What Can Contribute to Weakening of Poly(Vinyl Alcohol) Cryogels Used for Cell (Self)Immobilization?
Abstract
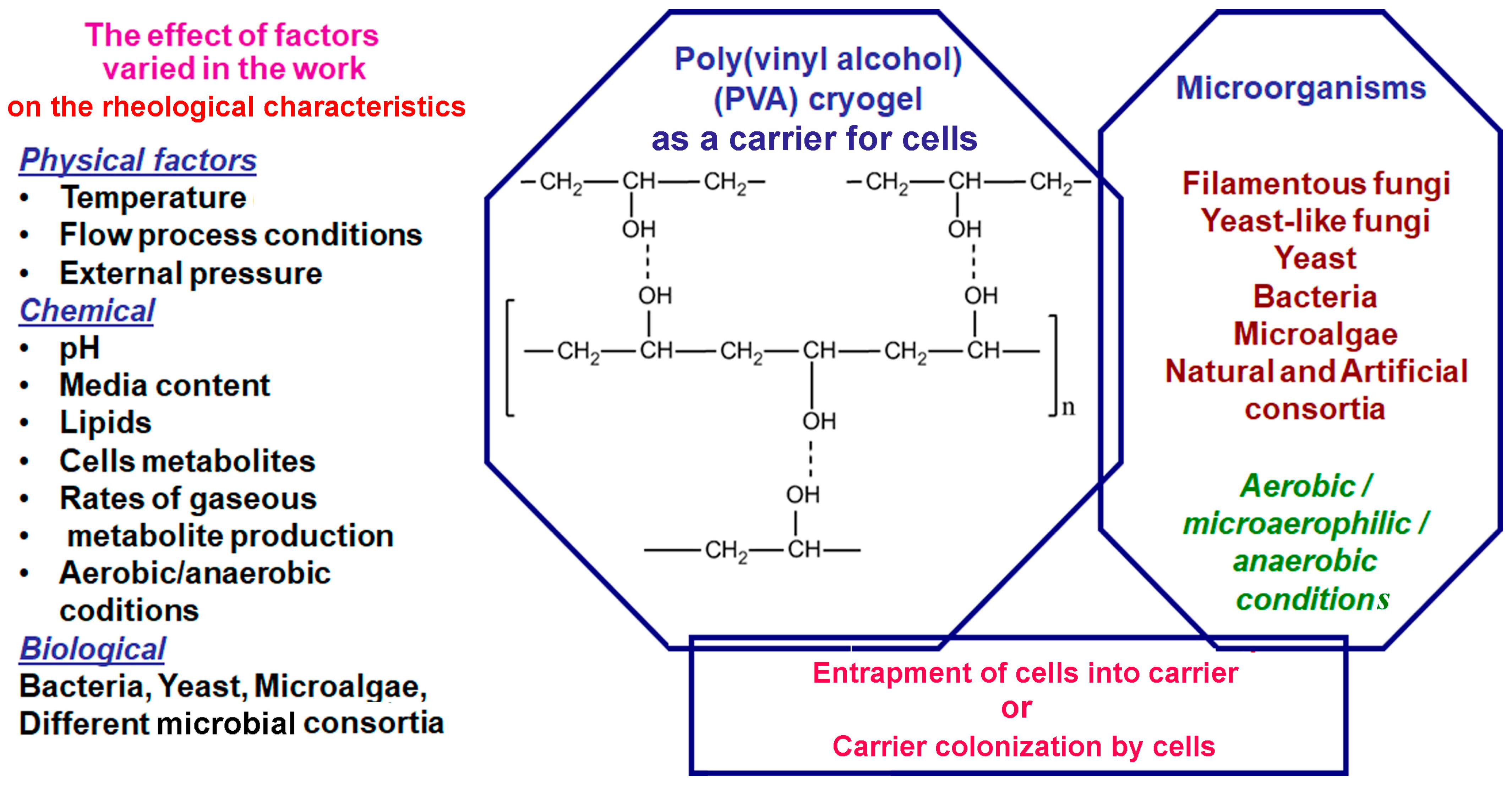
1. Introduction
- −
- The presence of components of various wastes (for example, salt) used as media for exposing the polymer matrices under discussion [19];
- −
- −
- Reduction in ambient humidity and drying of PVA cryogels [21].
