Streptococcus thermophilus: Metabolic Properties, Functional Features, and Useful Applications
Abstract
1. Introduction
2. Streptococcus thermophilus Classification
3. Metabolomic Compounds of Streptococcus thermophilus
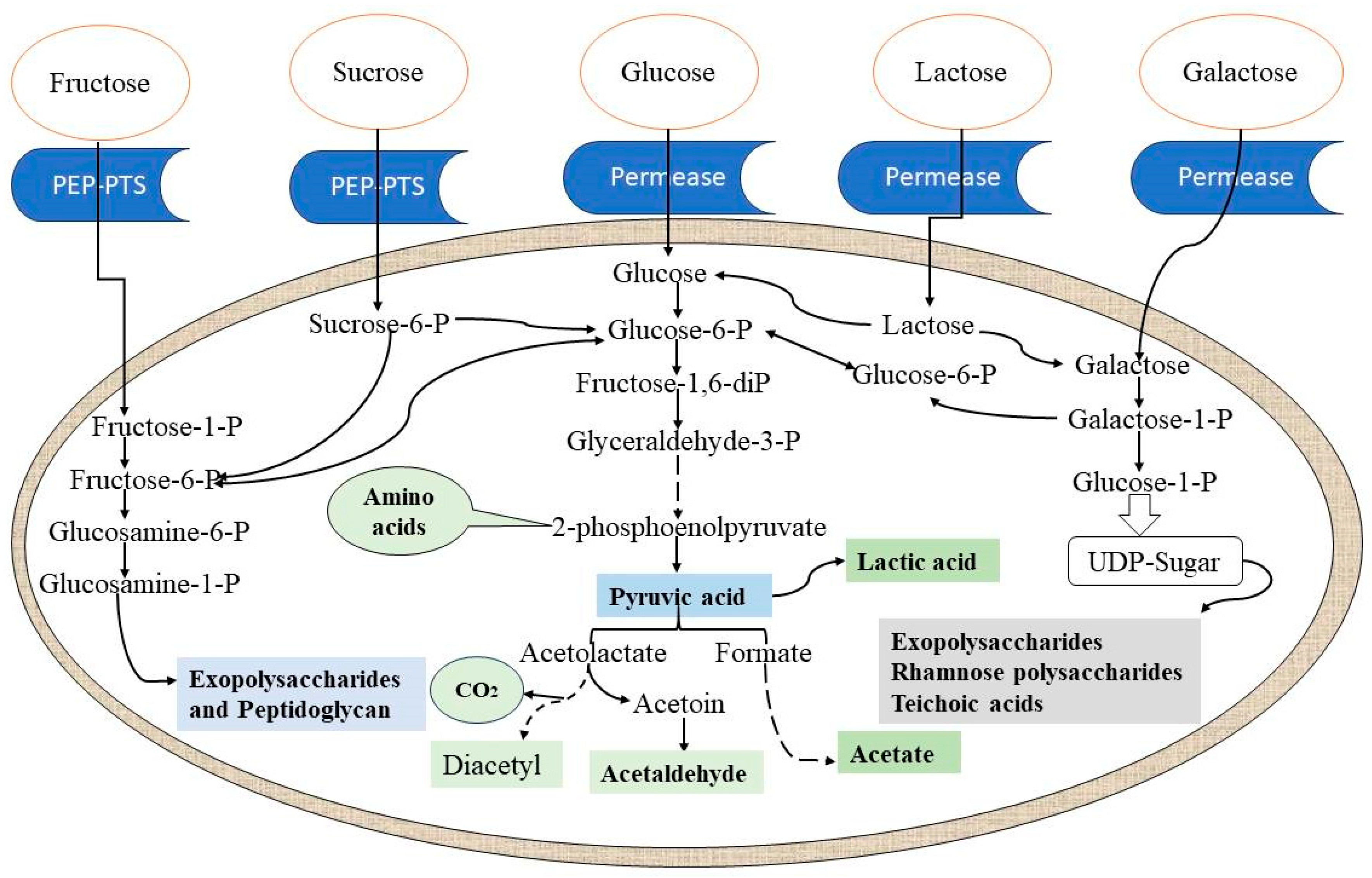
3.1. Organic Acids
3.2. Polysaccharides
3.3. Aromatic Compounds
3.4. Bacteriocins
| Bacteriocin Name | Type/Class | Molecular Weight (Da)/Amino Acids | Key Characteristics | Activity Spectrum (Examples) | References |
|---|---|---|---|---|---|
| Thermophilin 13 | Class IIb (Two-peptide bacteriocin) | 5776 (62 aa, ThmA); 3910 (43 aa, ThmB) | Requires two peptides (ThmA and ThmB) for activity. Genes characterized | Lt. monocytogenes, Bacillus spp., S. thermophilus | [67,70] |
| Thermophilin 110 | Unspecified (likely Class I or II) | - | Heat-stable, broad-spectrum. Production can be influenced by growth media. | Pediococcus acidilactici, other spoilage and food-borne pathogenic bacteria | [65,93] |
| Bacteriocin from S. thermophilus 81 | Unspecified | ~32 amino acids | Heat-labile but pH-stable (3–10). Activity not affected by 6 months storage at 4 °C. Inactivated by detergents and proteolytic enzymes. | Bacillus spp., Lt. monocytogenes, Sl. typhimurium, E. coli, Yersinia pseudotuberculosis, Y. enterocolitica, L. delbrueckii subsp. bulgaricus. | [70] |
| BlpU (from strain B59671) | Class II (encoded within blp gene cluster) | ~5–6 kDa (heat-stable peptide) | Broad-spectrum. Production regulated by quorum sensing (BlpC induction peptide). Inactivated by protease treatment. | Enterococcus faecalis, E. faecium, L. helveticus, S. mutans, S. salivarius, B. cereus, S. pyogenes | [94] |
| Bacteriocin from S. thermophilus LMD-9 (Thermophilin 9 related) | Class II (Multiple peptides, blp locus dependent) | - | Inhibitory spectrum dependent on multiple peptides and the activity of BlpGSt. Quorum-sensing regulated. | [95] | |
| Bacteriocin from S. thermophilus ACA-DC 0001 | Unspecified | - | Heat-unstable (activity lost at 60 °C for 1 h). | Not specified, but likely similar to other St. thermophilus bacteriocins. | [96] |
| Bacteriocin from S. thermophilus CHCC 3534 | Unspecified | 14.4 to 18.4 kDa (partially purified) | Heat-stable, pH-resistant, inactivated by proteolytic enzymes, resistant to α-amylase and lipase. | Broad antimicrobial spectrum against St. aureus Sl. typhimurium. | [97] |
| Bacteriocin from S. thermophilus ST109 | Class II (blp gene cluster encoded) | ~5–6 kDa (heat-stable) | Production regulated by BlpC (quorum sensing). Inactivated by protease and α-amylase. | Lactobacilli, enterococci, S. pyogenes. | [70] |
4. Application of Streptococcus thermophilus
4.1. In Food
4.2. In Medicine
4.3. In Biotechnology
5. Recent Trends of Streptococcus thermophilus Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAD | Antibiotic-associated diarrhea |
| AMR | Antimicrobial resistance |
| CFU | Colony-forming units |
| Da | Dalton |
| EFSA | European Food Safety Authority |
| FDA | Food and Drug Administration |
| GDH | Glutamate dehydrogenase |
| GRAS | Generally Recognized as Safe |
| LAB | Lactic acid bacteria |
| MDR | Multidrug-resistant |
| MRSA | Methicillin-resistant St. aureus |
| PDO | Protected Designations of Origin |
| PEP | Phosphoenolpyruvate |
| PTS | Phosphotransferase system |
| SHMT | Serine hydroxymethyl transferase |
References
- Patel, A.; Shah, N.; Verma, D.K. Lactic Acid Bacteria (LAB) Bacteriocins: An Ecological and Sustainable Biopreservative Approach to Improve the Safety and Shelf-life of Foods. In Microorganisms in Sustainable Agriculture, Food and the Environment; Verma, D.K., Srivastav, P.P., Eds.; Innovations in Agricultural Microbiology; CRC Press: Boca Raton, FL, USA; Apple Academic Press: Palm Bay, FL, USA, 2017; Volume 1, pp. 197–258. [Google Scholar]
- Al-Sahlany, S.T.G.; Niamah, A.K. Bacterial Viability, Antioxidant Stability, Antimutagenicity and Sensory Properties of Onion Types Fermentation by Using Probiotic Starter during Storage. Nutr. Food Sci. 2022, 52, 901–916. [Google Scholar] [CrossRef]
- Niamah, A.K.; Al-Sahlany, S.T.G.; Verma, D.K.; Shukla, R.M.; Patel, A.R.; Tripathy, S.; Singh, S.; Baranwal, D.; Singh, A.K.; Utama, G.L.; et al. Emerging Lactic Acid Bacteria Bacteriocins as Anti-Cancer and Anti-Tumor Agents for Human Health. Heliyon 2024, 10, e37054. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Srivastav, P.P. Microorganisms in Sustainable Agriculture, Food and the Environment; CRC Press: Boca Raton, FL, USA; Apple Academic Press: Palm Bay, FL, USA, 2017; ISBN 9781771884792. [Google Scholar] [CrossRef]
- Verma, D.K.; Patel, A.R.; Srivastav, P.P.; Mohapatra, B.; Niamah, A.K. Microbiology for Food and Health Technological Developments and Advances; CRC Press: Boca Raton, FL, USA; Apple Academic Press: Palm Bay, FL, USA, 2020; ISBN 9781771888134. [Google Scholar] [CrossRef]
