Monono. 2025. "Adopting Biochar as Immobilization Support for Hyper Ammonia-Producing Bacteria Proliferation" Applied Microbiology 5, no. 4: 111. https://doi.org/10.3390/applmicrobiol5040111
APA StyleBitrus, C., Hammed, A., Ayodele, T., Alarape, K., Chandra Sarker, N., Clementson, C., & Monono, E. (2025). Adopting Biochar as Immobilization Support for Hyper Ammonia-Producing Bacteria Proliferation. Applied Microbiology, 5(4), 111. https://doi.org/10.3390/applmicrobiol5040111