2. Materials and Methods
2.1. Materials
2.2. Microorganisms and Conditions of Their Cultivation
2.3. Immobilization and Application of Microorganisms
2.4. Rheological Characteristics of PVS Cryogel
2.5. Analytical Methods
3. Results
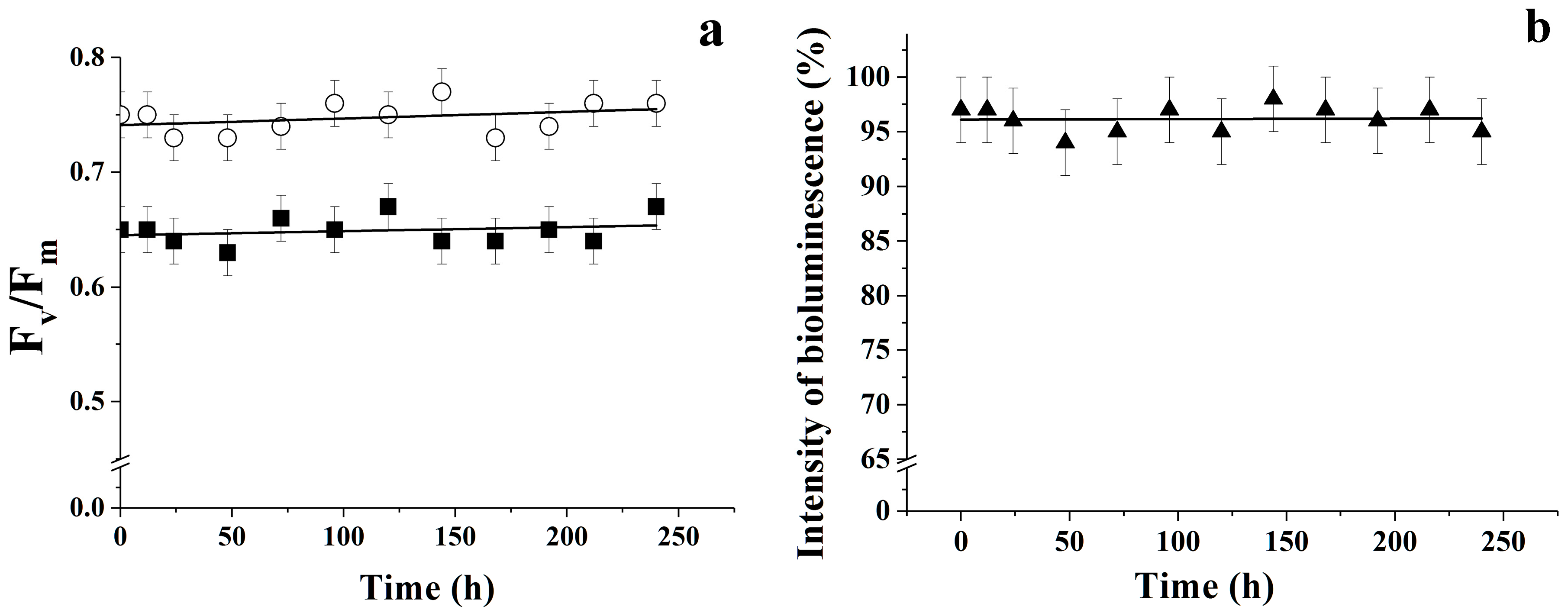
3.1. Changes in the Properties of PVA Cryogels in Presence of Microorganisms Operating Under Aerobic and Microaerophilic Conditions
3.2. Changes in the Properties of PVA Cryogels in the Presence of Microorganisms Operating Under Anaerobic Conditions
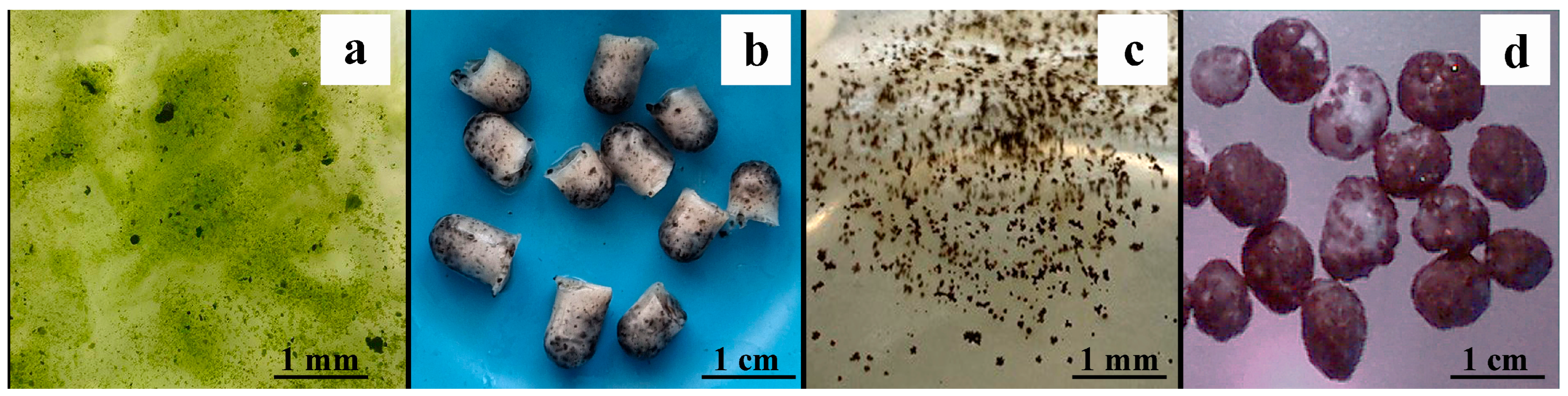
3.3. The Effect of Microbial Colonization of PVA Cryogels on Their Characteristics
3.4. Changes in the Strength Characteristics of PVA Cryogels as Part of BCs Used Under Flow Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| BCs | biocatalysts |
| Ch | carbohydrates |
| DW | dry weight |
| E | elasticity of cryogels samples |
| F | intensity of chlorophyll fluorescence |
| GRAS | generally recognized as safe |
| Lip | lipids |
| PBS | phosphate-buffered saline |
| Pmax | maximal pressure |
| Pr | proteins |
| PVA | poly(vinyl alcohol) |
| SD | standard deviation |
| Sl | salts |
References
- GRAS Notice (GRN) No. 767. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory (accessed on 28 July 2025).