- Raman, J.; Kim, J.S.; Choi, K.R.; Eun, H.; Yang, D.; Ko, Y.J.; Kim, S.J. Application of Lactic Acid Bacteria (LAB) in Sustainable Agriculture: Advantages and Limitations. Int. J. Mol. Sci. 2022, 23, 7784. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Patel, A.R.; Billoria, S.; Kaushik, G.; Kaur, M. Microbial Biotechnology in Food Processing and Health Advances, Challenges, and Potential; CRC Press: Boca Raton, FL, USA; Apple Academic Press: Palm Bay, FL, USA, 2022; ISBN 9781774637289. [Google Scholar]
- Hosseini, E.; Tsegay, Z.T.; Smaoui, S.; Varzakas, T. Lactic Acid Bacteria in Vinegar Fermentation: Diversity, Functionality and Health Benefits. Foods 2025, 14, 698. [Google Scholar] [CrossRef]
- Loo, J.S.; Oslan, S.N.H.; Mokshin, N.A.S.; Othman, R.; Amin, Z.; Dejtisakdi, W.; Prihanto, A.A.; Tan, J.S. Comprehensive Review of Strategies for Lactic Acid Bacteria Production and Metabolite Enhancement in Probiotic Cultures: Multifunctional Applications in Functional Foods. Fermentation 2025, 11, 241. [Google Scholar] [CrossRef]
- Al-Sahlany, S.T.G.; Khassaf, W.H.; Niamah, A.K.; Abd Al-Manhel, A.J. Date Juice Addition to Bio-Yogurt: The Effects on Physicochemical and Microbiological Properties during Storage, as Well as Blood Parameters in Vivo. J. Saudi Soc. Agric. Sci. 2023, 22, 71–77. [Google Scholar] [CrossRef]
- Niamah, A.K.; Al-fekaiki, D.F.; Thyab Gddoa Al-Sahlany, S.; Verma, D.K.; Patel, A.R.; Singh, S. Investigating the Effect of Addition of Probiotic Microorganisms (Bacteria or Yeast) to Yoghurt on the Viability and Volatile Aromatic Profiles. J. Food Meas. Charact. 2023, 17, 5463–5473. [Google Scholar] [CrossRef]
- Han, M.; Wu, Y.; Guo, X.; Jiang, L.; Wang, X.; Gai, Z. Milk Fermentation by Monocultures or Co-Cultures of Streptococcus thermophilus Strains. Front. Bioeng. Biotechnol. 2022, 10, 1097013. [Google Scholar] [CrossRef]
- Toit, M.D.; Huch, M.; Cho, G.S.; Franz, C.M. The genus streptococcus. Lactic Acid Bacteria: Biodiversity and Taxonomy; Wiley: Hoboken, NJ, USA, 2014; pp. 457–505. [Google Scholar]
- Wu, T.; Guo, S.; Kwok, L.Y.; Zhang, H.; Wang, J. Temperature-Dependent Metabolic Interactions between Streptococcus thermophilus and Lactobacillus delbrueckii ssp. Bulgaricus in Milk Fermentation: Insights from Gas Chromatography–Ion Mobility Spectrometry Metabolomics. J. Dairy Sci. 2025, 108, 242–256. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, T.; Qu, X.; Hu, T.; Jiang, X.; Zhao, C. New Insights into Various Production Characteristics of Streptococcus thermophilus Strains. Int. J. Mol. Sci. 2016, 17, 1701. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Ibrahim, S.A. Lactic Acid Bacteria: An Essential Probiotic and Starter Culture for the Production of Yoghurt. Int. J. Food Sci. Technol. 2022, 57, 7008–7025. [Google Scholar] [CrossRef]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic Acid Bacteria in Raw-Milk Cheeses: From Starter Cultures to Probiotic Functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef] [PubMed]
- Tarrah, A. Characterization and Assessment of Health-Related Probiotic Properties of Newly Isolated Lactic Acid Bacteria and Study of Their Technological Potential by In-Silico, In-Vitro, and In-Vivo Approaches. PhD Thesis, Department of Agronomy, Animals, Food, Natural Resources, and Environment, University of Padua, Padua, Italy, 2019. [Google Scholar]
- Iyer, R.; Tomar, S.K.; Uma Maheswari, T.; Singh, R. Streptococcus thermophilus Strains: Multifunctional Lactic Acid Bacteria. Int. Dairy J. 2010, 20, 133–141. [Google Scholar] [CrossRef]
- Arioli, S.; Della Scala G’ Martinović, A.; Scaglioni, L.; Mazzini, S.; Volonté, F.; Pedersen, M.B.; Mora, D. In Streptococcus thermophilus, ammonia from urea hydrolysis paradoxically boosts acidification and reveals a new regulatory mechanism of glycolysis. Microbiol. Spectr. 2022, 10, e02760-21. [Google Scholar] [CrossRef]
- Roux, E.; Nicolas, A.; Valence, F.; Siekaniec, G.; Chuat, V.; Nicolas, J.; Le Loir, Y.; Guédon, E. The Genomic Basis of the Streptococcus thermophilus Health-Promoting Properties. BMC Genom. 2022, 23, 210. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, G.M.; García-Garibay, J.M.; Cruz-Guerrero, A.E.; Gómez-Ruiz, L.D.C.; Ayala-Niño, A.; Castañeda-Ovando, A.; González-Olivares, L.G. Proteolytic System of Streptococcus Thermophilus. J. Microbiol. Biotechnol. 2018, 28, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, Z.; Cakir-Kiefer, C.; Girardet, J.M.; Lecomte, X.; Paris, C.; Galia, W.; Dary, A.; Miclo, L. New Insights into the Proteolytic System of Streptococcus Thermophilus: Use of Isracidin to Characterize Cell-Associated Extracellular Peptidase Activities. J. Agric. Food Chem. 2015, 63, 7522–7531. [Google Scholar] [CrossRef]
- Ulmer, A.; Erdemann, F.; Mueller, S.; Loesch, M.; Wildt, S.; Jensen, M.L.; Gaspar, P.; Zeidan, A.A.; Takors, R. Differential Amino Acid Uptake and Depletion in Mono-Cultures and Co-Cultures of Streptococcus thermophilus and Lactobacillus delbrueckii Subsp. Bulgaricus in a Novel Semi-Synthetic Medium. Microorganisms 2022, 10, 1771. [Google Scholar] [CrossRef]
- Verma, D.K.; Al-Sahlany, S.T.G.; Niamah, A.K.; Thakur, M.; Shah, N.; Singh, S.; Baranwal, D.; Patel, A.R.; Utama, G.L.; Aguilar, C.N. Recent Trends in Microbial Flavour Compounds: A Review on Chemistry, Synthesis Mechanism and Their Application in Food. Saudi J. Biol. Sci. 2022, 29, 1565–1576. [Google Scholar] [CrossRef]
- Niamah, A.K.; Gddoa Al-Sahlany, S.T.; Abdul-Sada, H.K.; Prabhakar, P.; Tripathy, S.; Dadrwal, B.K.; Singh, S.; Verma, D.K.; Gupta, A.K.; Shukla, R.M.; et al. Phytophagous Probiotic Foods: Exploring the Intersection of Characteristics, Quality Implications, Health Benefits, and Market Dynamics. Trends Food Sci. Technol. 2024, 154, 104795. [Google Scholar] [CrossRef]
- Liu, G.; Qiao, Y.; Zhang, Y.; Leng, C.; Chen, H.; Sun, J.; Fan, X.; Li, A.; Feng, Z. Metabolic Profiles of Carbohydrates in Streptococcus thermophilus During PH-Controlled Batch Fermentation. Front. Microbiol. 2020, 11, 1131. [Google Scholar] [CrossRef] [PubMed]
- Barboza, P.S.; Parker, K.L.; Hume, I.D. Carbohydrates: Sugars, fiber and fermentation. In Integrative Wildlife Nutrition; Springer: Berlin/Heidelberg, Germany, 2009; pp. 97–118. [Google Scholar]
- Poolman, B.; Royer, T.J.; Mainzer, S.E.; Schmidt, B.F. Lactose transport system of Streptococcus thermophilus: A hybrid protein with homology to the melibiose carrier and enzyme III of phosphoenolpyruvate-dependent phosphotransferase systems. J. Bacteriol. 1989, 171, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liang, Y.; Zhang, S.; Xu, Z. Effect of Sugar Transporter on Galactose Utilization in Streptococcus Thermophilus. Front. Microbiol. 2023, 14, 1267237. [Google Scholar] [CrossRef]
- Solopova, A.; Bachmann, H.; Teusink, B.; Kok, J.; Kuipers, O.P. Further Elucidation of Galactose Utilization in Lactococcus Lactis MG1363. Front. Microbiol. 2018, 9, 1803. [Google Scholar] [CrossRef]
- Evivie, S.E.; Abdelazez, A.; Li, B.; Bian, X.; Li, W.; Du, J.; Huo, G.; Liu, F. In Vitro Organic Acid Production and in Vivo Food Pathogen Suppression by Probiotic S. thermophilus and L. bulgaricus. Front. Microbiol. 2019, 10, 782. [Google Scholar] [CrossRef]
- Ge, Y.; Yu, X.; Zhao, X.; Liu, C.; Li, T.; Mu, S.; Zhang, L.; Chen, Z.; Zhang, Z.; Song, Z.; et al. Fermentation Characteristics and Postacidification of Yogurt by Streptococcus thermophilus CICC 6038 and Lactobacillus delbrueckii Ssp. Bulgaricus CICC 6047 at Optimal Inoculum Ratio. J. Dairy Sci. 2024, 107, 123–140. [Google Scholar] [CrossRef]
- Yamauchi, R.; Fujisawa, M.; Koyanagi, S.; Muramatsu, A.; Kobayashi, T.; Wada, Y.; Akama, K.; Tanaka, M.; Kurashige, H.; Sato, A.; et al. Formate-Producing Capacity Provided by Reducing Ability of Streptococcus thermophilus Nicotinamide Adenine Dinucleotide Oxidase Determines Yogurt Acidification Rate. J. Dairy Sci. 2023, 106, 6710–6722. [Google Scholar] [CrossRef]
- Wa, Y.; Chanyi, R.M.; Nguyen, H.T.H.; Gu, R.; Day, L.; Altermann, E. Extracellular Polysaccharide Extraction from Streptococcus thermophilus in Fermented Milk. Microbiol. Spectr. 2022, 10, e0228021. [Google Scholar] [CrossRef]
- Yue, Y.; Han, J.; Shen, X.; Zhu, F.; Liu, Y.; Zhang, W.; Xia, W.; Wu, M. Structural Characteristics, Immune-Activating Mechanisms in Vitro, and Immunomodulatory Effects in Vivo of the Exopolysaccharide EPS53 from Streptococcus thermophilus XJ53. Carbohydr. Polym. 2024, 340, 122259. [Google Scholar] [CrossRef]
- Tiwari, S.; Kavitake, D.; Devi, P.B.; Halady Shetty, P. Bacterial Exopolysaccharides for Improvement of Technological, Functional and Rheological Properties of Yoghurt. Int. J. Biol. Macromol. 2021, 183, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Brüls, M.; Foroutanparsa, S.; Maljaars, C.E.P.; Olsthoorn, M.; Tas, R.P.; Voets, I.K. Investigating the Impact of Exopolysaccharides on Yogurt Network Mechanics and Syneresis through Quantitative Microstructural Analysis. Food Hydrocoll. 2024, 150, 109629. [Google Scholar] [CrossRef]
- Jiménez-Guzmán, J.; Flores-Nájera, A.; Cruz-Guerrero, A.E.; García-Garibay, M. Use of an Exopolysaccharide-Producing Strain of Streptococcus thermophilus in the Manufacture of Mexican Panela Cheese. LWT 2009, 42, 1508–1512. [Google Scholar] [CrossRef]
- Purwandari, U.; Shah, N.P.; Vasiljevic, T. Effects of Exopolysaccharide-Producing Strains of Streptococcus thermophilus on Technological and Rheological Properties of Set-Type Yoghurt. Int. Dairy J. 2007, 17, 1344–1352. [Google Scholar] [CrossRef]