- Michurov, D.A.; Andreasyan, G.A.; Lozinsky, V.I. Cryostructuring of polymeric systems: 68. evaluation of poly(vinyl alcohol) composite cryogels filled with poly(3-hydroxybutyric acid)-based microspheres of different porous morphology as potential delivery systems for drugs of various water-solubility. Gels 2024, 10, 734. [Google Scholar] [CrossRef]
- Lytkina, D.N.; Fedorishin, D.A.; Kalachikova, P.M.; Plyaskina, A.A.; Babeshin, A.R.; Kurzina, I.A. Cryo-structured materials based on polyvinyl alcohol and hydroxyapatite for osteogenesis. J. Funct. Biomater. 2021, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Ari, B.; Sahiner, M.; Demirci, S.; Sahiner, N. Poly(vinyl alcohol)-tannic acid cryogel matrix as antioxidant and antibacterial material. Polymers 2021, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Berillo, D.; Malika, T.; Baimakhanova, B.B.; Sadanov, A.K.; Berezin, V.E.; Trenozhnikova, L.P.; Baimakhanova, G.B.; Amangeldi, A.A.; Kerimzhanova, B. An Overview of Microorganisms Immobilized in a Gel Structure for the Production of Precursors, Antibiotics, and Valuable Products. Gels 2024, 10, 646. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wu, E.; Lou, X.; Deng, Q.; Hou, X.; Lv, C.; Hu, Q. Anthraquinone removal by a metal-organic framework/polyvinyl alcohol cryogel-immobilized laccase: Effect and mechanism exploration. Chem. Eng. J. 2021, 418, 129473. [Google Scholar] [CrossRef]
- Maslova, O.; Stepanov, N.; Senko, O.; Efremenko, E. Production of various organic acids from different renewable sources by immobilized cells in the regimes of separate hydrolysis and fermentation (SHF) and simultaneous saccharification and fermentation (SFF). Bioresour. Technol. 2019, 272, 1–9. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Ivshina, I.B.; Kamenskikh, T.N.; Bulicheva, M.V.; Stukova, G.I. Survival of cryogel-immobilized Rhodococcus strains in crude oil-contaminated soil and their impact on biodegradation efficiency. Int. Biodeterior. Biodegrad. 2013, 84, 118–125. [Google Scholar] [CrossRef]
- Berillo, D.; Al-Jwaid, A.; Caplin, J. Polymeric materials used for immobilisation of bacteria for the bioremediation of contaminants in water. Polymers 2021, 13, 1073. [Google Scholar] [CrossRef]
- Altunina, L.K.; Fufaeva, M.S.; Filatov, D.A.; Svarovskaya, L.I.; Rozhdestvenskii, E.A.; Gan-Erdene, T. Effect of cryogel on soil properties. Eurasian Soil. Sci. 2014, 47, 425–431. [Google Scholar] [CrossRef]
- Vasiliev, N.K.; Ivanov, A.A.; Sokurov, V.V.; Shatalina, I.N.; Vasilyev, K.N. Strength properties of ice–soil composites created by method of cryotropic gel formation. Cold Reg. Sci. Technol. 2012, 70, 94–97. [Google Scholar] [CrossRef]
- Fufaeva, M.S.; Fisenko, D.V.; Manzhay, V.N.; Bondaletov, V.G.; Altunina, L.K. Hydrophobic cryogels based on polyvinyl alcohol and polymeric oil resins. J. Sib. Fed. Univ. Chem. 2019, 12, 166–176. [Google Scholar] [CrossRef]
- Mohamed, H.; Shah, A.M.; Nazir, Y.; Naz, T.; Nosheen, S.; Song, Y. Biodegradation of poly (vinyl alcohol) by an orychophragmus rhizosphere-associated fungus Penicillium brevicompactum OVR-5, and its proposed PVA biodegradation pathway. World J. Microbiol. Biotechnol. 2022, 38, 10. [Google Scholar] [CrossRef]
- Demir, D.; Özdemir, S.; Gonca, S.; Bölgen, N. Novel styrax liquidus loaded chitosan/polyvinyl alcohol cryogels with antioxidant and antimicrobial properties. J. Appl. Polym. Sci. 2022, 139, 52033. [Google Scholar] [CrossRef]
- Kula, H.; Motaung, S.; Razwinani, M.; Adeyemi, S.A.; Ubanako, P.; Ngema, L.M.; Fonkui, T.Y.; Ndinteh, D.T.; Kumar, P.; Choonara, Y.E.; et al. Polyvinyl alcohol-based cryogels: Design, characterization, and in vitro biological studies. J. Appl. Polym. Sci. 2025, 142, e57508. [Google Scholar] [CrossRef]
- Radosavljević, M.; Lević, S.; Belović, M.; Pejin, J.; Djukić-Vuković, A.; Mojović, L.; Nedović, V. Encapsulation of Lactobacillus rhamnosus in polyvinyl alcohol for the production of L(+)-lactic acid. Process Biochem. 2021, 100, 149–160. [Google Scholar] [CrossRef]
- Salehiyan, R.; Soleymani Eil Bakhtiari, S. A review on rheological approaches as a perfect tool to monitor thermal degradation of biodegradable polymers. Korea-Aust. Rheol. J. 2024, 36, 295–317. [Google Scholar] [CrossRef]
- Nigro, L.; Magni, S.; Ortenzi, M.A.; Gazzotti, S.; Della Torre, C.; Binelli, A. Are “liquid plastics” a new environmental threat? The case of polyvinyl alcohol. Aquat. Toxicol. 2022, 248, 106200. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Sushko, N.I.; Zagorskaya, S.A. Effect of salt concentration on the structure of Poly(vinyl alcohol) cryogels obtained from aqueous salt solutions. J. Appl. Spectrosc. 2015, 82, 40–45. [Google Scholar] [CrossRef]
- Baniasadi, H.; Madani, Z.; Ajdary, R.; Rojas, O.J.; Seppälä, J. Ascorbic acid-loaded polyvinyl alcohol/cellulose nanofibril hydrogels as precursors for 3D printed materials. Mater. Sci. Eng. C 2021, 130, 112424. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, J.P.; Pott, R.W. Transparent polyvinyl-alcohol cryogel as immobilisation matrix for continuous biohydrogen production by phototrophic bacteria. Biotechnol. Biofuels 2020, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.N.; Lyagin, I.V.; Maslova, O.V.; Senko, O.V.; Stepanov, N.A.; Aslanli, A.G. Catalytic degradation of microplastics. Russ. Chem. Rev. 2023, 92, RCR5069. [Google Scholar] [CrossRef]
- Maslova, O.; Senko, O.; Stepanov, N.; Gladchenko, M.; Gaydamaka, S.; Akopyan, A.; Evseeva, E.; Anisimov, A.; Efremenko, E. Sulfur containing mixed wastes in anaerobic processing by new immobilized synthetic consortia. Bioresour. Technol. 2022, 362, 127794. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Zubov, A.L.; Titova, E.F. Swelling behavior of poly (vinyl alcohol) cryogels employed as matrices for cell immobilization. Enzym. Microb. Technol. 1996, 18, 561–569. [Google Scholar] [CrossRef]
- Castro, A.P.; Laity, P.; Shariatzadeh, M.; Wittkowske, C.; Holland, C.; Lacroix, D. Combined numerical and experimental biomechanical characterization of soft collagen hydrogel substrate. J. Mater. Sci. Mater. Med. 2016, 27, 79. [Google Scholar] [CrossRef] [PubMed]
- Sisman-Aydin, G.; Simsek, K. Municipal wastewater effects on the performance of nutrient removal, and lipid, carbohydrate, and protein productivity of blue-green algae Chroococcus turgidus. Sustainability 2022, 14, 17021. [Google Scholar] [CrossRef]
- Magrí, A.; Vanotti, M.B.; Szögi, A.A. Anammox sludge immobilized in polyvinyl alcohol (PVA) cryogel carriers. Bioresour. Technol. 2012, 114, 231–240. [Google Scholar] [CrossRef]
- Stepanov, N.; Senko, O.; Aslanli, A.; Maslova, O.; Efremenko, E. Enhanced biogas production from glucose and glycerol by artificial consortia of anaerobic sludge with immobilized yeast. Fermentation 2025, 11, 352. [Google Scholar] [CrossRef]
- Stepanov, N.; Senko, O.; Perminova, I.; Efremenko, E. A new approach to assess the effect of various humic compounds on the metabolic activity of cells participating in methanogenesis. Sustainability 2019, 11, 3158. [Google Scholar] [CrossRef]