- Lévesque, C.; Duplessis, M.; Labonté, J.; Labrie, S.; Fremaux, C.; Tremblay, D.; Moineau, S. Genomic Organization and Molecular Analysis of Virulent Bacteriophage 2972 Infecting an Exopolysaccharide-Producing Streptococcus thermophilus Strain. Appl. Environ. Microbiol. 2005, 71, 4057–4068. [Google Scholar] [CrossRef]
- Che, H.; Zhang, H.; Tian, Y.; Lai, P.F.H.; Xia, Y.; Wang, S.; Ai, L. Exopolysaccharide from Streptococcus thermophilus as Stabilizer in Fermented Dairy: Binding Kinetics and Interactions with Casein of Milk. Int. J. Biol. Macromol. 2019, 140, 1018–1025. [Google Scholar] [CrossRef]
- Xu, Z.; Li, X.; Tian, X.; Yang, S.; Li, Y.; Li, Z.; Guo, T.; Kong, J. Characterization of the Antioxidant Activities of the Exopolysaccharides Produced by Streptococcus thermophilus CGMCC 7.179. LWT 2023, 173, 114256. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Ma, X.; Yi, H.; Zhang, L.; Liu, T. Exploration of the Key Factors Influencing the Viscosity of Exopolysaccharides Produced by Streptococcus thermophilus in Milk Fermentation through Comparative Studies. Int. J. Biol. Macromol. 2025, 315, 144347. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Liang, M.; Liu, J.; Liu, Y.; Renye, J.A.; Qi, P.X.; Ren, D. Characterization of an Exopolysaccharide (EPS-3A) Produced by Streptococcus thermophilus ZJUIDS-2-01 Isolated from Traditional Yak Yogurt. Int. J. Biol. Macromol. 2021, 192, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Xiong, Z.; Zhang, H.; Xie, F.; Song, X.; Xia, Y.; Song, Z.; Chen, G.; Ai, L. Chemical Components, Molecular Characterization and Rheological Properties of Exopolysaccharides Produced by Streptococcus thermophilus Strains. LWT 2025, 215, 117203. [Google Scholar] [CrossRef]
- Wang, X.; Lin, T.; Lin, Y.; Xu, C.; Ma, K.; Zhang, C.; Ji, F.; Mahsa, G.C.; Rui, X.; Li, W. Chemical Composition and in Vitro Intestinal Probiotic Properties Analysis of Exopolysaccharides from Screened Streptococcus thermophilus 90301. Int. J. Biol. Macromol. 2025, 311, 143882. [Google Scholar] [CrossRef]
- Sun, N.; Liu, H.; Liu, S.; Zhang, X.; Chen, P.; Li, W.; Xu, X.; Tian, W. Purification, Preliminary Structure and Antitumor Activity of Exopolysaccharide Produced by Streptococcus thermophilus CH9. Molecules 2018, 23, 2898. [Google Scholar] [CrossRef]
- Padmanabhan, A.; Shah, N.P. Structural Characterization of Exopolysaccharide from Streptococcus thermophilus ASCC 1275. J. Dairy Sci. 2020, 103, 6830–6842. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Ren, F. A Role of Exopolysaccharide Produced by Streptococcus thermophilus in the Intestinal Inflammation and Mucosal Barrier in Caco-2 Monolayer and Dextran Sulphate Sodium-Induced Experimental Murine Colitis. Molecules 2019, 24, 513. [Google Scholar] [CrossRef]
- Ren, W.; Xia, Y.; Wang, G.; Zhang, H.; Zhu, S.; Ai, L. Bioactive Exopolysaccharides from a S. Thermophilus Strain: Screening, Purification and Characterization. Int. J. Biol. Macromol. 2016, 86, 402–407. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Niamah, A.K. Identification and Antioxidant Activity of Hyaluronic Acid Extracted from Local Isolates of Streptococcus Thermophilus. Mater. Today Proc. 2022, 60, 1523–1529. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Niamah, A.K. Production and Optimization of Hyaluronic Acid Extracted from Streptococcus thermophilus Isolates. Arch. Razi Inst. 2022, 77, 2295–2305. [Google Scholar] [CrossRef]
- Hols, P.; Hancy, F.; Fontaine, L.; Grossiord, B.; Prozzi, D.; Leblond-Bourget, N.; Decaris, B.; Bolotin, A.; Delorme, C.; Ehrlich, S.D.; et al. New Insights in the Molecular Biology and Physiology of Streptococcus thermophilus Revealed by Comparative Genomics. FEMS Microbiol. Rev. 2005, 29, 435–463. [Google Scholar] [PubMed]
- Hu, T.; Cui, Y.; Zhang, Y.; Qu, X.; Zhao, C. Genome Analysis and Physiological Characterization of Four Streptococcus thermophilus Strains Isolated from Chinese Traditional Fermented Milk. Front. Microbiol. 2020, 11, 184. [Google Scholar] [CrossRef] [PubMed]
- Chaves, A.C.S.D.; Fernandez, M.; Lerayer, A.L.S.; Mierau, I.; Kleerebezem, M.; Hugenholtz, J. Metabolic Engineering of Acetaldehyde Production by Streptococcus Thermophilus. Appl. Environ. Microbiol. 2002, 68, 5656–5662. [Google Scholar] [CrossRef] [PubMed]
- Bennama, R.; Ladero, V.; Alvarez, M.A.; Fernández, M.; Bensoltane, A. Influence of Lactose and Sucrose on Growth and Acetaldehyde Production by Three Strains of Streptococcus thermophilus; InTech: London, UK, 2012. [Google Scholar]
- Peralta, G.H.; Bergamini, C.V.; Hynes, E.R. Aminotransferase and Glutamate Dehydrogenase Activities in Lactobacilli and Streptococci. Braz. J. Microbiol. 2016, 47, 741–748. [Google Scholar] [CrossRef]
- Helinck, S.; Le Bars, D.; Moreau, D.; Yvon, M. Ability of Thermophilic Lactic Acid Bacteria to Produce Aroma Compounds from Amino Acids. Appl. Environ. Microbiol. 2004, 70, 3855–3861. [Google Scholar] [CrossRef]
- Zhao, X.; Ge, Y.; Yu, X.; Liu, C.; Li, H.; Wang, X.; Yao, S. Fermentation Characteristics of Fermented Milk with Streptococcus thermophilus CICC 6063 and Lactobacillus Helveticus CICC 6064 and Volatile Compound Dynamic Profiles during Fermentation and Storage. Molecules 2024, 29, 1257. [Google Scholar] [CrossRef]
- Ceruso, M.; Liu, Y.; Gunther, N.W.; Pepe, T.; Anastasio, A.; Qi, P.X.; Tomasula, P.M.; Renye, J.A. Anti-Listerial Activity of Thermophilin 110 and Pediocin in Fermented Milk and Whey. Food Control 2021, 125, 107941. [Google Scholar] [CrossRef]
- Verma, D.K.; Thakur, M.; Singh, S.; Tripathy, S.; Gupta, A.K.; Baranwal, D.; Patel, A.R.; Shah, N.; Utama, G.L.; Niamah, A.K.; et al. Bacteriocins as Antimicrobial and Preservative Agents in Food: Biosynthesis, Separation and Application. Food Biosci. 2022, 46, 101594. [Google Scholar] [CrossRef]
- Aslam, M.; Shahid, M.; Rehman, F.U.; Naveed, N.H.; Batool, A.I.; Sharif, S.; Asia, A. Purification and Characterization of Bacteriocin Isolated from Streptococcus Thermophilus. Afr. J. Microbiol. Res. 2011, 5, 2642–2648. [Google Scholar] [CrossRef]
- Kaminarides, S.; Aktypis, A.; Koronios, G.; Massouras, T.; Papanikolaou, S. Effect of ‘in Situ’ Produced Bacteriocin Thermophilin T on the Microbiological and Physicochemical Characteristics of Myzithra Whey Cheese. Int. J. Dairy Technol. 2018, 71, 213–222. [Google Scholar] [CrossRef]
- McAnulty, M.J.; Guron, G.K.; Oest, A.M.; Miller, A.L.; Renye, J.A. The Quorum Sensing Peptide BlpC Regulates the Transcription of Genes Outside Its Associated Gene Cluster and Impacts the Growth of Streptococcus Thermophilus. Front. Microbiol. 2023, 14, 1304136. [Google Scholar] [CrossRef]
- Renye, J.A.; Somkuti, G.A.; Qi, P.X.; Steinberg, D.H.; McAnulty, M.J.; Miller, A.L.; Guron, G.K.; Oest, A.M. BlpU is a broad-spectrum bacteriocin in Streptococcus thermophilus. Front. Microbiol. 2024, 15, 1409359. [Google Scholar] [CrossRef] [PubMed]
- Salini, F.; Iacumin, L.; Comi, G.; Dicks, L.M. Thermophilin 13: In silico analysis provides new insight in genes involved in Bacteriocin production. Microorganisms 2023, 11, 611. [Google Scholar] [CrossRef]
- Renye, J.A., Jr.; Mendez-Encinas, M.A.; White, A.K.; Miller, A.L.; McAnulty, M.J.; Yadav, M.P.; Hotchkiss, A.T.; Guron, G.K.; Oest, A.M.; Martinez-Robinson, K.G.; et al. Antimicrobial activity of thermophilin 110 against the opportunistic pathogen Cutibacterium acnes. Biotechnol. Lett. 2023, 45, 1365–1379. [Google Scholar] [CrossRef]
- Kabuki, T.; Uenishi, H.; Watanabe, M.; Seto, Y.; Nakajima, H. Characterization of a bacteriocin, Thermophilin 1277, produced by Streptococcus thermophilus SBT1277. J. Appl. Microbiol. 2007, 102, 971–980. [Google Scholar] [CrossRef]
- Ivanova, I.; Miteva, V.; Stefanova, T.S.; Pantev, A.; Budakov, I.; Danova, S.; Moncheva, P.; Nikolova, I.; Dousset, X.; Boyaval, P. Characterization of a bacteriocin produced by Streptococcus thermophilus 81. Int. J. Food Microbiol. 1998, 42, 147–158. [Google Scholar] [CrossRef]
- Evivie, S.E.; Ogwu, M.C.; Abdelazez, A.; Bian, X.; Liu, F.; Li, B.; Huo, G. Suppressive effects of Streptococcus thermophilus KLDS 3.1003 on some foodborne pathogens revealed through in vitro, in vivo and genomic insights. Food Funct. 2020, 11, 6573–6587. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.K.; Yusra, M.B.; Mohsin Enas, O.Z.; Jawad, F.G. The Antibacterial Effectiveness of Bacteriocin Output via Streptococcus thermophilus Versus Viral Pathogens and Spores. Agric. Sci. Dig. A Res. J. 2025, 383, 1–8. [Google Scholar] [CrossRef]
- Marciset, O.; Jeronimus-Stratingh, M.C.; Mollet, B.; Poolman, B. Thermophilin 13, a nontypical antilisterial poration complex bacteriocin, that functions without a receptor. J. Biol. Chem. 1997, 272, 14277–14284. [Google Scholar] [CrossRef]
- Kapse, N.; Pisu, V.; Dhakephalkar, T.; Margale, P.; Shetty, D.; Wagh, S.; Dagar, S.; Dhakephalkar, P.K. Unveiling the probiotic potential of Streptococcus thermophilus MCC0200: Insights from in vitro studies corroborated with genome analysis. Microorganisms 2024, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.D.; Nuttall, J.P. Preclinical Safety Evaluation. Microbicides for Prevention of HIV Infection; Springer: Berlin/Heidelberg, Germany, 2013; Volume 383, pp. 55–78. [Google Scholar] [CrossRef]
- Benítez-Chao, D.F.; León-Buitimea, A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. Bacteriocins: An overview of antimicrobial, toxicity, and biosafety assessment by in vivo models. Front. Microbiol. 2021, 12, 630695. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef]