- Krainiukov, A.; Liu, J.; Kravchenko, E.; Chang, D. Performance of silty sand reinforced with aqueous solution of polyvinyl alcohol subjected to freeze-thaw cycles. Cold Reg. Sci. Technol. 2020, 174, 103054. [Google Scholar] [CrossRef]
- Crețu, B.E.B.; Dodi, G.; Gardikiotis, I.; Balan, V.; Nacu, I.; Stoica, I.; Stoleru, E.; Rusu, A.G.; Ghilan, A.; Nita, L.E.; et al. Bioactive composite cryogels based on poly(vinyl alcohol) and a polymacrolactone as tissue engineering scaffolds: In vitro and in vivo studies. Pharmaceutics 2023, 15, 2730. [Google Scholar] [CrossRef]
- Ryabushko, L.; Miroshnichenko, E.; Blaginina, A.; Shiroyan, A.; Lishaev, D. Diatom and cyanobacteria communities on artificial polymer substrates in the Crimean coastal waters of the Black Sea. Mar. Pollut. Bull. 2021, 169, 112521. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Wang, H.Y. Highly effective removal of microplastics by microalgae Scenedesmus abundans. Chem. Eng. J. 2022, 435, 135079. [Google Scholar] [CrossRef]
- Efremenko, E.; Senko, O.; Stepanov, N.; Maslova, O.; Lomakina, G.; Ugarova, N. Luminescent analysis of ATP: Modern objects and processes for sensing. Chemosensors 2022, 10, 493. [Google Scholar] [CrossRef]
- Jecu, L.; Gheorghe, A.; Rosu, A.; Raut, I.; Grosu, E.; Ghiurea, M. Ability of fungal strains to degrade PVA based materials. J. Polym. Environ. 2010, 18, 284–290. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Jecu, L.; Gheorghe, A.; Raut, I.; Stroescu, M.; Ghiurea, M.; Danila, M.; Jipa, I.; Fruth, V. Biodegradation of poly(vinyl alcohol) and bacterial cellulose composites by Aspergillus niger. J. Polym. Environ. 2011, 19, 69–79. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Guo, H. Research progress of polyvinyl alcohol water-resistant film materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Rahman, L.; Goswami, J. Poly(vinyl alcohol) as sustainable and eco-friendly packaging: A review. J. Package Technol. Res. 2023, 7, 1–10. [Google Scholar] [CrossRef]
- Suleiman, G.S.A.; Zeng, X.; Chakma, R.; Wakai, I.Y.; Feng, Y. Recent advances and challenges in thermal stability of PVA-based film: A review. Polym. Adv. Technol. 2024, 35, e6327. [Google Scholar] [CrossRef]
- Rolsky, C.; Kelkar, V. Degradation of polyvinyl alcohol in US wastewater treatment plants and subsequent nationwide emission estimate. Int. J. Env. Res. Public Health 2021, 18, 6027. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Zhao, H.; Liu, X.; Ertürk, A.S.; Elmaci, G.; Zhao, P.; Meng, X. Synergistic adsorption and oxidative degradation of polyvinyl alcohol by acidified OMS-2: Catalytic mechanism, degradation pathway and toxicity evaluation. Sep. Purif. Technol. 2022, 302, 122047. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Okay, O. Basic principles of cryotropic gelation. In Polymeric Cryogels; Advances in polymer science; Springer: Cham, Switzerland, 2014; pp. 49–101. [Google Scholar] [CrossRef]
- Quispe-Siccha, R.M.; Medina-Sandoval, O.I.; Estrada-Tinoco, A.; Pedroza-Pérez, J.A.; Martínez-Tovar, A.; Olarte-Carrillo, I.; Ceron-Maldonado, R.; Reding-Bernal, A.; López-Alvarenga, J.C. Development of polyvinyl alcohol hydrogels for controlled glucose release in biomedical applications. Gels 2024, 10, 668. [Google Scholar] [CrossRef]