- Reynolds, S.; Pandey, M.; Dooley, J.; Calcutt, A.; Batzloff, M.; Ozberk, V.; Mills, J.L.; Good, M. Preclinical safety and immunogenicity of Streptococcus pyogenes (Strep A) peptide vaccines. Sci. Rep. 2021, 11, 127. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Hu, J.S.; Liu, D.M.; Tang, J.; Liang, M.H.; Wu, J.J.; Xiong, J. Assessment of the safety and metabolism characteristics of Streptococcus thermophilus DMST-H2 based on complete genome and phenotype analysis. LWT 2023, 184, 114907. [Google Scholar] [CrossRef]
- Hua, Z.; Liu, S.; Yang, G.; Hou, X.; Fang, Y. Next-generation probiotics: Innovations in safety assessments. Curr. Opin. Food Sci. 2025, 61, 101238. [Google Scholar] [CrossRef]
- Nisar, S.; Shah, A.H.; Nazir, R. The clinical praxis of bacteriocins as natural anti-microbial therapeutics. Arch. Microbiol. 2024, 206, 451. [Google Scholar] [CrossRef] [PubMed]
- Niamah, A.K.; Al-Sahlany, S.T.G.; Ibrahim, S.A.; Verma, D.K.; Thakur, M.; Singh, S.; Patel, A.R.; Aguilar, C.N.; Utama, G.L. Electro-hydrodynamic processing for encapsulation of probiotics: A review on recent trends, technological development, challenges and future prospect. Food Biosci. 2021, 44, 101458. [Google Scholar] [CrossRef]
- Tripathy, S.; Verma, D.K.; Gupta, A.K.; Srivastav, P.P.; Patel, A.R.; González, M.L.C.; Utama, G.L.; Aguilar, C.N. Nanoencapsulation of biofunctional components as a burgeoning nanotechnology-based approach for functional food development: A review. Biocatal. Agric. Biotechnol. 2023, 53, 102890. [Google Scholar] [CrossRef]
- Bahrami, S.; Andishmand, H.; Pilevar, Z.; Hashempour-Baltork, F.; Torbati, M.; Dadgarnejad, M.; Rastegar, H.; Mohammadi, S.A.; Azadmard-Damirchi, S. Innovative perspectives on bacteriocins: Advances in classification, synthesis, mode of action, and food industry applications. J. Appl. Microbiol. 2024, 135, lxae274. [Google Scholar] [CrossRef]
- Tripathy, S.; Verma, D.K.; Srivastav, P.P. Encapsulation of enzymes-based on biopolymers and biochemical systems: Progress and perspective. In Enzymatic Processes for Food Valorization; Chávez González, M.L., Buenrostro Figueroa, J.J., Verma, D.K., Aguilar, C.N., Eds.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Prabhakar, P.; Tripathy, S.; Verma, D.K.; Singh, S.; Thakur, M.; Singh, A.K.; Srivastav, P.P.; Banerjee, M.; Patel, A.R.; González, M.L.C.; et al. Trends and Advances in Liposome Formulation Technology with an Emphasis on Ensuring Safety and Quality in Food and Drug Applications. Food Biosci. 2025, 69, 106913. [Google Scholar] [CrossRef]
- Fahim, H.A.; Khairalla, A.S.; El-Gendy, A.O. Nanotechnology: A valuable strategy to improve bacteriocin formulations. Front. Microbiol. 2016, 7, 1385. [Google Scholar] [CrossRef]
- Eghbal, N.; Viton, C.; Gharsallaoui, A. Nano and microencapsulation of bacteriocins for food applications: A review. Food Biosci. 2022, 50, 102173. [Google Scholar] [CrossRef]
- Parada Fabián, J.C.; Álvarez Contreras, A.K.; Natividad Bonifacio, I.; Hernández Robles, M.F.; Vázquez Quiñones, C.R.; Quiñones Ramírez, E.I.; Vázquez Salinas, C. Toward safer and sustainable food preservation: A comprehensive review of bacteriocins in the food industry. Biosci. Rep. 2025, 45, 277–302. [Google Scholar] [CrossRef]
- Farrukh, M.; Munawar, A.; Nawaz, Z.; Hussain, N.; Hafeez, A.B.; Szweda, P. Antibiotic resistance and preventive strategies in foodborne pathogenic bacteria: A comprehensive review. Food Sci. Biotechnol. 2025, 34, 2101–2129. [Google Scholar] [CrossRef]
- Abebe, A.A.; Birhanu, A.G. Methicillin resistant Staphylococcus aureus: Molecular mechanisms underlying drug resistance development and novel strategies to combat. Infect. Drug Resist. 2023, 16, 7641–7662. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Badawy, N.K.; Ashraf, Y.; Zatioun, A.A.; Masriya, H.H.; Ammar, M.M.; Mohamed, N.A.; Mourad, S.; Assy, A.M. Combating antibiotic resistance: Mechanisms, multidrug-resistant pathogens, and novel therapeutic approaches: An updated review. Pharmaceuticals 2025, 18, 402. [Google Scholar] [CrossRef]
- Somkuti, G.A.; Renye, J.A. Effect of a BlpC-Based Quorum-Sensing Induction Peptide on Bacteriocin Production in Streptococcus Thermophilus. J. Food Res. 2014, 4, 88. [Google Scholar] [CrossRef]
- Fontaine, L.; Boutry, C.; Guédon, E.; Guillot, A.; Ibrahim, M.; Grossiord, B.; Hols, P. Quorum-Sensing Regulation of the Production of Blp Bacteriocins in Streptococcus Thermophilus. J. Bacteriol. 2007, 189, 7195–7205. [Google Scholar] [CrossRef]
- Fontaine, L.; Hols, P. The Inhibitory Spectrum of Thermophilin 9 from Streptococcus thermophilus LMD-9 Depends on the Production of Multiple Peptides and the Activity of BlpGSt, a Thiol-Disulfide Oxidase. Appl. Environ. Microbiol. 2008, 74, 1102–1110. [Google Scholar] [CrossRef]
- Aktypis, A.; Kalantzopoulos, G. Purification and Characterization of Thermophilin ST-1, a Novel Bacteriocin Produced by Streptococcus thermophilus ACA-DC 0001. Le Lait 2003, 83, 365–378. [Google Scholar] [CrossRef]