- Liang, X.; Zhong, H.J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.M.; He, J. Polyvinyl alcohol (PVA)-based hydrogels: Recent progress in fabrication, properties, and multifunctional applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef]
- Efremenko, E.; Stepanov, N.; Senko, O.; Maslova, O.; Volikov, A.; Zhirkova, A.; Perminova, I. Strategies for variable regulation of methanogenesis efficiency and velocity. Appl. Microbiol. Biotechnol. 2022, 106, 6833–6845. [Google Scholar] [CrossRef]
- Dangi, M.B.; Urynowicz, M.A.; Schultz, C.L.; Budhathoki, S. A comparison of the soil natural oxidant demand exerted by permanganate, hydrogen peroxide, sodium persulfate, and sodium percarbonate. Environ. Chall. 2022, 7, 100456. [Google Scholar] [CrossRef]
- Liu, X.; He, S.; Yang, Y.; Yao, B.; Tang, Y.; Luo, L.; Zhi, D.; Wan, Z.; Wang, L.; Zhou, Y. A review on percarbonate-based advanced oxidation processes for remediation of organic compounds in water. Environ. Res. 2021, 200, 111371. [Google Scholar] [CrossRef] [PubMed]
- Fufaeva, M.S.; Kim, Y.E.; Ovsyanikova, V.S.; Manzhai, V.N.; Altunina, L.K. Preparation and properties od biodegradable cryogels based on polyvinyl alcohol and potato starch for use in soil erosion control. CSD 2023, 31, 584–589. [Google Scholar] [CrossRef]
- Malka, E.; Margel, S. Engineering of PVA/PVP hydrogels for agricultural applications. Gels 2023, 9, 895. [Google Scholar] [CrossRef] [PubMed]

| Medium and Its Content * | Process Conditions |
|---|---|
| Starch processing wastewater, g/L: Lip-5; Ch-50; Pr-20; Sl-3 | Process: Wastewater treatment with multi-enzyme complexes of filamentous fungi. Conditions: The use of each type of model wastewater for cell cultivation was carried out on a shaker Lab-Therm (Adolf Kühner, Switzerland) under aerobic conditions, 180 rpm, 28 °C. Concentration of each BC was 20 g DW/L. The media were refreshed after each working cycle (30 h). |
| Meat processing wastewater, g/L: Lip-10; Ch-35; Pr-1; Sl-12.3 | |
| Wastewater from abattoirs, g/L: Lip-8; Ch-30; Pr-20; Sl-4.3 | |
| Mixed dairy processing, g/L: Lip-21; Ch-0.1; Pr-0.5; Sl-10.3 | |
| Soybean processing factory, g/L: Lip-0.5; Ch-0.1; Pr-0.9; Sl-3 | |
| Apple cake, g/g: Ch-45.9; Pr-6.2 | |
| Domestic wastewater, g/L: Lip-0.08; Ch-0.04; Pr-0.06 | Process: Wastewater treatment and accumulation of microalgae biomass. Conditions: BC concentration—3.5 g DW/L; 28 °C. The media were refreshed after each working cycle (3 days). |
| Dairy wastewater, g/L: Lip-0.35; Ch-0.56; Pr-0.28 | |
| Farming wastewater, g/L: Lip-0.17; Ch-0.27; Pr-0.13 | |
| Treated potato pulp: for its treatment (enzymatic hydrolysis), the α-amylase of Aspergillus oryzae (Sigma-Aldrich Inc., Missouri, United States) was added to raw material (5 mg per 1 g of dry biomass). The hydrolysate contained 25 g/L glucose. | Process: Biotransformation of waste and production of pullulan with A. pullulanus. Conditions: BC concentration–12 g DW/L; 200 rpm, 26 °C. The media were refreshed after each working cycle (50 h). |
| The treated beet pulp was obtained by enzymatic hydrolysis of 50 g/L of dried beet pulp in 0.1 M citrate buffer (pH 5.0) with a complex enzyme preparation consisting of cellulases and β-xylosidase isolated from Penicillium canescens cells (5 mg protein/g substrate) for 48 h at 50 °C and constant stirring (250 rpm) (IRC-1-U Adolf Kuhnner, Apparaebau, Switzerland). | Process: Biotransformation of waste and production of dextran and bacterial cellulose by L. mesenteroides and K. xylinus cells, correspondently. Conditions for L. mesenteroides cells: BC concentration—30 g DW/L; 28 °C, 200 rpm, working cycle—24 h; Conditions for K. xylinus cells: BC concentration-12 g DW/L; 28 °C, working cycle-140 h. |