- Khali, R.K. Evidence for Probiotic Potential of a Capsular-Producing Streptococcus thermophilus CHCC 3534 Strain. Afr. J. Microbiol. Res. 2009, 3, 27–34. [Google Scholar]
- Paul, R.H.; Frestedt, J.; Magurany, K. GRAS from the Ground up: Review of the Interim Pilot Program for GRAS Notification. Food Chem. Toxicol. 2017, 105, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Niamah, A.K. Physicochemical and Microbial Characteristics of Yogurt with Added Saccharomyces Boulardii. Curr. Res. Nutr. Food Sci. 2017, 5, 300–307. [Google Scholar] [CrossRef]
- Gasser, C.; Garault, P.; Chervaux, C.; Monnet, V.; Faurie, J.M.; Rul, F. Co-Utilization of Saccharides in Mixtures: Moving toward a New Understanding of Carbon Metabolism in Streptococcus Thermophilus. Food Microbiol. 2022, 107, 104080. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.P.; Cu, X.; Xu, K.; Peng, J.H.; Liu, H.R.; Zhao, R.T.; Wang, Z.; Wang, T.; Xu, Z.S. The Effect of Glutathione Biosynthesis of Streptococcus thermophilus ST-1 on Cocultured Lactobacillus Delbrueckii Ssp. Bulgaricus ATCC11842. J. Dairy Sci. 2023, 106, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.C.; Cardarelli, H.R. Mozzarella Cheese Stretching: A Minireview. Food Technol. Biotechnol. 2021, 59, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, G.; Taspinar, T.; Busetta, G.; Mastrangelo, S.; Portolano, B.; Sardina, M.T.; Gaglio, R.; Erten, H.; Settanni, L. Description of Ewiss Cheese, a New Ewe Milk Cheese Processed by Swiss Cheese Manufacturing Techniques: Microbiological, Physicochemical, and Sensory Aspects. J. Dairy Sci. 2024, 107, 6614–6628. [Google Scholar] [CrossRef]
- Grizon, A.; Theil, S.; Callon, C.; Gerber, P.; Helinck, S.; Dugat-Bony, E.; Bonnarme, P.; Chassard, C. Genetic and Technological Diversity of Streptococcus thermophilus Isolated from the Saint-Nectaire PDO Cheese-Producing Area. Front. Microbiol. 2023, 14, 1245510. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Figueroa, R.H.; López-Malo, A.; Palou, E.; Mani-López, E. Reduced-Fat Cream Cheese with Lactobacillus Acidophilus LA-5 as a Probiotic: Physicochemical, Sensory, and Fermentation Characteristics. ACS Food Sci. Technol. 2025, 5, 1699–1709. [Google Scholar] [CrossRef]
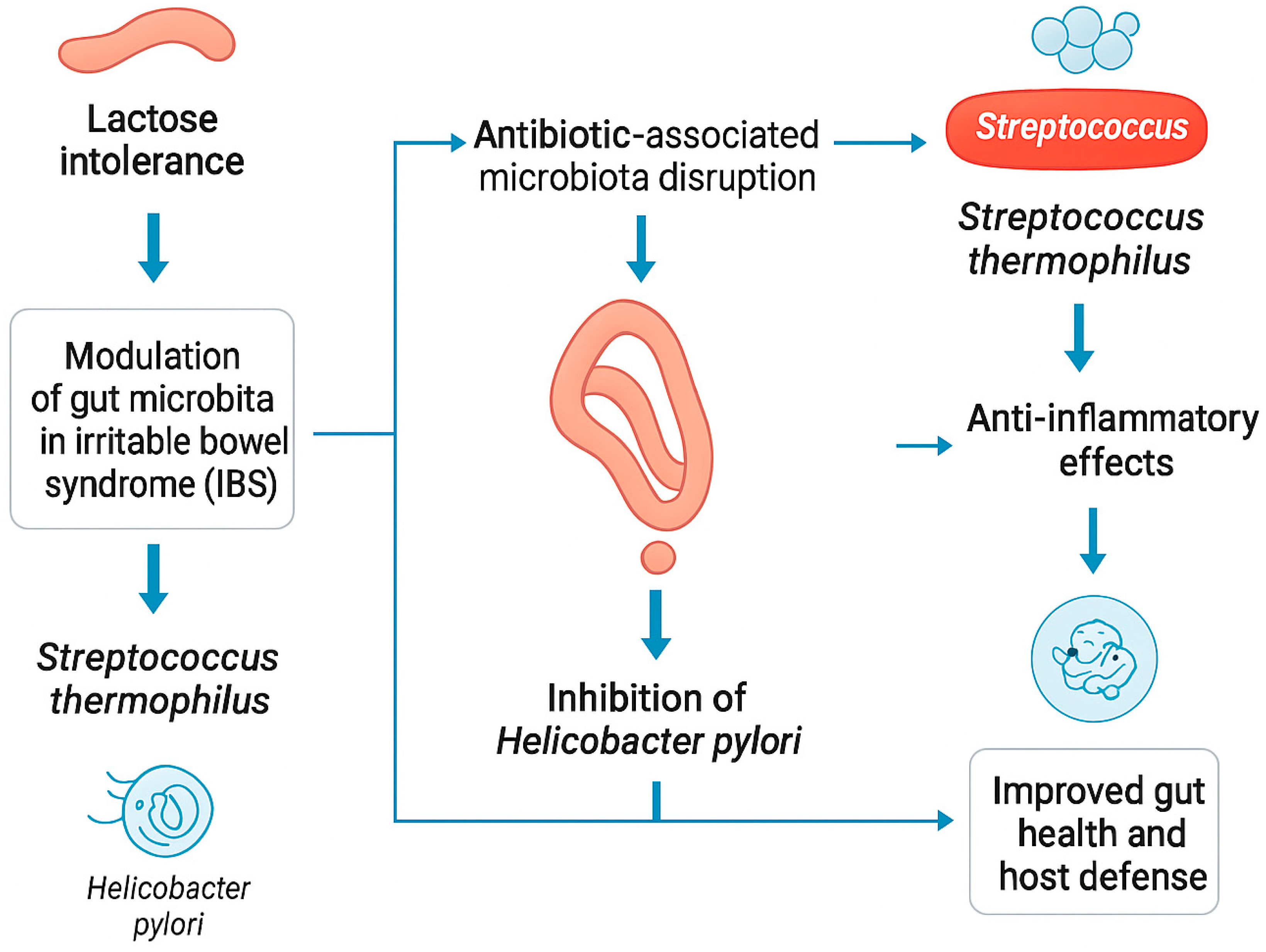
- Rul, F.; Ben-Yahia, L.; Chegdani, F.; Wrzosek, L.; Thomas, S.; Noordine, M.L.; Gitton, C.; Cherbuy, C.; Langella, P.; Thomas, M. Impact of the Metabolic Activity of Streptococcus thermophilus on the Colon Epithelium of Gnotobiotic Rats. J. Biol. Chem. 2011, 286, 10288–10296. [Google Scholar] [CrossRef]
- Drouault, S.; Anba, J.; Corthier, G. Streptococcus thermophilus Is Able to Produce a β-Galactosidase Active during Its Transit in the Digestive Tract of Germ-Free Mice. Appl. Environ. Microbiol. 2002, 68, 938–941. [Google Scholar] [CrossRef]
- Hu, J.S.; Huang, Y.Y.; Kuang, J.H.; Yu, J.J.; Zhou, Q.Y.; Liu, D.M. Streptococcus Thermophiles DMST-H2 Promotes Recovery in Mice with Antibiotic-Associated Diarrhea. Microorganisms 2020, 8, 1650. [Google Scholar] [CrossRef]
- Kopacz, K.; Phadtare, S. Probiotics for the Prevention of Antibiotic-Associated Diarrhea. Healthcare 2022, 10, 1450. [Google Scholar] [CrossRef]
- Cusumano, G.; Flores, G.A.; Venanzoni, R.; Angelini, P. The Impact of Antibiotic Therapy on Intestinal Microbiota: Dysbiosis, Antibiotic Resistance, and Restoration Strategies. Antibiotics 2025, 14, 371. [Google Scholar] [CrossRef]
- Martinović, A.; Cocuzzi, R.; Arioli, S.; Mora, D. Streptococcus Thermophilus: To Survive, or Not to Survive the Gastrointestinal Tract, That Is the Question! Nutrients 2020, 12, 2175. [Google Scholar] [CrossRef]
- Chlebicz-Wójcik, A.; Śliżewska, K. Probiotics, Prebiotics, and Synbiotics in the Irritable Bowel Syndrome Treatment: A Review. Biomolecules 2021, 11, 1154. [Google Scholar] [CrossRef]
- Horvat, I.B.; Gobin, I.; Kresović, A.; Hauser, G. How Can Probiotic Improve Irritable Bowel Syndrome Symptoms? World J. Gastrointest. Surg. 2021, 13, 923–940. [Google Scholar] [CrossRef]
- Tongtawee, T.; Dechsukhum, C.; Leeanansaksiri, W.; Kaewpitoon, S.; Kaewpitoon, N.; Loyd, R.A.; Matrakool, L.; Panpimanmas, S. Improved Helicobacter Pylori Eradication Rate of Tailored Triple Therapy by Adding Lactobacillus Delbrueckii and Streptococcus thermophilus in Northeast Region of Thailand: A Prospective Randomized Controlled Clinical Trial. Gastroenterol. Res. Pract. 2015, 2015, 518018. [Google Scholar] [CrossRef]
- Han, F.; Wu, G.; Zhang, Y.; Zheng, H.; Han, S.; Li, X.; Cai, W.; Liu, J.; Zhang, W.; Zhang, X.; et al. Streptococcus thermophilus Attenuates Inflammation in Septic Mice Mediated by Gut Microbiota. Front. Microbiol. 2020, 11, 598010. [Google Scholar] [CrossRef]