| Crude glycerol, obtained as a by-product of biodiesel production from the lipids of C. vulgaris biomass, was used for P. tannophilus cell cultivation. KOH in CH3OH was used for lipid transesterification of microalgal lipids, and the reaction was carried out for 10 min at 60 °C. The product was cooled and separated by centrifugation at 5000 rpm for 5 min. Crude glycerol feedstock contained 81.9% glycerol, 6.5% ash, 0.6% CH3OH, and 11% H2O. | Process: Ethanol production. Conditions for P. tannophilus cells: BC concentration—12 g DW/L; 28 °C, 200 rpm, working cycle-24 h. |
| Soil with pesticide (paraoxon), humidity 90 ± 5%, pesticide concentration—100 mg paraoxon/kg of soil | Process: Soil decontamination from pesticides Conditions: The immobilized cells were buried in the soil to a depth of 15 cm. BC concentration was 20 g DW/kg soil. A new dose of pesticide was added every 24 h. The soil moisture was constantly controlled and maintained. |
| Medium with benzothiophene sulfone (0.15 mM) was prepared using 0.1 M phosphate buffer (pH 7.2) with addition of 5 g/L ethanol | Process: Biotransformation of sulfur-containing waste from chemical and biocatalytic desulfurization of hydrocarbon raw materials. Conditions: BC concentration—20 g DW/L; 35 °C, anaerobic conditions |
| Medium | pHstart/ pHfinal | Immobilized BCs | Modulus of Elasticity (kPa) * | ||||
|---|---|---|---|---|---|---|---|
| Concentration of PVA Solution, % | Cell Biomass | Time of Exposition of BCs in the Medium, h | |||||
| 30 | 90 | 300 | 480 | ||||
| Starch processing | 4.3/6.5 | 11 | Filamentous fungi Rhizopus oryzae | 60.3 ± 2.3 | 69.2 ± 6.4 | 77.1 ± 6.1 | 79.6 ± 3.9 |
| Meat processing | 7.5/5.7 | 59.8 ± 3.3 | 56.2 ± 3.4 | 44.4 ± 5.5 | 37.7 ± 7.4 | ||
| Wastewater from abattoirs | 6.0/5.1 | 58.7 ± 6.3 | 54.3 ± 5.5 | 50.1 ± 6.6 | 46.3 ± 4.5 | ||
| Wastewater from mixed dairy processing | 5.9/4.5 | 56.4 ± 6.5 | 30.1 ± 5.0 | 29.9 ± 3.9 | 26.0 ± 4.2 | ||
| Wastewater from soybean processing factory | 6.3/4.2 | 58.5 ± 9.5 | 62.8 ± 9.2 | 77.1 ± 6.1 | 75.2 ± 5.1 | ||
| 6.3 | 11 | Without cells | 56.3 ± 3.5 | 56.1 ± 3.1 | 56.8 ± 3.3 | 58.3 ± 2.9 | |
| Apple cake | 5.5/4.2 | 10 | Filamentous fungi Aspergillus foetidus | 61.7 ± 9.2 | 65.6 ± 9.7 | 67.8 ± 7.2 | 70.1 ± 8.2 |
| Domestic wastewater | 6.9/9.5 | 7 | Microalgae Chlorella vulgaris | 20.1 ± 2.4 | 26.3 ± 2.7 | 35.2 ± 2.3 | 42.7 ± 2.2 |
| Dairy wastewater | 6.8/9.2 | 17.4 ± 1.7 | 16.2 ± 1.6 | 14.1 ± 1.7 | 10.4 ± 1.1 | ||
| Farming wastewater | 6.5/9.0 | 18.3 ± 1.8 | 17.5 ± 1.5 | 16.9 ± 1.5 | 16.4 ± 1.2 | ||
| Treated potato pulp | 5.5/5.4 | 11 | Yeast-like fungi Aureobasidium pullulanus | 68.6 ± 3.1 | 90.6 ± 4.8 | 87.4 ± 4.8 | 89.2 ± 5.2 |
| Treated beet pulp | 7.0/5.4 | 12 | Bacteria Leuconostoc mesenteroides | 78.8 ± 3.1 | 79.3 ± 2.3 | 79.1 ± 2.6 | 78.7 ± 3.4 |
| 5.5/3.4 | 12 | Bacteria Komagataeibacter xylinus | 79.2 ± 3.4 | 80.2 ± 3.2 | 79.7 ± 3.3 | 81.7 ± 4.1 | |
| Crude glycerol | 5.6/3.2 5.6/4.7 | 79.2 ± 3.4 | 76.2 ± 4.1 | 77.4 ± 3.7 | 78.2 ± 2.9 | ||
| 12 | Yeast Pachysolen tannophilus | 63.4 ± 3.5 | 65.5 ± 3.3 | 67.2 ± 3.0 | 66.4 ± 2.9 | ||
| Soil with pesticide (paraoxon) | 6.4/6.4 | 12 | Bacteria Pseudomonas sp. | 74.2 ± 3.1 | 72.7 ± 3.0 | 68.9 ± 2.8 | 68.8 ± 2.3 |
| 12 | Artificial bacterial consortium of Pseudomonas sp. and Rhodococcus erythropolis | 72.5 ± 2.7 | 70.6 ± 2.7 | 65.9 ± 3.3 | 66.7 ± 2.9 | ||