- Lee, G.A.; Chang, Y.W.; Lin, W.L.; Yang, Y.C.S.H.; Chen, W.J.; Huang, F.H.; Liu, Y.R. Modulatory Effects of Heat-Inactivated Streptococcus thermophilus Strain 7 on the Inflammatory Response: A Study on an Animal Model with TLR3-Induced Intestinal Injury. Microorganisms 2023, 11, 278. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The Role of Gut Microbiota in Immune Homeostasis and Autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, V.; Kazou, M.; Blom, J.; Pot, B.; Papadimitriou, K.; Tsakalidou, E. Comparative Genomics of Streptococcus thermophilus Support Important Traits Concerning the Evolution, Biology and Technological Properties of the Species. Front. Microbiol. 2019, 10, 2916. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, Z.; Liang, J.; Dou, J.; Guo, F.; Xu, Z.; Wang, T. Advances in Genetic Tools and Their Application in Streptococcus Thermophilus. Foods 2023, 12, 3119. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, X.; Gagnaire, V.; Lortal, S.; Dary, A.; Genay, M. Streptococcus Thermophilus, an Emerging and Promising Tool for Heterologous Expression: Advantages and Future Trends. Food Microbiol. 2016, 53, 2–9. [Google Scholar] [CrossRef]
- Mao, B.; Guo, W.; Chen, M.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H.; Cui, S. Effects of Streptococcus thermophilus Fermentation on the Flavors and Antioxidant Properties of Barley Juice. Fermentation 2023, 9, 623. [Google Scholar] [CrossRef]
- Allouche, R.; Hafeez, Z.; Dary-Mourot, A.; Genay, M.; Miclo, L. Streptococcus Thermophilus: A Source of Postbiotics Displaying Anti-Inflammatory Effects in THP 1 Macrophages. Molecules 2024, 29, 1552. [Google Scholar] [CrossRef] [PubMed]
- Tavsanli, H.; Mus, T.E.; Cetinkaya, F.; Ayanoglu, E.; Cibik, R. Isolation of Lactobacillus Delbrueckii Subsp. Bulgaricus and Streptococcus thermophilus from Nature: Technological Characterisation and Antibiotic Resistance. Czech J. Food Sci. 2021, 39, 305–311. [Google Scholar] [CrossRef]


| Strain Types | Growth Conditions | Sub Units | Function | References |
|---|---|---|---|---|
| S. thermophilus CGMCC 7.179 | The bacterium was cultured in LM17 broth for a duration of 24 h at a temperature of 42 °C. | Mannose, glucuronic acid, galacturonic acid, glucose and N-acetylglucosamine | Antioxidant activity | [43] |
| S. thermophilus S6-13 | The strain was cultured in MRS broth at a temperature of 37 °C for a duration of 16 h. | Glucose, galactose, and N-acetylglucosamine | Higher viscosity of yogurt | [44] |
| S. thermophilus ZJUIDS-2-01 | The strain was cultured in both MRS broth and M17 at 37 °C for 12 h. | Glucose, galactose, N-acetyl-D-galactosamine, and rhamnose | Antioxidant activity and antibacterial properties | [45] |
| S. thermophilus NQ12 | The strain underwent anaerobic incubation for a duration of 24 h at a temperature of 40 °C in an M17 medium supplemented with 0.1% lactose. | Galactosamine, galactose, glucose and mannose | Viscosity factor | [46] |
| S. thermophilus 90301 | 2% (v/v) of bacteria were cultured in MRS medium with an inoculation rate maintained for 24 h at a temperature of 37 °C. | Mannose, rhamnose, glucosamine, glucose, and galactose | Potential prebiotic | [47] |
| S. thermophilus CH9 | The strain was incubated at 40 °C for a duration of 24 h on a modified skimmed milk-based medium. | Fucose, ribose, rhamnose, arabinose, xylose, sorbose, glucose, and galactose | Antitumor activity | [48] |
| S. thermophilus ASCC 1275 | Inoculate 1% of the strain into M17 broth that supplemented with 1% lactose, and incubate at 37 °C for a duration of 18 h. | Glucose, galactose, and mannose | - | [49] |
| S. thermophilus MN-BM-A01 | The strain was cultured in skimmed milk at a temperature of 37 °C for a duration of 24 h. | Rhamnose, glucose, galactose, and mannose | Decrease in disease activity index and reduction in colonic epithelial cell injury | [50] |
| S. thermophilus CRL1190 | The strain was cultured in a reconstituted skim milk medium. | Galactose and glucose | Immunoregulatory factor | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghailan, A.Z.; Niamah, A.K. Streptococcus thermophilus: Metabolic Properties, Functional Features, and Useful Applications. Appl. Microbiol. 2025, 5, 101. https://doi.org/10.3390/applmicrobiol5040101
Ghailan AZ, Niamah AK. Streptococcus thermophilus: Metabolic Properties, Functional Features, and Useful Applications. Applied Microbiology. 2025; 5(4):101. https://doi.org/10.3390/applmicrobiol5040101
Chicago/Turabian StyleGhailan, Alyaa Zaidan, and Alaa Kareem Niamah. 2025. "Streptococcus thermophilus: Metabolic Properties, Functional Features, and Useful Applications" Applied Microbiology 5, no. 4: 101. https://doi.org/10.3390/applmicrobiol5040101
APA StyleGhailan, A. Z., & Niamah, A. K. (2025). Streptococcus thermophilus: Metabolic Properties, Functional Features, and Useful Applications. Applied Microbiology, 5(4), 101. https://doi.org/10.3390/applmicrobiol5040101