| Without cells | 71.7 ± 2.4 | 71.2 ± 1.7 | 68.9 ± 1.4 | 68.1 ± 1.2 | |||
| Microorganisms in BC | Process | Period of BC Use, Days | Pmax, Atm * | Accumulated gases | Velocity of Gas Accumulation, mL/g BC/day | Damaged Granules, % of Total Amount | Type of Damage in Granules |
|---|---|---|---|---|---|---|---|
| Clostridium acetobutylicum | Production of organic solvents | 4 | 2.6 | H2 | 1.12 ÷ 1.20 | 15 ± 1 | Granule rupture |
| Saccharomyces cerevisiae | Ethanol production | 1095 | 4.0 | CO2 | 0.92 ÷ 1.01 | 95 ± 5 | Deformation of granules |
| Saccharomyces cerevisiae | Treatment of wastewater | 3 | 1.1 | CO2 | 0.18 ÷ 0.20 | 2 ± 0.2 | No significant changes |
| Artificial methanogenic consortium | Conversion of sulfones to hydrogen sulfide within methanogenesis | 1095 | 1.6 | Biogas (H2, CH4, CO2, H2S) | 0.10 ÷ 0.21 | 1 ± 0.1 | No significant changes |
| Natural methanogenic consortium | Treatment of wastewater | 1095 | 1.7 | Biogas (H2, CH4, CO2) | 0.17 ÷ 0.25 | 1 ± 0.1 | No significant changes |
| Desulfovibrio desulfuricans | Conversion of sulfones to hydrogen sulfide within methanogenesis | 3 | 0.3 | H2S | 0.01 ÷ 0.03 | 1 ± 0.1 | No significant changes |
| Concentration of PVA Solution Used for Cryogel Formation, % | Average Pore Size, µm | Microorganisms | |||
|---|---|---|---|---|---|
| Microalgae Chlorella vulgaris | Bacteria Pseudomonas putida | Methanogenic Anaerobic Consortium | Filamentous Fungi Aspergillus niger | ||
| [ATP], ×10−11 mole/g PVA cryogel colonized by cells | |||||
| 7 | 70 ± 4 | 7.3 ± 0.2 | 14.2 ± 0.1 | 0.07 ± 0.01 | 57.3 ± 2.4 |
| 9 | 40 ± 3 | 12.7 ± 0.6 | 17.3 ± 0.1 | 0.04 ± 0 | 56.8 ± 2.1 |
| 11 | 25 ± 2 | 19.2 ± 1.2 | 19.6 ± 0.1 | 0.01 ± 0 | 46.9 ± 1.9 |
| 13 | 15 ± 1 | 14.1 ± 0.7 | 21.2 ± 0.1 | 0.01 ± 0 | 40.5 ± 1.3 |
| * Shear modulus, kPa | |||||
| 7 | 70 ± 4 | 3.0 ± 0.07 | 2.7 ± 0.06 | 2.8 ± 0.02 | 2.4 ± 0.02 |
| 9 | 40 ± 3 | 3.3 ± 0.07 | 3.2 ± 0.06 | 3.3 ± 0.08 | 3.0 ± 0.04 |
| 11 | 25 ± 2 | 3.8 ± 0.1 | 3.8 ± 0.1 | 3.7 ± 0.1 | 3.5 ± 0.1 |
| 13 | 15 ± 1 | 4.1 ± 0.1 | 4.2 ± 0.1 | 4.2 ± 0.1 | 4.0 ± 0.1 |
| Microalgae | Initial Value | After Exposition in the Flow | ||||
|---|---|---|---|---|---|---|
| [ATP], mole/g BC | F0 | Fv/Fm | [ATP], mole/g BC | F0 | * Fv/Fm | |
| C. vulgaris | (2.0 ± 0.1) × 10−11 | 1.79 | 0.71 | (1.9 ± 0.2) × 10−11 | 1.71 | 0.70 |
| T. weissflogii | (1.4 ± 0.3) × 10−9 | 1.75 | 0.63 | (1.2 ± 0.3) × 10−9 | 1.69 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senko, O.; Stepanov, N.; Maslova, O.; Efremenko, E. What Can Contribute to Weakening of Poly(Vinyl Alcohol) Cryogels Used for Cell (Self)Immobilization? Appl. Microbiol. 2025, 5, 97. https://doi.org/10.3390/applmicrobiol5030097
Senko O, Stepanov N, Maslova O, Efremenko E. What Can Contribute to Weakening of Poly(Vinyl Alcohol) Cryogels Used for Cell (Self)Immobilization? Applied Microbiology. 2025; 5(3):97. https://doi.org/10.3390/applmicrobiol5030097
Chicago/Turabian StyleSenko, Olga, Nikolay Stepanov, Olga Maslova, and Elena Efremenko. 2025. "What Can Contribute to Weakening of Poly(Vinyl Alcohol) Cryogels Used for Cell (Self)Immobilization?" Applied Microbiology 5, no. 3: 97. https://doi.org/10.3390/applmicrobiol5030097
APA StyleSenko, O., Stepanov, N., Maslova, O., & Efremenko, E. (2025). What Can Contribute to Weakening of Poly(Vinyl Alcohol) Cryogels Used for Cell (Self)Immobilization? Applied Microbiology, 5(3), 97. https://doi.org/10.3390/applmicrobiol5030097







